Functional Alterations in the Olfactory Neuronal Circuit Occur before Hippocampal Plasticity Deficits in the P301S Mouse Model of Tauopathy: Implications for Early Diagnosis and Translational Research in Alzheimer’s Disease
Abstract
1. Introduction
2. Results
2.1. Network Oscillations and Connectivity
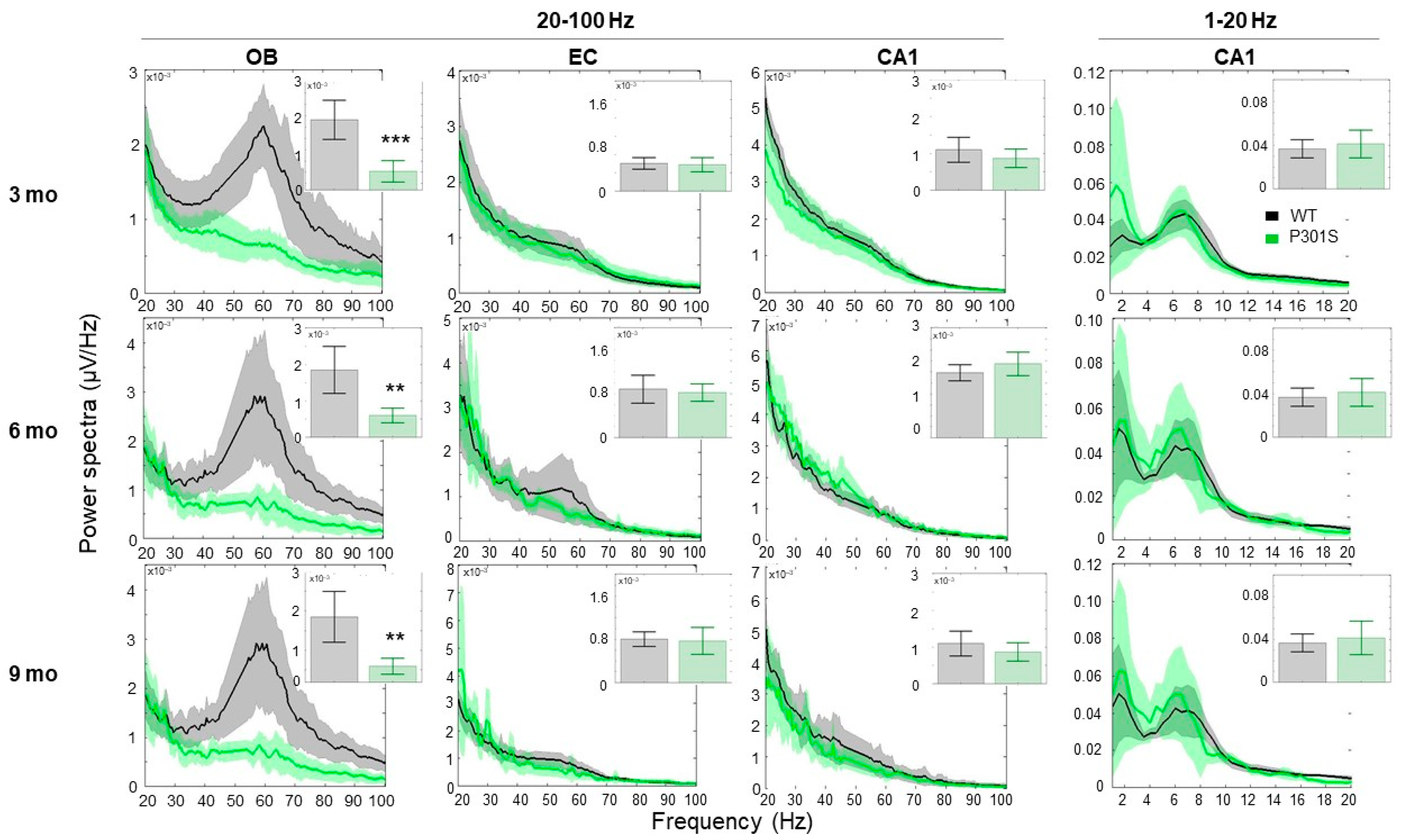
2.1.1. P301S Animals Display Early Reduction in the Gamma Frequency Oscillations, Specifically in the OB Area
2.1.2. P301S Animals Show Severe Early Impairments in the Theta-Gamma Phase Amplitude Coupling in the OB Region
2.2. In Vivo Electrophysiology
2.2.1. Establishing Criteria, Pharmacology, and Sensitivity of the Tetanization Protocol to Modulation of the Glutamatergic NMDA Signaling
2.2.2. Plasticity Assessment in Three, Six and Nine-Month-Old P301S Mice and WT Littermates
3. Discussion
3.1. P301S Mice Exhibited a Prominent Reduction in Gamma Oscillations at an Early Age of Three Months that Was Maintained While Getting Older
3.2. P301S Mice Exhibited Early Connectivity Deficits at the OB Circuit
3.3. P301S Mice Exhibited Deficits in LTP Response at Six Months of Age
3.4. At Six Months of Age, a Clear Trend of Impairments Was Observed in EPSP I/O Values and a Significant Decay Was Observed in the LTP Response in P301S Mice
3.5. Translational Perspective
4. Materials and Methods
4.1. Animals
4.2. In Vivo Local Field Potential (LFP) Procedures
4.2.1. Surgery
4.2.2. Experimental Design, Recording, and Analysis
4.2.3. LFP Spectra
4.2.4. Phase-Amplitude Cross-Frequency Coupling
4.3. In-Vivo Electrophysiology Procedure
4.3.1. Surgery
4.3.2. Basal Synaptic Activity and Inclusion and Exclusion Criteria
4.3.3. LTP Induction
4.4. Genotype and Histological Confirmation of Recording Sites
4.5. Pharmacological Validation
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewczuk, P.; Mroczko, B.; Fagan, A.; Kornhuber, J. Biomarkers of Alzheimer’s disease and mild cognitive impairment: A current perspective. Adv. Med. Sci. 2015, 60, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Figurski, M.; Coart, E.; Blennow, K.; Soares, H.; Simon, A.J.; Lewczuk, P.; Dean, R.A.; et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011, 121, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Xie, S.; Chittams, J.; Ewbank, D.; Peskind, E.; Galasko, D.; Morris, J.C.; McKeel, D.W.; Farlow, M.; Weitlauf, S.L.; et al. Cerebrospinal fluid tau and beta-amyloid: How well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch. Neurol. 2003, 60, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Tapiola, T.; Alafuzoff, I.; Herukka, S.K.; Parkkinen, L.; Hartikainen, P.; Soininen, H.; Pirttilä, T. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009, 66, 382–389. [Google Scholar] [CrossRef]
- Hesse, C.; Rosengren, L.; Andreasen, N.; Davidsson, P.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 2001, 297, 187–190. [Google Scholar] [CrossRef]
- Ost, M. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 2006, 67, 1600–1604. [Google Scholar] [CrossRef]
- Walsh, C.; Drinkenburg, W.; Ahnaou, A. Neurophysiological assessment of neural network plasticity and connectivity: Progress towards early functional biomarkers for disease interception therapies in Alzheimer’s disease. Neurosci. Biobehav. Rev. 2017, 73, 340–358. [Google Scholar] [CrossRef]
