Ablation of Galectin-12 Inhibits Atherosclerosis through Enhancement of M2 Macrophage Polarization
Abstract
1. Introduction
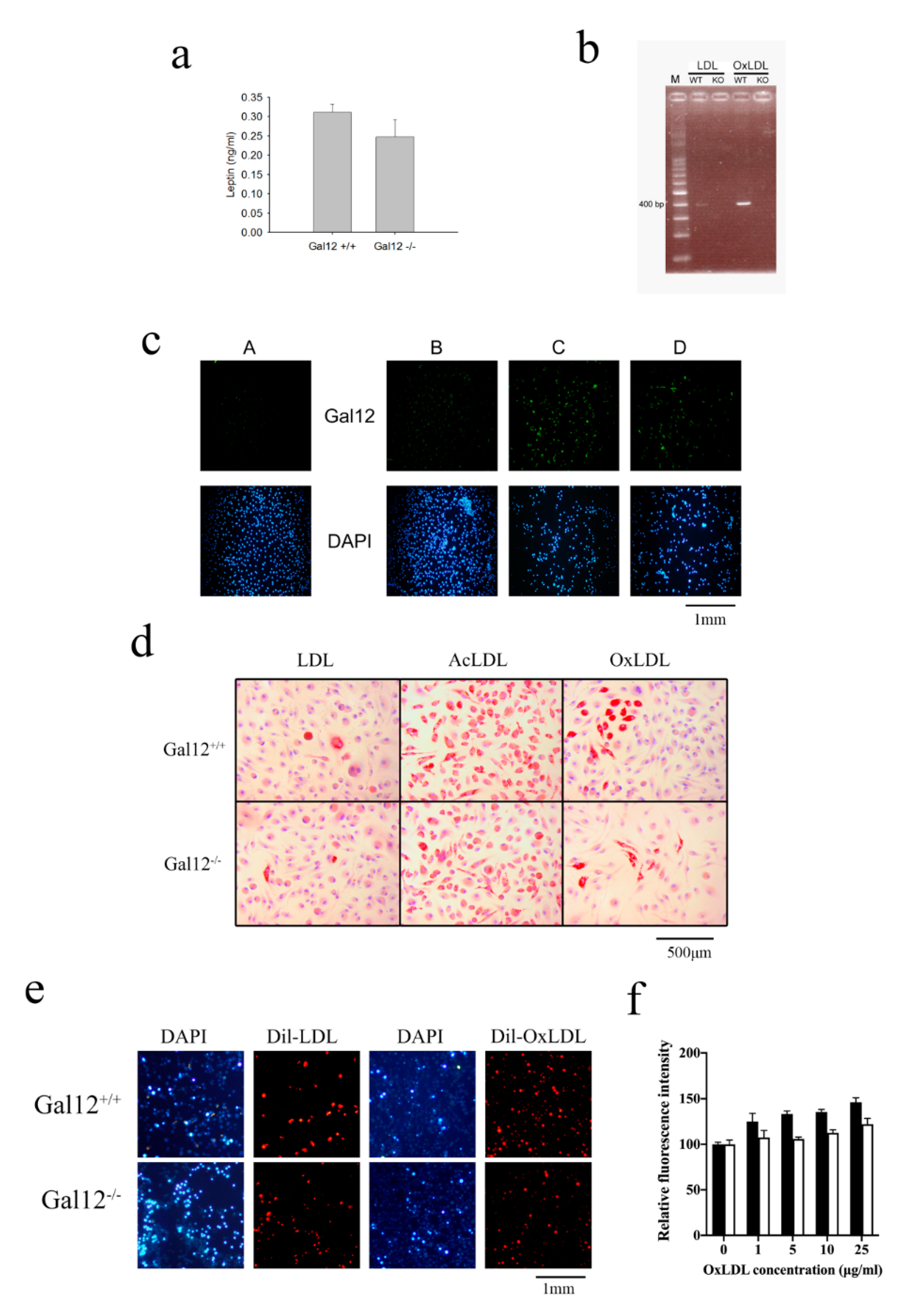
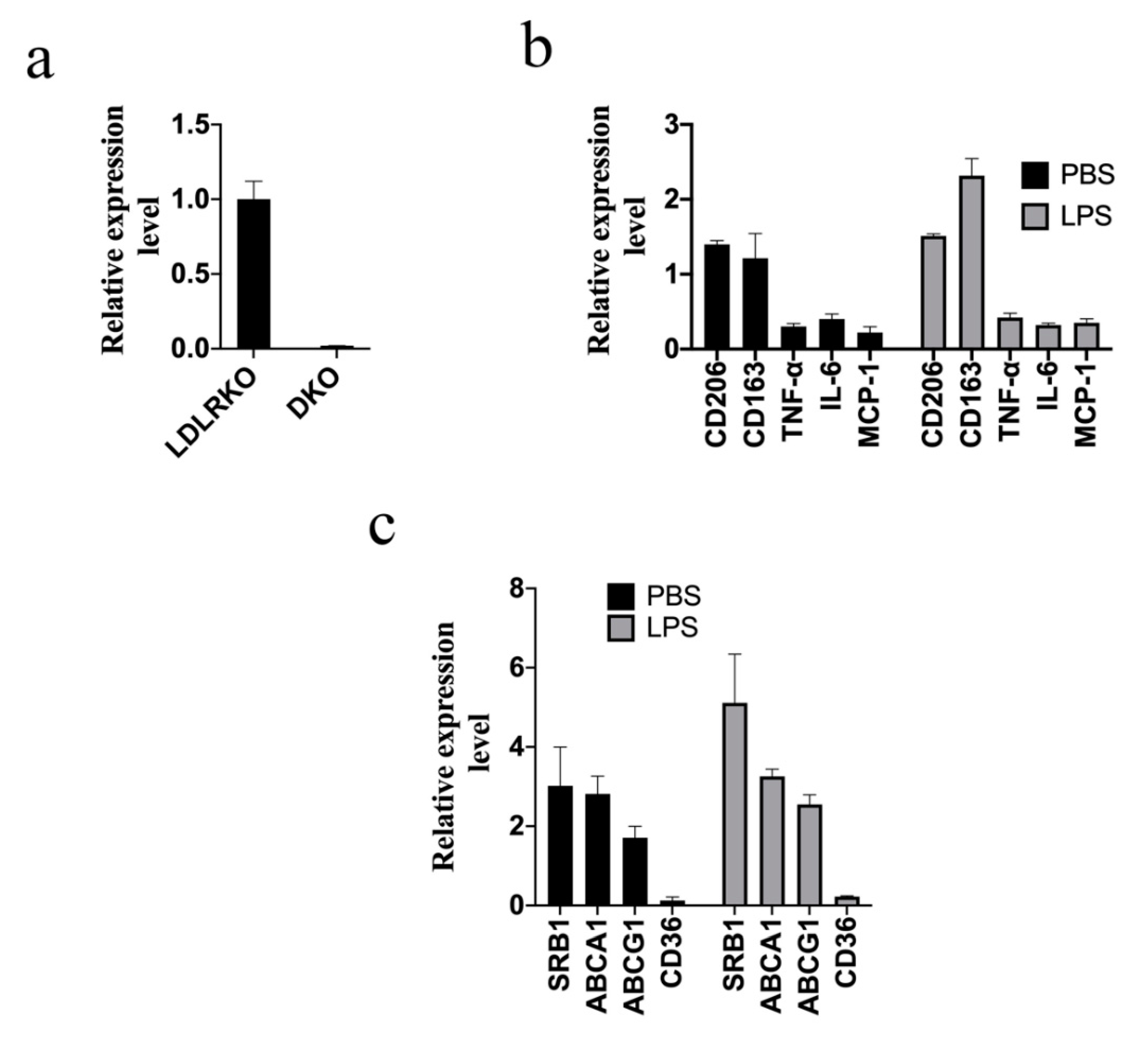
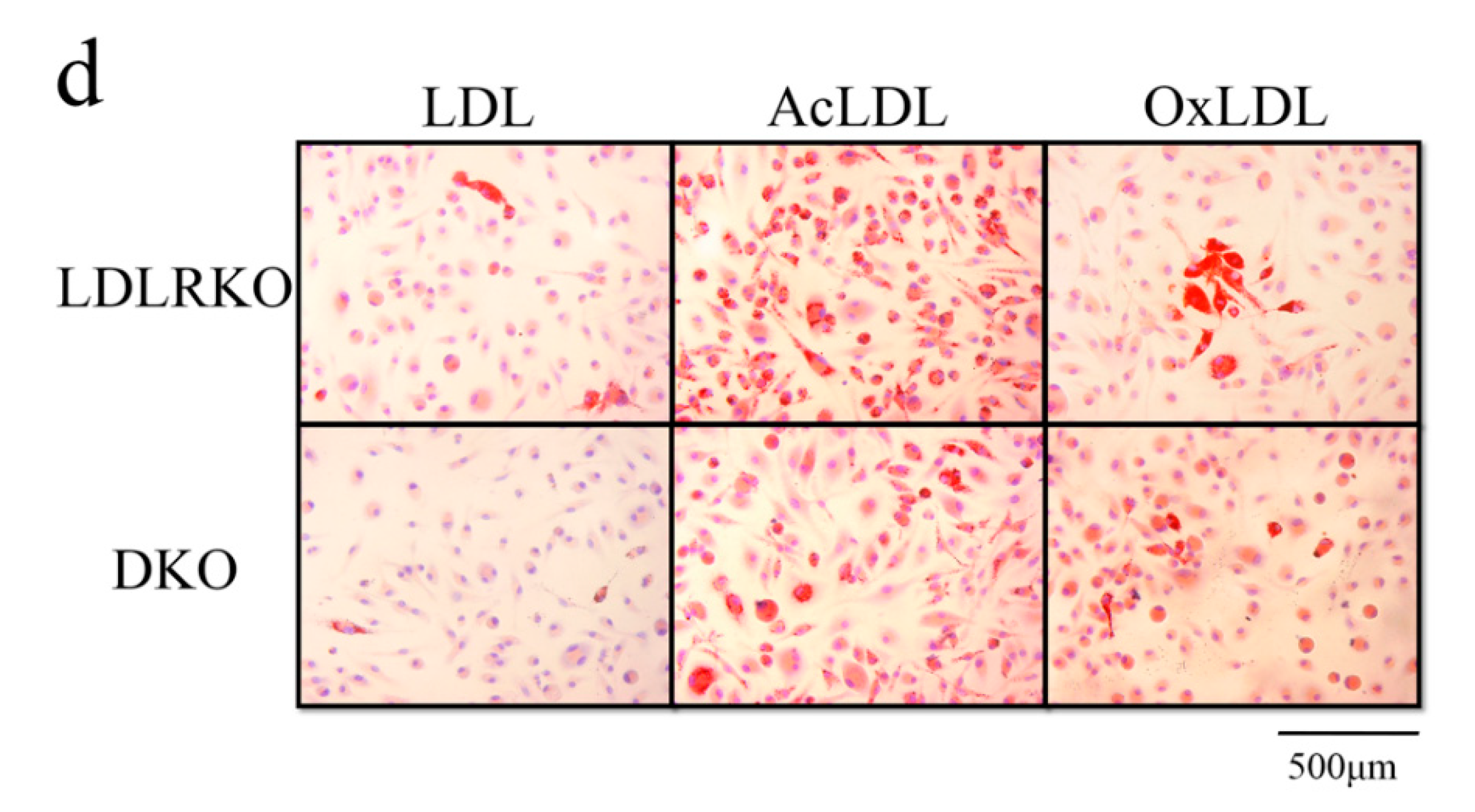
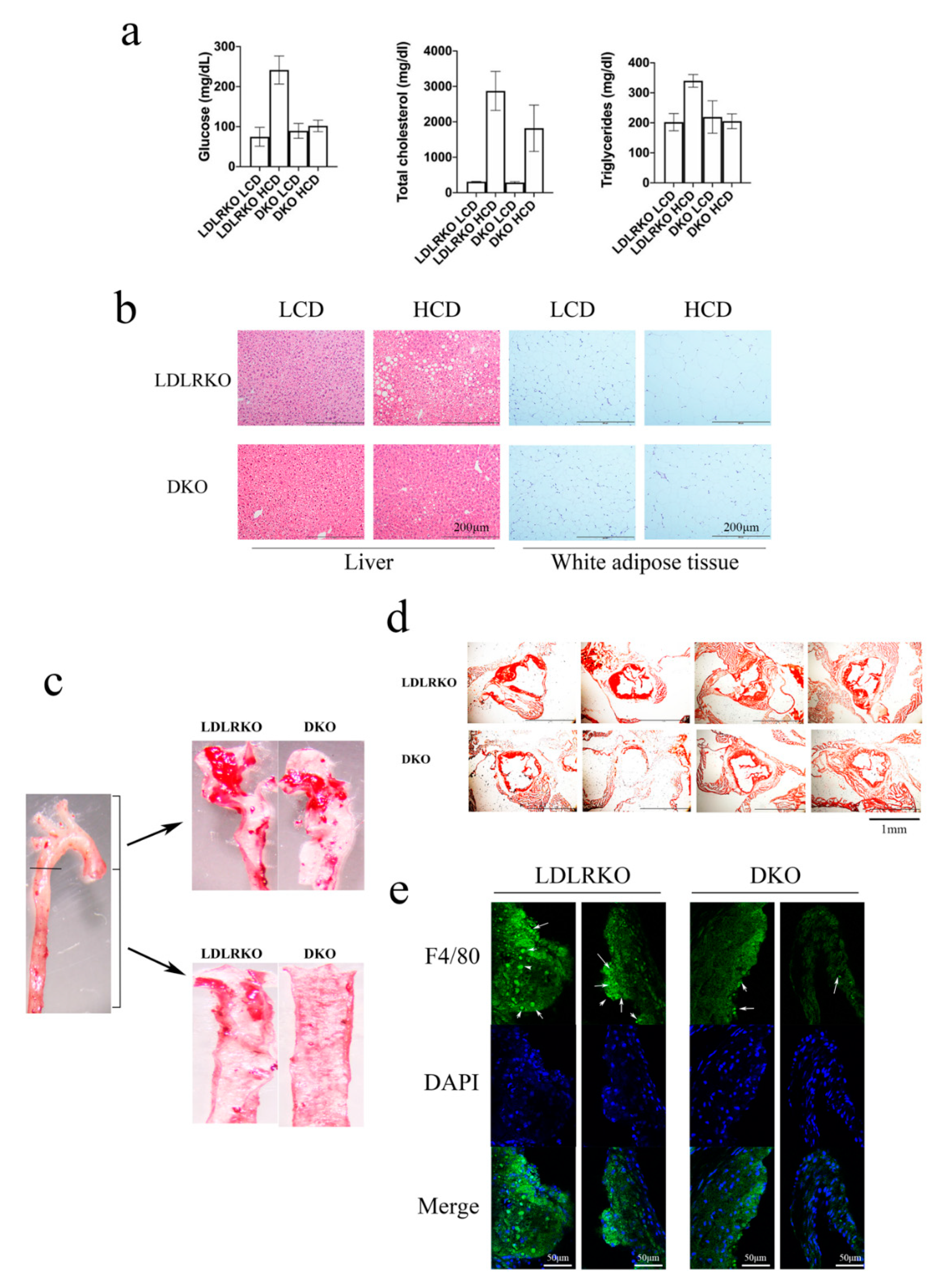
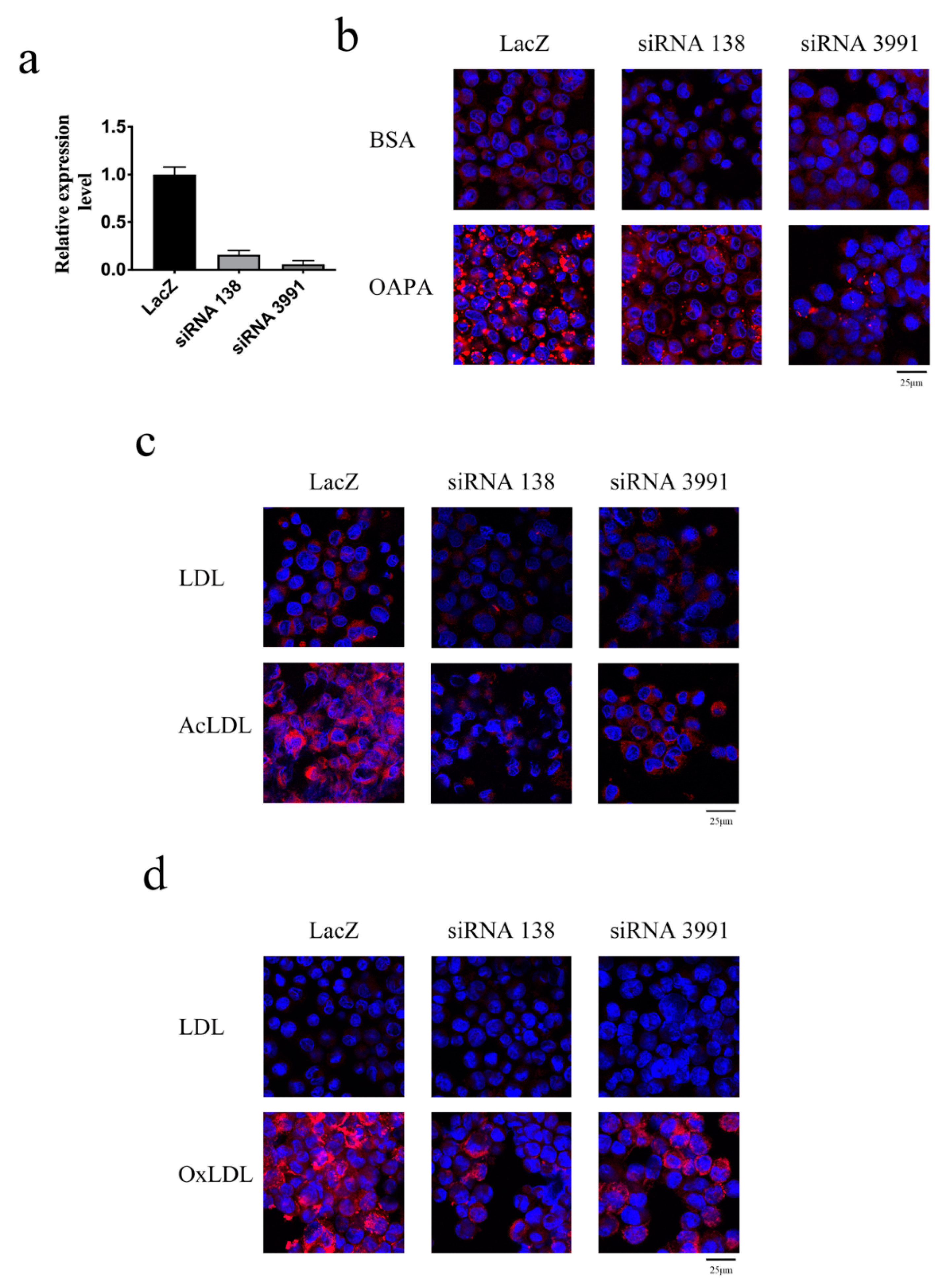
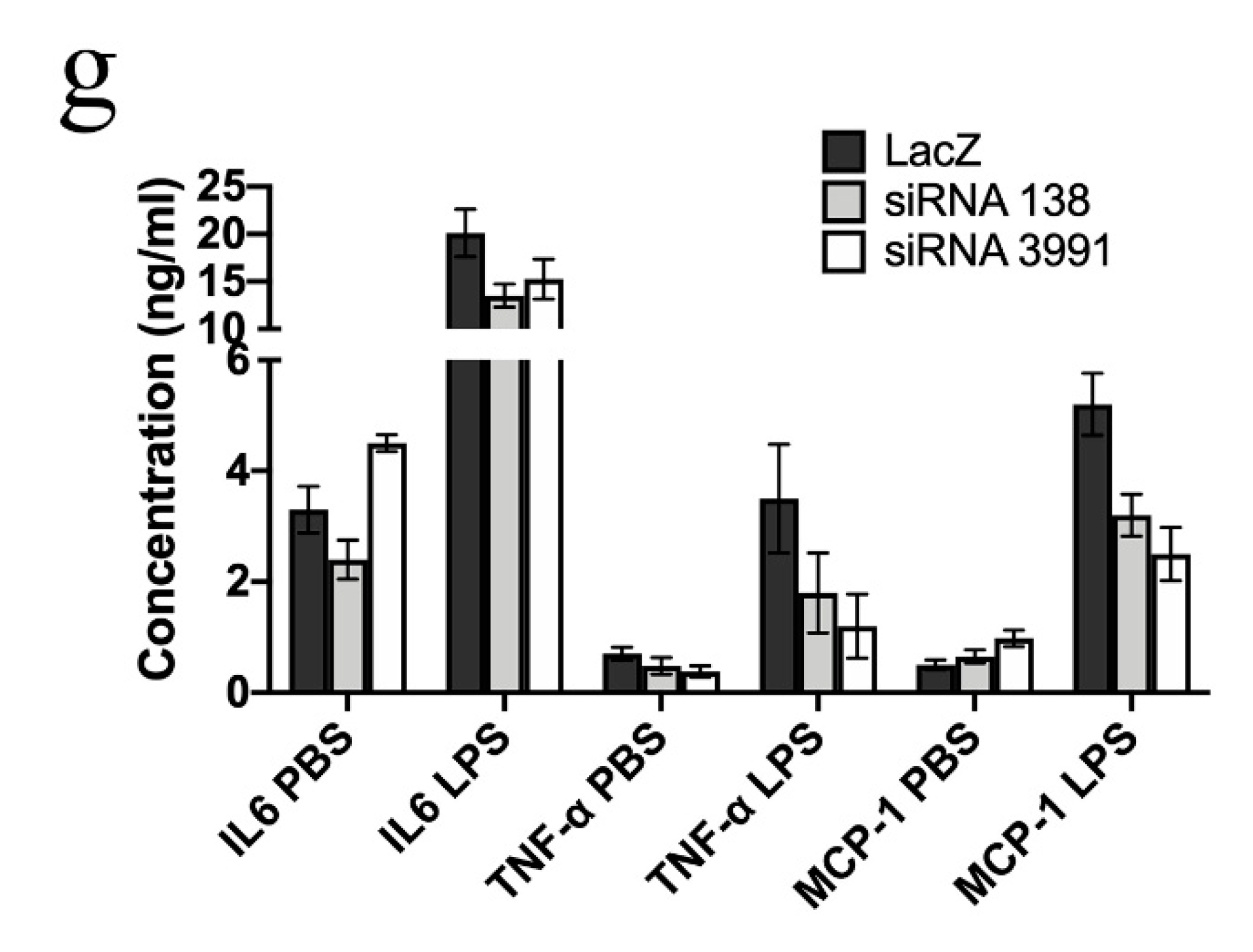
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Animal Care
4.2. Preparation of Bone Marrow-Derived Macrophages
4.3. Cytokine Enzyme-Linked Immunosorbent Assay
4.4. Quantitative Polymerase Chain Reaction
4.5. Immunofluorescence Staining
4.6. Lipid Droplet Staining
4.7. Generating Doxycycline-Inducible Galectin-12 Knockdown THP-1 Cells
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AcLDL | Acetylated LDL |
| ABCA1 | ATP Binding Cassette Subfamily A Member 1 |
| ABCG1 | ATP Binding Cassette Subfamily G Member 1 |
| CRD | carbohydrate recognition domain |
| BMDM | bone-marrow-derived macrophages |
| CD36 | cluster of differentiation 36 |
