Application of Antibodies to Neuronally Expressed Nogo-A Increases Neuronal Survival and Neurite Outgrowth
Abstract
1. Introduction
2. Results
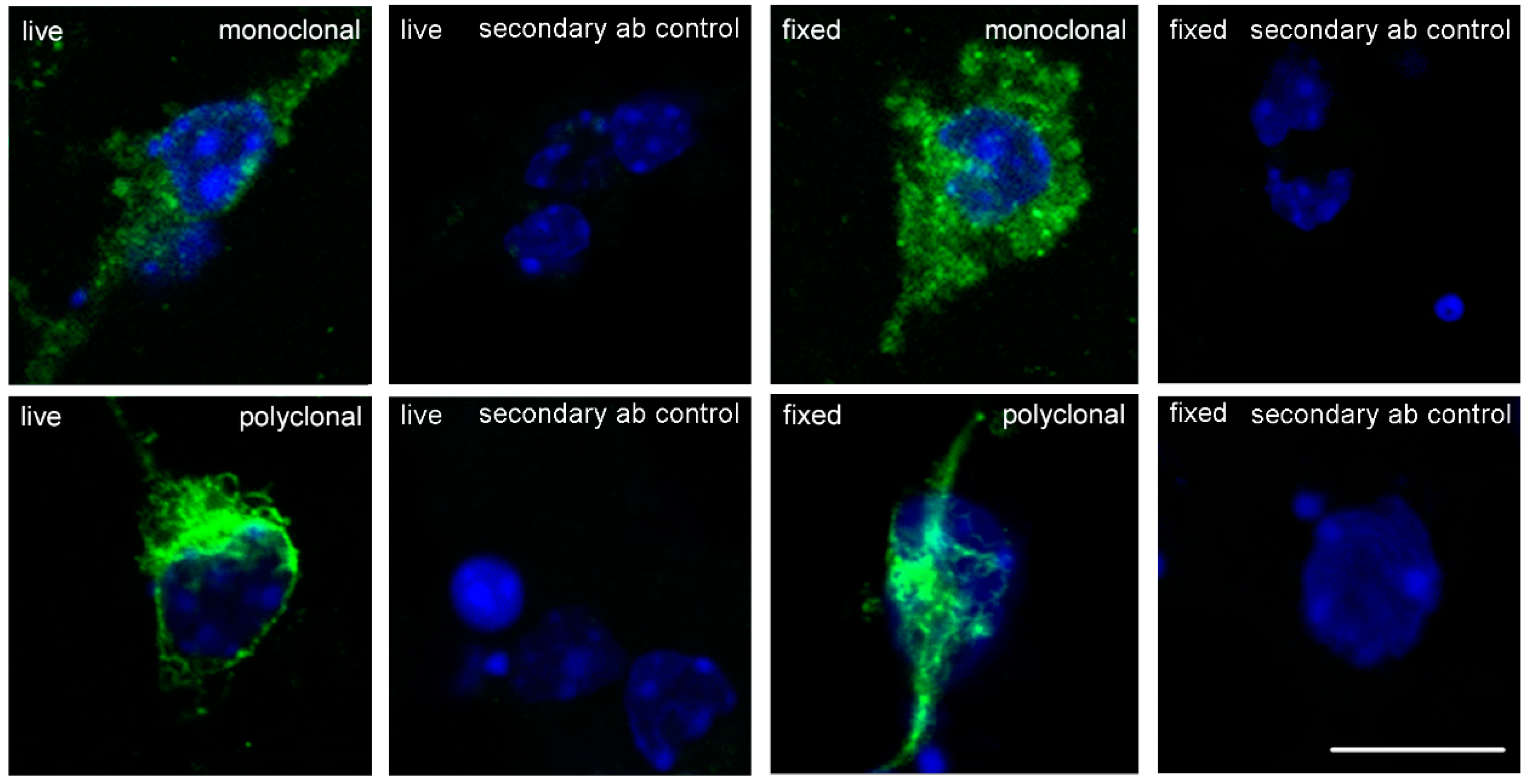
2.1. Nogo-A Is Expressed in Cultured Cerebellar Granule Neurons
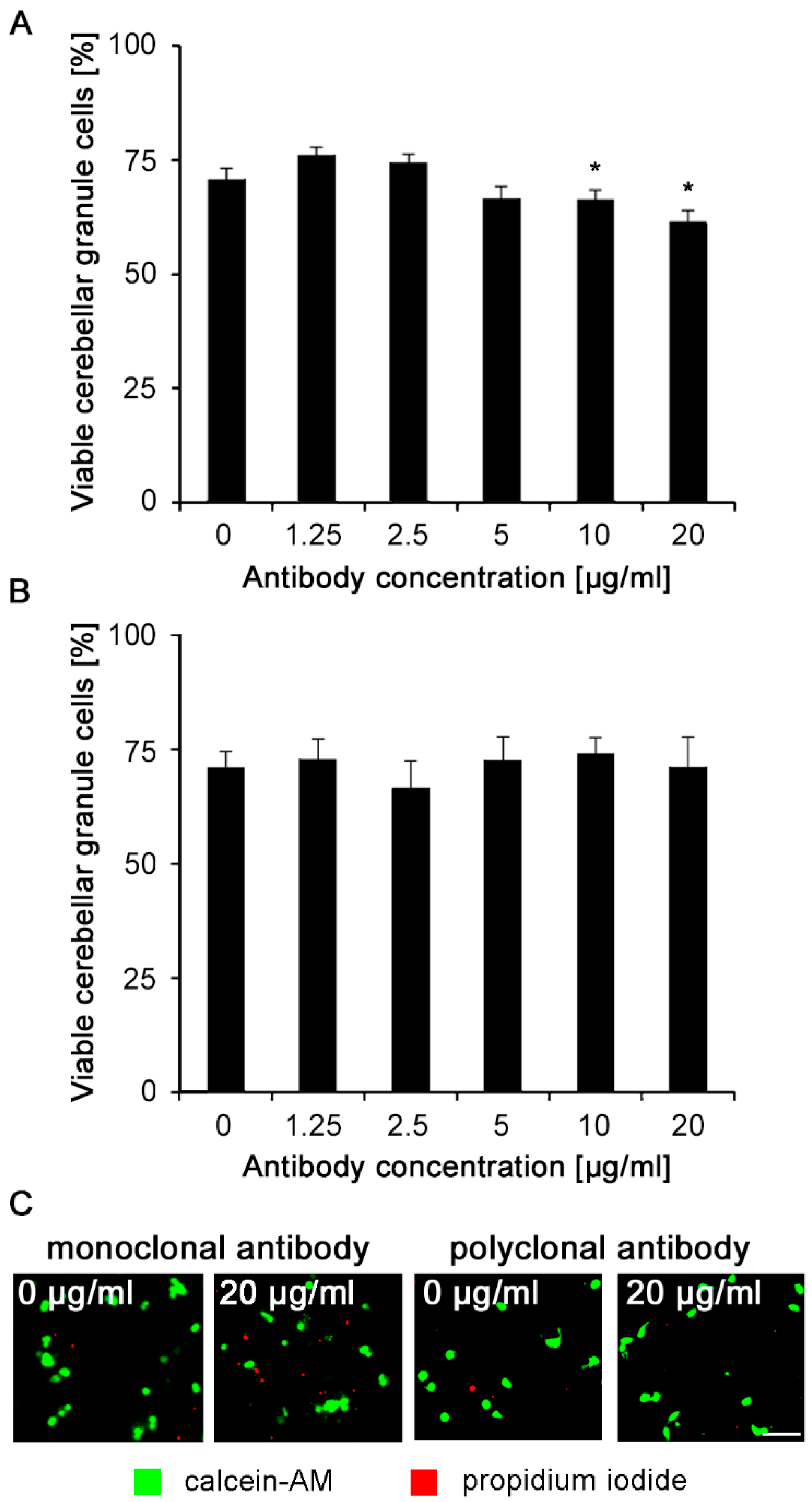
2.2. Nogo-A Antibodies Are Not Neurotoxic
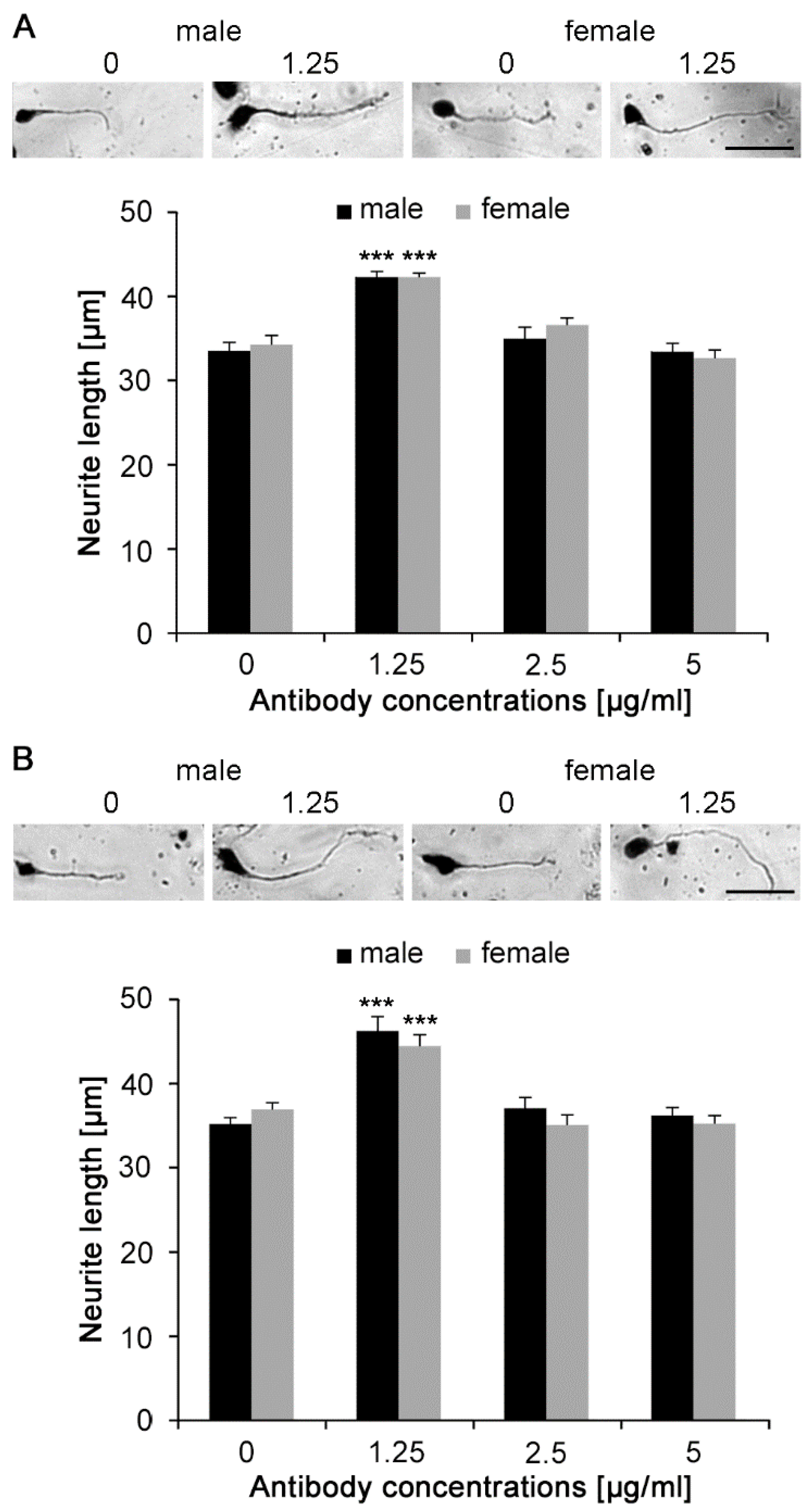
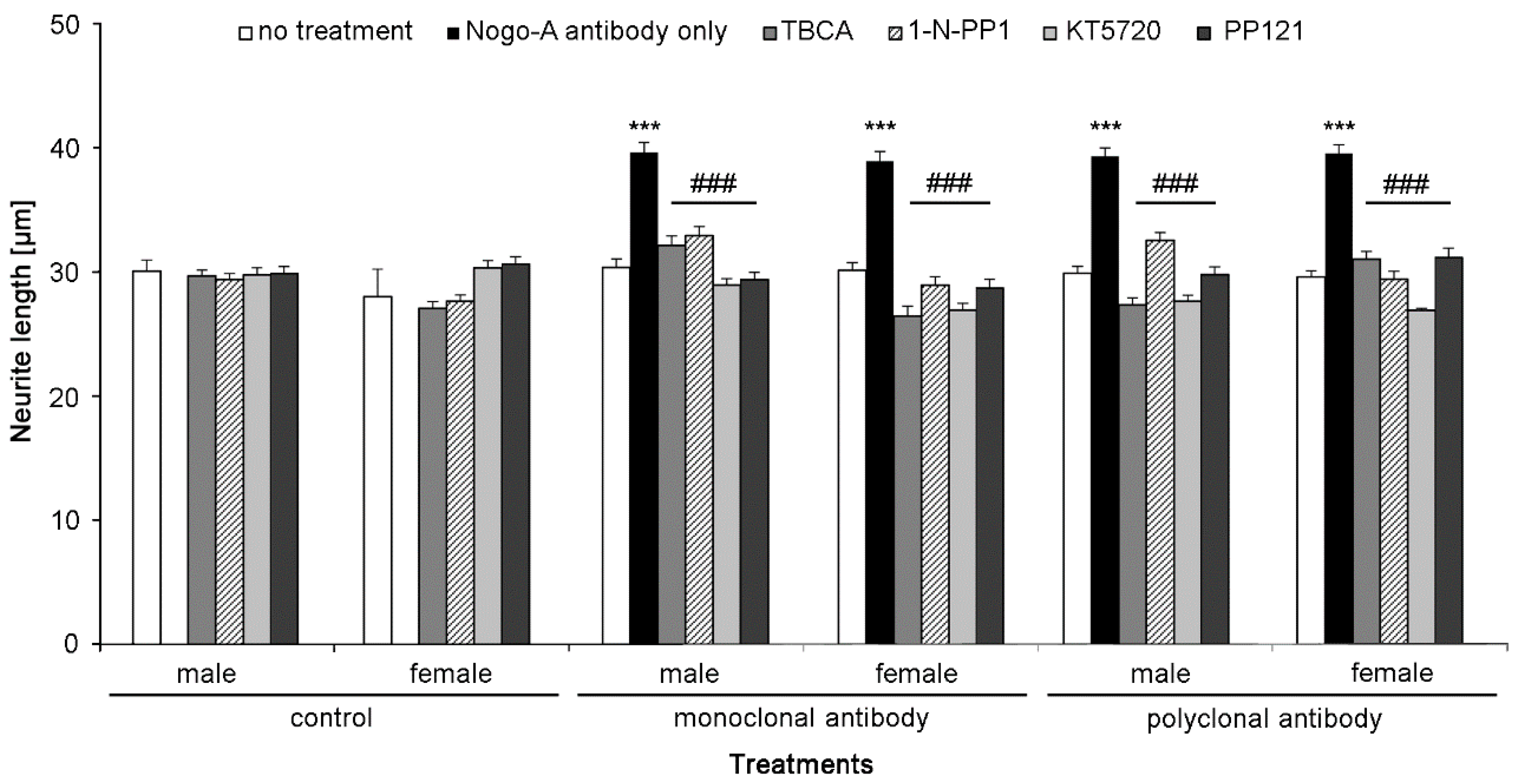
2.3. Nogo-A Antibodies Stimulate Neurite Outgrowth
2.4. Nogo-A Antibodies Promote Cell Survival under Oxidative Stress Conditions
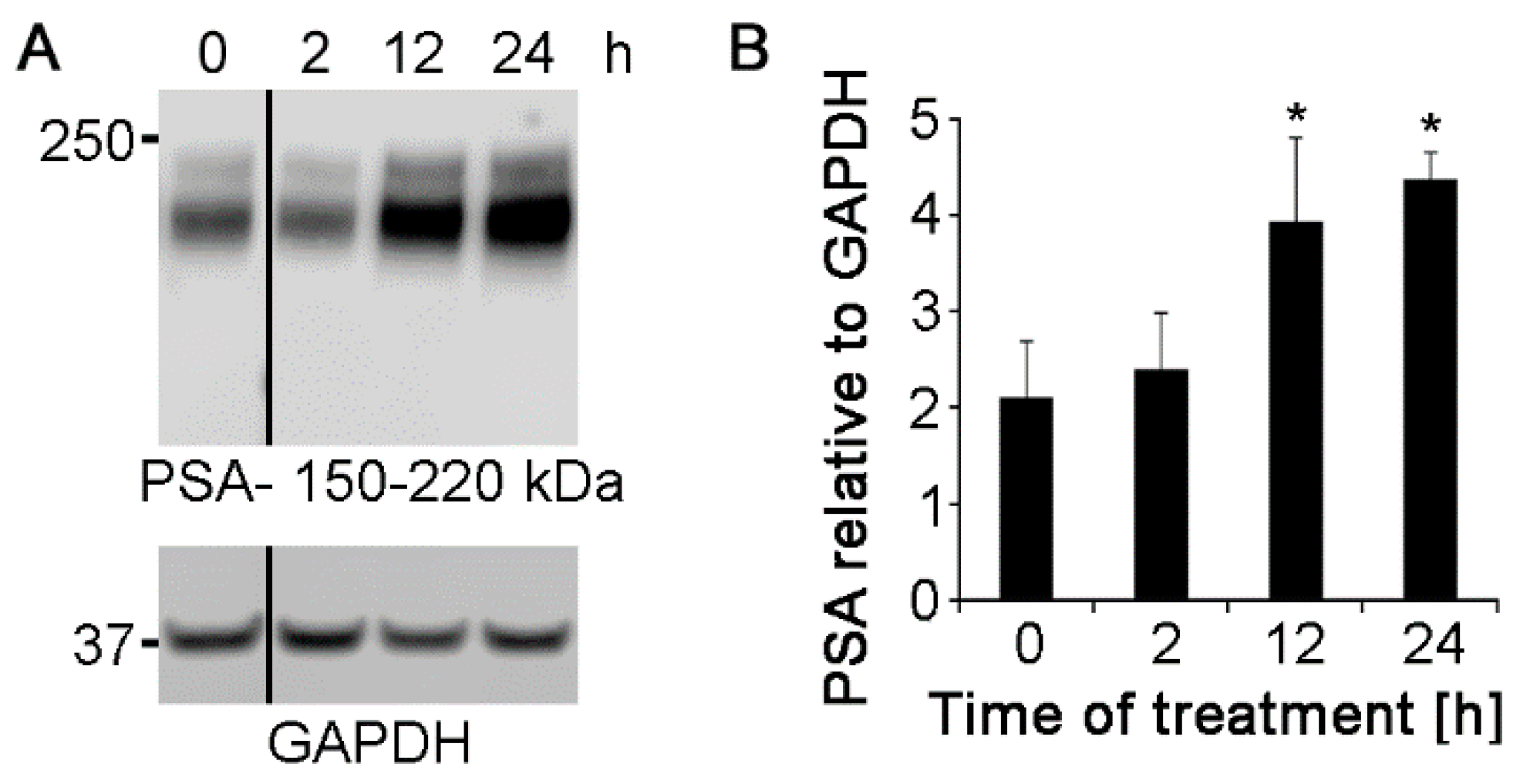
2.5. Polyclonal Nogo-A Antibody Increases the Expression of L1 Protein and Polysialic Acid Glycan
2.6. Nogo-A Antibodies Trigger Cell Signaling Pathways Required for Neurite Outgrowth
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibodies and Reagents
4.3. Preparation of Dissociated Cerebellar Granule Neurons
4.4. Immunostaining
4.5. Cell Survival
4.6. Neurite Outgrowth
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BCA | bicinchoninic acid |
| BSA | bovine serum albumin |
| CKII | casein kinase II |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DRG | dorsal root ganglion |
| EDTA | ethylenediaminetetraacetic acid |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| H2O2 | hydrogen peroxide |