- Killiany, R.J.; Hyman, B.T.; Gomez-Isla, T.M.D.P.; Moss, M.B.; Kikinis, R.; Jolesz, F.; Tanzi, R.; Jones, K.; Albert, M.S. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 2002, 58, 1188–1196. [Google Scholar] [CrossRef]
- Bathini, P.; Brai, E.; Auber, L.A. Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Res. Rev. 2019, 55, 100956. [Google Scholar] [CrossRef]
- Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 11–24. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, D.; Liu, L.; Zhang, H.; Zhou, Y. Olfactory dysfunction in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 2, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Rey, N.L.; Wesson, D.W.; Brundin, P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. 2018, 109, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Ohm, T.G.; Braak, H. Olfactory bulb changes in Alzheimer’s disease. Acta Neuropathol. 1987, 73, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Masurkar, A.V.; Devanand, D.P. Olfactory dysfunction in the elderly: Basic circuitry and alterations with normal aging and Alzheimer’s disease. Curr. Geriatr. Rep. 2014, 3, 91–100. [Google Scholar] [CrossRef]
- Christianson, T.J.; Kremers, W.K.; Mielke, M.M.; Machulda, M.M.; Vassilaki, M.; Alhurani, R.E.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016, 73, 93–101. [Google Scholar]
- Braak, H.; Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 1995, 16, 271–278. [Google Scholar] [CrossRef]
- Marui, W.; Iseki, E.; Nakai, T.; Miura, S.; Kato, M.; Uéda, K.; Kosaka, K. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J. Neurol. Sci. 2002, 95, 153–159. [Google Scholar] [CrossRef]
- Metzler-Baddeley, C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex 2007, 43, 583–600. [Google Scholar] [CrossRef]
- Bosman, C.A.; Lansink, C.S.; Pennartz, C.M. Functions of gamma-band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. Eur. J. Neurosci. 2014, 39, 1982–1999. [Google Scholar] [CrossRef]
- Buzsáki, G.; Wang, X.J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- Buzsáki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: LTP in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nadel, L.; Bohbot, V. Consolidation of memory. Hippocampus 2001, 11, 56–60. [Google Scholar] [CrossRef]
- Bugiani, O.; Murrell, J.R.; Giaccone, G.; Hasegawa, M.; Ghigo, G.; Tabaton, M.; Morbin, M.; Primavera, A.; Carella, F.; Solaro, C.; et al. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J. Neuropathol. Exp. Neurol. 1999, 58, 667–677. [Google Scholar] [CrossRef]
- Holmes, B.B.; Furman, J.L.; Mahan, T.E.; Yamasaki, T.R.; Mirbaha, H.; Eades, W.C.; Belaygorod, L.; Cairns, N.J.; Holtzman, D.M.; Diamond, M.I. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, E4376–E4385. [Google Scholar] [CrossRef]
- López-González, I.; Aso, E.; Carmona, M.; Armand-Ugon, M.; Blanco, R.; Naudí, A.; Cabré, R.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Neuroinflammatory gene regulation, mitochondrial function, oxidative stress, and brain lipid modifications with disease progression in tau P301S transgenic mice as a model of frontotemporal lobar degeneration-tau. J. Neuropathol. Exp. Neurol. 2015, 74, 975–999. [Google Scholar] [CrossRef]