| cAMP | cyclic adenosine monophosphate |
| DKO | galectin-12 and LDL receptor double knockout |
| HCD | high-cholesterol diet |
| Il-6 | interleukin-6 |
| LPS | lipopolysaccharide |
| LCD | low-cholesterol diet |
| LDL | low-density lipoproteins |
| LDLRKO | LDL receptor knockout |
| MCP-1 | monocyte chemoattractant protein-1 |
| OxLDL | oxidized LDL |
| PMA | phorbol myristate acetate |
| PKA | protein kinase A |
| SRB1 | scavenger receptor class B type 1 |
| TNF-α | tumor necrosis factor α |
| DAPI | 4′,6-diamidino-2-phenylindole |
References
- Liuzzo, G. Atherosclerosis: An inflammatory disease. Rays 2001, 26, 221–230. [Google Scholar]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P.; Schonbeck, U.; Yan, Z.Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Freitas Lima, L.C.; Braga Vde, A.; do Socorro de Franca Silva, M.; Cruz Jde, C.; Sousa Santos, S.H.; de Oliveira Monteiro, M.M.; Balarini Cde, M. Adipokines, diabetes and atherosclerosis: An inflammatory association. Front. Physiol. 2015, 6, 304. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Libby, P.; Okamoto, Y.; Rocha, V.Z.; Folco, E. Inflammation in atherosclerosis: Transition from theory to practice. Circ. J. 2010, 74, 213–220. [Google Scholar] [CrossRef]
- Wan, L.; Yang, R.Y.; Liu, F.T. Galectin-12 in Cellular Differentiation, Apoptosis and Polarization. Int. J. Mol. Sci. 2018, 19, 176. [Google Scholar] [CrossRef]
- Wan, L.; Lin, H.J.; Huang, C.C.; Chen, Y.C.; Hsu, Y.A.; Lin, C.H.; Lin, H.C.; Chang, C.Y.; Huang, S.H.; Lin, J.M.; et al. Galectin-12 enhances inflammation by promoting M1 polarization of macrophages and reduces insulin sensitivity in adipocytes. Glycobiology 2016, 26, 732–744. [Google Scholar] [CrossRef]
- Fasshauer, M.; Klein, J.; Lossner, U.; Paschke, R. Negative regulation of adipose-expressed galectin-12 by isoproterenol, tumor necrosis factor alpha, insulin and dexamethasone. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2002, 147, 553–559. [Google Scholar] [CrossRef]