| HBSS | Hanks balanced salt solution |
| PBS | phosphate buffered saline |
| PLL | poly-L-lysine |
| PLSD | protected least significant difference |
| SDS | sodium dodecyl sulfate |
| SEM | standard error of the mean |
| TBST | Tween-20 in Tris-base |
References
- Schwab, M.E.; Caroni, P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 1988, 8, 2381–2393. [Google Scholar] [CrossRef]
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef]
- Prinjha, R.; Moore, S.E.; Vinson, M.; Blake, S.; Morrow, R.; Christie, G.; Michalovich, D.; Simmons, D.L.; Walsh, F.S. Inhibitor of neurite outgrowth in humans. Nature 2000, 403, 383–384. [Google Scholar] [CrossRef]
- Simonen, M.; Pedersen, V.; Weinmann, O.; Schnell, L.; Buss, A.; Ledermann, B.; Christ, F.; Sansig, G.; van der Putten, H.; Schwab, M.E. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 2003, 38, 201–211. [Google Scholar] [CrossRef]
- Zemmar, A.; Chen, C.C.; Weinmann, O.; Kast, B.; Vajda, F.; Bozeman, J.; Isaad, N.; Zuo, Y.; Schwab, M.E. Oligodendrocyte- and neuron-specific Nogo-A restrict dendritic branching and spine density in the adult mouse motor cortex. Cereb Cortex 2018, 28, 2109–2117. [Google Scholar] [CrossRef]