- Yamada, K.; Cirrito, J.R.; Stewart, F.R.; Jiang, H.; Finn, M.B.; Holmes, B.B.; Binder, L.I.; Mandelkow, E.M.; Diamond, M.I.; Lee, V.M.Y.; et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J. Neurosci. 2011, 31, 13110–13117. [Google Scholar] [CrossRef]
- Briggs, D.I.; Defensor, E.; Ardestani, P.M.; Yi, B.; Halpain, M.; Seabrook, G.; Shamloo, M. Role of endoplasmic reticulum stress in learning and memory impairment and Alzheimer’s disease—Like neuropathology in the P301S and APP swe mouse models of tauopathy and amyloidosis. ENeuro 2017, 4, 1–25. [Google Scholar] [CrossRef]
- Holth, J.K.; Mahan, T.E.; Robinson, G.O.; Rocha, A.; Holtzman, D.M. Altered sleep and EEG power in the P301S Tau transgenic mouse model. Ann. Clin. Transl. Neurol. 2017, 15, 180–190. [Google Scholar] [CrossRef]
- Takeuchi, H.; Iba, M.; Inoue, H.; Higuchi, M.; Takao, K.; Tsukita, K.; Karatsu, Y.; Iwamoto, Y.; Miyakawa, T.; Suhara, T.; et al. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS ONE 2011, 6, e21050. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Buschler, A.; Goh, J.J.; Manahan-Vaughan, D. Frequency dependency of NMDA receptor-dependent synaptic plasticity in the hippocampal CA1 region of freely behaving mice. Hippocampus 2012, 22, 2238–2248. [Google Scholar] [CrossRef]
- Cooke, S.F.; Wu, J.; Plattner, F.; Errington, M.; Rowan, M.; Peters, M.; Hirano, A.; Bradshaw, K.D.; Anwyl, R.; Bliss, T.V.; et al. Autophosphorylation of αCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. J. Physiol. 2006, 574, 805–818. [Google Scholar] [CrossRef]
- Wong, E.H.; Kemp, J.A.; Priestley, T.; Knight, A.R.; Woodruff, G.N.; Iversen, L.L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc. Natl. Acad. Sci. USA 1986, 83, 7104–7108. [Google Scholar] [CrossRef]
- Dravid, S.M.; Erreger, K.; Yuan, H.; Nicholson, K.; Le, P.; Lyuboslavsky, P.; Almonte, A.; Murray, E.; Mosley, C.; Barber, J.; et al. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J. Physiol. 2007, 581, 107–128. [Google Scholar] [CrossRef]
- Abraham, W.C.; Mason, S.E. Effects of the NMDA receptor/channel antagonists CPP and MK801 on hippocampal field potentials and long-term potentiation in anesthetized rats. Brain Res. 1988, 462, 40–46. [Google Scholar] [CrossRef]
- Wiescholleck, V.; Manahan-Vaughan, D. PDE4 inhibition enhances hippocampal synaptic plasticity in vivo and rescues MK801-induced impairment of long-term potentiation and object recognition memory in an animal model of psychosis. Transl. Psychiatry 2012, 2, e89. [Google Scholar] [CrossRef]
- Flunkert, S.; Hierzer, M.; Löffler, T.; Rabl, R.; Neddens, J.; Duller, S.; Schofield, E.L.; Ward, M.A.; Posch, M.; Jungwirth, H.; et al. Elevated levels of soluble total and hyperphosphorylated tau result in early behavioral deficits and distinct changes in brain pathology in a new tau transgenic mouse model. Neurodegener. Dis. 2013, 11, 194–205. [Google Scholar] [CrossRef]