- Harrison, W.J.; Bull, J.J.; Seltmann, H.; Zouboulis, C.C.; Philpott, M.P. Expression of lipogenic factors galectin-12, resistin, SREBP-1, and SCD in human sebaceous glands and cultured sebocytes. J. Investig. Dermatol. 2007, 127, 1309–1317. [Google Scholar] [CrossRef]
- Hotta, K.; Funahashi, T.; Matsukawa, Y.; Takahashi, M.; Nishizawa, H.; Kishida, K.; Matsuda, M.; Kuriyama, H.; Kihara, S.; Nakamura, T.; et al. Galectin-12, an Adipose-expressed Galectin-like Molecule Possessing Apoptosis-inducing Activity. J. Biol. Chem. 2001, 276, 34089–34097. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Hsu, D.K.; Yu, L.; Chen, H.Y.; Liu, F.T. Galectin-12 is required for adipogenic signaling and adipocyte differentiation. J. Biol. Chem. 2004, 279, 29761–29766. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Hsu, D.K.; Yu, L.; Ni, J.; Liu, F.T. Cell cycle regulation by galectin-12, a new member of the galectin superfamily. J. Biol. Chem. 2001, 276, 20252–20260. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Yu, L.; Graham, J.L.; Hsu, D.K.; Lloyd, K.C.; Havel, P.J.; Liu, F.T. Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 18696–18701. [Google Scholar] [CrossRef]