- Vajda, F.; Jordi, N.; Dalkara, D.; Joly, S.; Christ, F.; Tews, B.; Schwab, M.E.; Pernet, V. Cell type-specific Nogo-A gene ablation promotes axonal regeneration in the injured adult optic nerve. Cell Death Differ. 2015, 22, 323–335. [Google Scholar] [CrossRef]
- Dimou, L.; Schnell, L.; Montani, L.; Duncan, C.; Simonen, M.; Schneider, R.; Liebscher, T.; Gullo, M.; Schwab, M.E. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J. Neurosci. 2006, 26, 5591–5603. [Google Scholar] [CrossRef]
- Caroni, P.; Schwab, M.E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 1988, 106, 1281–1288. [Google Scholar] [CrossRef]
- Kim, J.E.; Li, S.; GrandPre, T.; Qiu, D.; Strittmatter, S.M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 2003, 38, 187–199. [Google Scholar] [CrossRef]
- Zheng, B.; Ho, C.; Li, S.; Keirstead, H.; Steward, O.; Tessier-Lavigne, M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 2003, 38, 213–224. [Google Scholar] [CrossRef]
- Huber, A.B.; Weinmann, O.; Brosamle, C.; Oertle, T.; Schwab, M.E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002, 22, 3553–3567. [Google Scholar] [CrossRef]
- Wang, X.; Chun, S.J.; Treloar, H.; Vartanian, T.; Greer, C.A.; Strittmatter, S.M. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J. Neurosci. 2002, 22, 5505–5515. [Google Scholar] [CrossRef]