- Koss, D.J.; Robinson, L.; Mietelska-Porowska, A.; Gasiorowska, A.; Sepčić, K.; Turk, T.; Jaspars, M.; Niewiadomska, G.; Scott, R.H.; Platt, B.; et al. Polymeric alkylpyridinium salts permit intracellular delivery of human tau in rat hippocampal neurons: Requirement of tau phosphorylation for functional deficits. Cell. Mol. Life Sci. 2015, 72, 4613–4632. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.M.; William, C.M.; Adamowicz, D.H.; Pitstick, R.; Carlson, G.A.; Spires-Jones, T.L.; Hyman, B.T. Soluble tau species, not neurofibrillary aggregates, disrupt neural system integration in a tau transgenic model. J. Neuropathol. Exp. Neurol. 2011, 70, 588–595. [Google Scholar] [CrossRef]
- Polydoro, M. Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer’s disease model. Acta Neuropathol. 2014, 127, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Lagier, S.; Panzanelli, P.; Russo, R.E.; Nissant, A.; Bathellier, B.; Sassoè-Pognetto, M.; Fritschy, J.; Lledo, P.M. GABAergic inhibition at dendrodendritic synapses tunes oscillations in the olfactory bulb. Proc. Natl. Acad. Sci. USA 2007, 104, 7259–7264. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Manabe, H.; Narikiyo, K.; Onisawa, N. Olfactory consciousness and gamma oscillation couplings across the olfactory bulb, olfactory cortex, and orbitofrontal cortex. Front. Psychol. 2013, 4, 743. [Google Scholar] [CrossRef] [PubMed]
- Kay, L.M. Circuit oscillations in odor perception and memory. Prog. Brain Res. 2014, 208, 223–251. [Google Scholar]
- Stopfer, M.; Bhagavan, S.; Smith, B.H.; Laurent, G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 1997, 390, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Beshel, J.; Kopell, N.; Kay, L. Olfactory bulb gamma oscillations are enhanced with task demands. J. Neurosci. 2007, 27, 8358–8365. [Google Scholar] [CrossRef]
- Kay, L.M.; Beshel, J.; Brea, J.; Martin, C.; Rojas-Líbano, D.; Kopell, N. Olfactory oscillations: The what, how and what for. Trends Neurosci. 2009, 32, 207–214. [Google Scholar] [CrossRef]
- Gervais, R.; Buonviso, N.; Martin, C.; Ravel, N. What do electrophysiological studies tell us about processing at the olfactory bulb level? J. Physiol. Paris 2007, 101, 40–45. [Google Scholar] [CrossRef]
- Vanderwolf, C.H.; Zibrowski, E.M. Pyriform cortex beta-waves: Odor-specific sensitization following repeated olfactory stimulation. Brain Res. 2001, 892, 301–308. [Google Scholar] [CrossRef]
- Kay, L.M. Theta oscillations and sensorimotor performance. Proc. Natl. Acad. Sci. USA 2005, 102, 3863–3868. [Google Scholar] [CrossRef]
- Kadohisa, M.; Wilson, D.A. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 15206–15211. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.E. Accelerated aging of the GABAergic septohippocampal pathway and decreased hippocampal rhythms in a mouse model of Alzheimer’s disease. FASEB J. 2012, 26, 4458–4467. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.D.; Broughton, M.; Finnigan, S. Theta oscillations are affected by amnestic mild cognitive impairment and cognitive load. Int. J. Psychophysiol. 2008, 70, 75–81. [Google Scholar] [CrossRef]
- Babiloni, C.; Cassetta, E.; Binetti, G.; Tombini, M.; del Percio, C.; Ferreri, F.; Ferri, R.; Frisoni, G.; Lanuzza, B.; Nobili, F.; et al. Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer’s disease. Eur. J. Neurosci. 2007, 25, 3742–3757. [Google Scholar] [CrossRef]