- Hongo, S.; Watanabe, T.; Arita, S.; Kanome, T.; Kageyama, H.; Shioda, S.; Miyazaki, A. Leptin modulates ACAT1 expression and cholesterol efflux from human macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E474–E482. [Google Scholar] [CrossRef]
- Akiba, S.; Chiba, M.; Mukaida, Y.; Sato, T. Involvement of reactive oxygen species and SP-1 in fibronectin production by oxidized LDL. Biochem. Biophys. Res. Commun. 2003, 310, 491–497. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Yang, Y.; Yu, J.; Yang, J.; Yu, S.; Zhang, J.; Huang, L. C/EBPbeta Acts Upstream of NF-kappaB P65 Subunit in Ox-LDL-Induced IL-1beta Production by Macrophages. Cell Physiol. Biochem. 2018, 48, 1605–1615. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Arthur-Farraj, P. The Role of Cell Plasticity in Tissue Repair: Adaptive Cellular Reprogramming. Dev. Cell 2015, 34, 613–620. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Linton, M.F.; Fazio, S. Macrophages, inflammation, and atherosclerosis. Int. J. Obes. Relat. Metab. Disord. 2003, 27 (Suppl. 3), S35–S40. [Google Scholar] [CrossRef]
- Ohashi, R.; Mu, H.; Wang, X.; Yao, Q.; Chen, C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 2005, 98, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Matsui, K.; Murakami, Y.; Shimada, K. Monocyte chemoattractant protein-1 and coronary artery disease. Clin. Cardiol. 2002, 25, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S.; Lipton, B.A.; Rosenfeld, M.E.; Sarkioja, T.; Yoshimura, T.; Leonard, E.J.; Witztum, J.L.; Steinberg, D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1991, 88, 5252–5256. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Aiello, R.J.; Bourassa, P.A.; Lindsey, S.; Weng, W.; Natoli, E.; Rollins, B.J.; Milos, P.M. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arter. Thromb. Vasc. Biol. 1999, 19, 1518–1525. [Google Scholar]