- Schachner, M.; Faissner, A.; Kruse, J.; Lindner, J.; Meier, D.H.; Rathjen, F.G.; Wernecke, H. Cell-type specificity and developmental expression of neural cell-surface components involved in cell interactions and of structurally related molecules. Cold Spring Harb. Symp. Quant. Biol. 1983, 48 Pt 2, 557–568. [Google Scholar] [CrossRef]
- GrandPre, T.; Nakamura, F.; Vartanian, T.; Strittmatter, S.M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 2000, 403, 439–444. [Google Scholar] [CrossRef]
- Merkler, D.; Metz, G.A.; Raineteau, O.; Dietz, V.; Schwab, M.E.; Fouad, K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J. Neurosci. 2001, 21, 3665–3673. [Google Scholar] [CrossRef]
- Josephson, A.; Widenfalk, J.; Widmer, H.W.; Olson, L.; Spenger, C. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp. Neurol. 2001, 169, 319–328. [Google Scholar] [CrossRef]
- Hunt, D.; Coffin, R.S.; Prinjha, R.K.; Campbell, G.; Anderson, P.N. Nogo-A expression in the intact and injured nervous system. Mol. Cell Neurosci. 2003, 24, 1083–1102. [Google Scholar] [CrossRef]
- Pot, C.; Simonen, M.; Weinmann, O.; Schnell, L.; Christ, F.; Stoeckle, S.; Berger, P.; Rulicke, T.; Suter, U.; Schwab, M.E. Nogo-A expressed in Schwann cells impairs axonal regeneration after peripheral nerve injury. J. Cell Biol. 2002, 159, 29–35. [Google Scholar] [CrossRef]