- Scott, L.; Feng, J.; Kiss, T.; Needle, E.; Atchison, K.; Kawabe, T.T.; Milici, A.J.; Hajós-Korcsok, É.; Riddell, D.; Hajós, M. Age-dependent disruption in hippocampal θ oscillation in amyloid-β overproducing transgenic mice. Neurobiol. Aging 2012, 33, 1481-e13–1481-e23. [Google Scholar] [CrossRef]
- Peña, F. Beta-amyloid protein (25–35) disrupts hippocampal network activity: Role of Fyn-kinase. Hippocampus 2010, 20, 78–96. [Google Scholar] [CrossRef]
- Balleza-Tapia, H.; Huanosta-Gutiérrez, A.; Márquez-Ramos, A.; Arias, N.; Peña, F. Amyloid β oligomers decrease hippocampal spontaneous network activity in an age-dependent manner. Curr. Alzheimer Res. 2010, 7, 453–462. [Google Scholar] [CrossRef]
- Cayzac, S.; Mons, N.; Ginguay, A.; Allinquant, B.; Jeantet, Y.; Cho, Y.H. Altered hippocampal information coding and network synchrony in APP-PS1 mice. Neurobiol. Aging 2015, 36, 3200–3213. [Google Scholar] [CrossRef]
- Gutiérrez-Lerma, A.I.; Ordaz, B.; Peña-Ortega, F. Amyloid Beta peptides differentially affect hippocampal theta rhythms in vitro. Int. J. Pept. 2013, 2013, 328140. [Google Scholar] [CrossRef] [PubMed]
- Grove, L.M.; Kim, E.; Cooke, J.D.; Holmes, W.R. LTP in hippocampal area CA1 is induced by burst stimulation over a broad frequency range centered around delta. Learn. Mem. 2009, 16, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.A.; Ridler, T.; Murray, T.K.; Ward, M.A.; de Groot, E.; Goodfellow, M.; Phillips, K.G.; Randall, A.D.; Brown, J.T. Electrical and network neuronal properties are preferentially disrupted in dorsal, but not ventral, medial entorhinal cortex in a mouse model of tauopathy. J. Neurosci. 2016, 36, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Ahnaou, A.; Moechars, D.; Raeymaekers, L.; Biermans, R.; Manyakov, N.; Bottelbergs, A.; Wintmolders, C.; Van Kolen, K.; Van De Casteele, T.; Kemp, J.; et al. Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci. Rep. 2017, 7, 14189. [Google Scholar] [CrossRef]
- Ahnaou, A.; Walsh, C.; Manyakov, N.V.; Youssef, S.A.; Drinkenburg, W.H. Early electrophysiological disintegration of hippocampal neural networks in a novel locus coeruleus tau-seeding mouse model of Alzheimer’s disease. Neural. Plast. 2019, 2019, 6981268. [Google Scholar] [CrossRef]
- Drago, V.; Babiloni, C.; Bartrés-Faz, D.; Caroli, A.; Bosch, B.; Hensch, T.; Didic, M.; Klafki, H.W.; Pievani, M.; Jovicich, J.; et al. Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. J. Alzheimers Dis. 2011, 26, 159–199. [Google Scholar] [CrossRef]
- Velayudhan, L.; Pritchard, M.; Powell, J.F.; Proitsi, P.; Lovestone, S. Smell identification function as a severity and progression marker in Alzheimer’s disease. Int. Psychogeriatr. 2013, 25, 1157–1166. [Google Scholar] [CrossRef]
- Wilson, R.S.; Arnold, S.E.; Schneider, J.A.; Boyle, P.A.; Buchman, A.S.; Bennett, D.A. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann. Acad. Sci. 2009, 1170, 730–735. [Google Scholar] [CrossRef]
- Woodward, M.R.; Amrutkar, C.V.; Shah, H.C.; Benedict, R.H.B.; Rajakrishnan, S.; Doody, R.S.; Yan, L.; Szigeti, K. Validation of olfactory deficit as a biomarker of Alzheimer disease. Neurol. Clin. Pract. 2016, 7, 5–14. [Google Scholar] [CrossRef]
- Bazzigaluppi, P.; Beckett, T.L.; Koletar, M.M.; Lai, A.Y.; Joo, I.L.; Brown, M.E.; Carlen, P.L.; McLaurin, J.; Stefanovic, B. Early-stage attenuation of phase-amplitude coupling in the hippocampus and medial prefrontal cortex in a transgenic rat model of Alzheimer’s disease. J. Neurochem. 2018, 144, 669–679. [Google Scholar] [CrossRef]