- Schmandke, A.; Schmandke, A.; Schwab, M.E. Nogo-A: Multiple Roles in CNS Development, Maintenance, and Disease. Neuroscientist 2014, 20, 372–386. [Google Scholar] [CrossRef]
- Cassens, C.; Kleene, R.; Xiao, M.F.; Friedrich, C.; Dityateva, G.; Schafer-Nielsen, C.; Schachner, M. Binding of the receptor tyrosine kinase TrkB to the neural cell adhesion molecule (NCAM) regulates phosphorylation of NCAM and NCAM-dependent neurite outgrowth. J. Biol. Chem. 2010, 285, 28959–28967. [Google Scholar] [CrossRef]
- Lee, J.K.; Chan, A.F.; Luu, S.M.; Zhu, Y.; Ho, C.; Tessier-Lavigne, M.; Zheng, B. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J. Neurosci. 2009, 29, 8649–8654. [Google Scholar] [CrossRef]
- Lee, J.K.; Geoffroy, C.G.; Chan, A.F.; Tolentino, K.E.; Crawford, M.J.; Leal, M.A.; Kang, B.; Zheng, B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 2010, 66, 663–670. [Google Scholar] [CrossRef]
- Bartsch, U.; Bandtlow, C.E.; Schnell, L.; Bartsch, S.; Spillmann, A.A.; Rubin, B.P.; Hillenbrand, R.; Montag, D.; Schwab, M.E.; Schachner, M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 1995, 15, 1375–1381. [Google Scholar] [CrossRef]