- Goutagny, R.; Gu, N.; Cavanagh, C.; Jackson, J.; Chabot, J.; Quirion, R.; Krantic, S.; Williams, S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2013, 37, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Salimpour, Y.; Anderson, W.S. Cross-frequency coupling based neuromodulation for treating neurological disorders. Front. Neurosci. 2019, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Tanninen, S.E.; Nouriziabari, B.; Morrissey, M.D.; Bakir, R.; Dayton, R.D.; Klein, R.L.; Takehara-Nishiuchi, K. Entorhinal tau pathology disrupts hippocampal-prefrontal oscillatory coupling during associative learning. Neurobiol. Aging 2017, 58, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Nusser, Z. AMPA and NMDA receptors: Similarities and differences in their synaptic distribution. Curr. Opin. Neurobiol. 2000, 10, 337–341. [Google Scholar] [CrossRef]
- Elie, A.; Prezel, E.; Guérin, C.; Denarier, E.; Ramirez-Rios, S.; Serre, L.; Andrieux, A.; Fourest-Lieuvin, A.; Blanchoin, L.; Arnal, I. Tau co-organizes dynamic microtubule and actin networks. Sci. Rep. 2015, 5, 9964. [Google Scholar] [CrossRef]
- Fulga, T.A.; Elson-Schwab, I.; Khurana, V.; Steinhilb, M.L.; Spires, T.L.; Hyman, B.T.; Feany, M.B. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat. Cell Biol. 2007, 9, 139–148. [Google Scholar] [CrossRef]
- Schindowski, K.; Bretteville, A.; Leroy, K.; Bégard, S.; Brion, J.P.; Hamdane, M.; Buée, L. Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am. J. Pathol. 2006, 169, 599–616. [Google Scholar] [CrossRef]
- Rosenmann, H.; Grigoriadis, N.; Eldar-Levy, H.; Avital, A.; Rozenstein, L.; Touloumi, O.; Behar, L.; Ben-Hur, T.; Avraham, Y.; Berry, E.; et al. A novel transgenic mouse expressing double mutant tau driven by its natural promoter exhibits tauopathy characteristics. Exp. Neurol. 2008, 212, 71–84. [Google Scholar] [CrossRef]
- Hoover, R.B.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Applebaum, S.L.; Giberson, R.; Siksorski, L.; Rosenberg, L. Smell identification ability: Changes with age. Science 1984, 226, 1441–1443. [Google Scholar] [CrossRef]
- Price, J.L. Olfactory system. In The Human Nervous System; Academic Press: New York, NY, USA, 1990; pp. 979–998. [Google Scholar]
- Conti, M.Z.; Vicini-Chilovi, B.; Riva, M.; Zanetti, M.; Liberini, P.; Padovani, A.; Rozzini, L. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer’s disease. Arch. Clin. Neuropsychol. 2013, 28, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Chen, H.; Deal, J.A.; Sharrett, A.R.; Gross, A.; Knopman, D.; Griswold, M.; Heiss, G.; Mosley, T.H. Olfactory function and neurocognitive outcomes in old age: The atherosclerosis risk in communities neurocognitive study. Alzheimers Dement. 2018, 14, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Jones-Gotman, M.; De Sousa, K.; Chertkow, H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2008, 29, 693–706. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Wu, X.; Li, J.; Yang, J.; Tu, C.; Ye, X.; Ling, S. Olfactory deficit is associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice. Brain Res. Bull. 2019, 148, 34–45. [Google Scholar] [CrossRef]