- Guitart, K.; Loers, G.; Buck, F.; Bork, U.; Schachner, M.; Kleene, R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 2016, 64, 896–910. [Google Scholar] [CrossRef]
- Loers, G.; Astafiev, S.; Hapiak, Y.; Saini, V.; Mishra, B.; Gul, S.; Kaur, G.; Schachner, M.; Theis, T. The polysialic acid mimetics idarubicin and irinotecan stimulate neuronal survival and neurite outgrowth and signal via protein kinase C. J. Neurochem. 2017, 142, 392–406. [Google Scholar] [CrossRef]
- Loers, G.; Chen, S.; Grumet, M.; Schachner, M. Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J. Neurochem. 2005, 92, 1463–1476. [Google Scholar] [CrossRef]
- Petrinovic, M.M.; Duncan, C.S.; Bourikas, D.; Weinman, O.; Montani, L.; Schroeter, A.; Maerki, D.; Sommer, L.; Stoeckli, E.T.; Schwab, M.E. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development 2010, 137, 2539–2550. [Google Scholar] [CrossRef]
- Meves, J.M.; Geoffroy, C.G.; Kim, N.D.; Kim, J.J.; Zheng, B. Oligodendrocytic but not neuronal Nogo restricts corticospinal axon sprouting after CNS injury. Exp. Neurol. 2018, 309, 32–43. [Google Scholar] [CrossRef]
- Theis, T.; Johal, A.S.; Kabat, M.; Basak, S.; Schachner, M. Enhanced neuronal survival and neurite outgrowth triggered by novel small organic compounds mimicking the LewisX glycan. Mol. Neurobiol. 2018, 55, 8203–8215. [Google Scholar] [CrossRef]