- Yang, S.; Kuan, W.L.; Spillantini, M.G. Progressive tauopathy in P301S tau transgenic mice is associated with a functional deficit of the olfactory system. Eur. J. Neurosci. 2016, 44, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Losacco, J.; Ramirez-Gordillo, D.; Gilmer, J.; Restrepo, D. Learning improves decoding of odor identity with phase-referenced oscillations in the olfactory bulb. Elife 2020, 9, e52583. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Martorell, A.J.; Paulson, A.L.; Suk, H.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory gamma stimulation ameliorates Alzheimer’s—Associated pathology and improves cognition. Cell 2019, 177, 256–271. [Google Scholar] [CrossRef]
- Huang, S.M.; Mouri, A.; Kokubo, H.; Nakajima, R.; Suemoto, T.; Higuchi, M.; Staufenbiel, M.; Noda, Y.; Yamaguchi, H.; Nabeshima, T.; et al. Neprilysin-sensitive synapse-associated amyloid-β peptide oligomers impair neuronal plasticity and cognitive function. J. Biol. Chem. 2006, 281, 17941–17951. [Google Scholar] [CrossRef]
- Namgung, U.; Valcourt, E.; Routtenberg, A. Long-term potentiation in vivo in the intact mouse hippocampus. Brain Res. 1995, 689, 85–92. [Google Scholar] [CrossRef]
- Goh, J.J.; Manahan-Vaughan, D. Synaptic depression in the CA1 region of freely behaving mice is highly dependent on afferent stimulation parameters. Front. Integr. Neurosci. 2013, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahnaou, A.; Rodriguez-Manrique, D.; Biermans, R.; Embrechts, S.; Manyakov, N.V.; Drinkenburg, W.H. Functional Alterations in the Olfactory Neuronal Circuit Occur before Hippocampal Plasticity Deficits in the P301S Mouse Model of Tauopathy: Implications for Early Diagnosis and Translational Research in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 5431. https://doi.org/10.3390/ijms21155431
Ahnaou A, Rodriguez-Manrique D, Biermans R, Embrechts S, Manyakov NV, Drinkenburg WH. Functional Alterations in the Olfactory Neuronal Circuit Occur before Hippocampal Plasticity Deficits in the P301S Mouse Model of Tauopathy: Implications for Early Diagnosis and Translational Research in Alzheimer’s Disease. International Journal of Molecular Sciences. 2020; 21(15):5431. https://doi.org/10.3390/ijms21155431
Chicago/Turabian StyleAhnaou, Abdallah, Daniela Rodriguez-Manrique, Ria Biermans, Sofie Embrechts, Nikolay V. Manyakov, and Wilhelmus H. Drinkenburg. 2020. "Functional Alterations in the Olfactory Neuronal Circuit Occur before Hippocampal Plasticity Deficits in the P301S Mouse Model of Tauopathy: Implications for Early Diagnosis and Translational Research in Alzheimer’s Disease" International Journal of Molecular Sciences 21, no. 15: 5431. https://doi.org/10.3390/ijms21155431
APA StyleAhnaou, A., Rodriguez-Manrique, D., Biermans, R., Embrechts, S., Manyakov, N. V., & Drinkenburg, W. H. (2020). Functional Alterations in the Olfactory Neuronal Circuit Occur before Hippocampal Plasticity Deficits in the P301S Mouse Model of Tauopathy: Implications for Early Diagnosis and Translational Research in Alzheimer’s Disease. International Journal of Molecular Sciences, 21(15), 5431. https://doi.org/10.3390/ijms21155431