- Garcia-Maya, M.; Anderson, A.A.; Kendal, C.E.; Kenny, A.V.; Edwards-Ingram, L.C.; Holladay, A.; Saffell, J.L. Ligand concentration is a driver of divergent signaling and pleiotropic cellular responses to FGF. J. Cell Physiol. 2006, 206, 386–393. [Google Scholar] [CrossRef]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural cell adhesion molecules of the immunoglobulin superfamily regulate synapse formation, maintenance, and function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef]
- Welte, C.; Engel, S.; Stuermer, C.A. Upregulation of the zebrafish Nogo-A homologue, Rtn4b, in retinal ganglion cells is functionally involved in axon regeneration. Neural Dev. 2015, 10, 6. [Google Scholar] [CrossRef]
- Bodrikov, V.; Welte, C.; Wiechers, M.; Weschenfelder, M.; Kaur, G.; Shypitsyna, A.; Pinzon-Olejua, A.; Bastmeyer, M.; Stuermer, C.A.O. Substrate properties of zebrafish Rtn4b/Nogo and axon regeneration in the zebrafish optic nerve. J. Comp. Neurol. 2017, 525, 2991–3009. [Google Scholar] [CrossRef]
- Jones, J.A.; Starkey, J.R.; Kleinhofs, A. Toxicity and mutagenicity of sodium azide in mammalian cell cultures. Mutat. Res. 1980, 77, 293–299. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, V.; Theis, T.; Johal, A.S.; Seth, A.; Gore, J.; Arsha, N.; Patel, M.; Hao, H.B.; Kurian, N.; Schachner, M. Application of Antibodies to Neuronally Expressed Nogo-A Increases Neuronal Survival and Neurite Outgrowth. Int. J. Mol. Sci. 2020, 21, 5417. https://doi.org/10.3390/ijms21155417
Nagaraj V, Theis T, Johal AS, Seth A, Gore J, Arsha N, Patel M, Hao HB, Kurian N, Schachner M. Application of Antibodies to Neuronally Expressed Nogo-A Increases Neuronal Survival and Neurite Outgrowth. International Journal of Molecular Sciences. 2020; 21(15):5417. https://doi.org/10.3390/ijms21155417
Chicago/Turabian StyleNagaraj, Vini, Thomas Theis, Anmol Singh Johal, Arihant Seth, Jada Gore, Neha Arsha, Mukti Patel, Helen Baixia Hao, Nikki Kurian, and Melitta Schachner. 2020. "Application of Antibodies to Neuronally Expressed Nogo-A Increases Neuronal Survival and Neurite Outgrowth" International Journal of Molecular Sciences 21, no. 15: 5417. https://doi.org/10.3390/ijms21155417
APA StyleNagaraj, V., Theis, T., Johal, A. S., Seth, A., Gore, J., Arsha, N., Patel, M., Hao, H. B., Kurian, N., & Schachner, M. (2020). Application of Antibodies to Neuronally Expressed Nogo-A Increases Neuronal Survival and Neurite Outgrowth. International Journal of Molecular Sciences, 21(15), 5417. https://doi.org/10.3390/ijms21155417