- Takeya, M.; Yoshimura, T.; Leonard, E.J.; Takahashi, K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum. Pathol. 1993, 24, 534–539. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Ahmad, Z.; Iskandar, M.A.; Tuomilehto, J.; Al-Mulla, F.; Ahmad, R. TNF-alpha Induces a Pro-Inflammatory Phenotypic Shift in Monocytes through ACSL1: Relevance to Metabolic Inflammation. Cell Physiol. Biochem. 2019, 52, 397–407. [Google Scholar]
- Tieu, B.C.; Lee, C.; Sun, H.; Lejeune, W.; Recinos, A., 3rd; Ju, X.; Spratt, H.; Guo, D.C.; Milewicz, D.; Tilton, R.G.; et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Investig. 2009, 119, 3637–3651. [Google Scholar] [CrossRef]
- Xue, H.; Yang, R.Y.; Tai, G.; Liu, F.T. Galectin-12 inhibits granulocytic differentiation of human NB4 promyelocytic leukemia cells while promoting lipogenesis. J. Leukoc. Biol. 2016, 100, 657–664. [Google Scholar] [CrossRef]
- Satish, M.; Saxena, S.K.; Agrawal, D.K. Adipokine Dysregulation and Insulin Resistance with Atherosclerotic Vascular Disease: Metabolic Syndrome or Independent Sequelae? J. Cardiovasc. Transl. Res. 2019, 12, 415–424. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation. 2004, 109 (Suppl. 1), III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Dai, D.; Wang, X.; Ding, Z.; Li, C.; Mehta, J.L. GLP-1 agonists inhibit ox-LDL uptake in macrophages by activating protein kinase A. J. Cardiovasc. Pharmacol. 2014, 64, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.W.; Ma, X.; Li, X.X.; Liu, X.H.; Xiao, J.; Mo, Z.C.; Xiang, J.; Liao, D.F.; Tang, C.K. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis 2009, 204, e35–e43. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Xiao, J.; Mo, Z.C.; Yin, K.; Jiang, J.; Cui, L.B.; Tan, C.Z.; Tang, Y.L.; Liao, D.F.; Tang, C.K. Contribution of D4-F to ABCA1 expression and cholesterol efflux in THP-1 macrophage-derived foam cells. J. Cardiovasc. Pharmacol. 2010, 56, 309–319. [Google Scholar] [CrossRef]
- Alexeyev, M.F.; Fayzulin, R.; Shokolenko, I.N.; Pastukh, V. A retro-lentiviral system for doxycycline-inducible gene expression and gene knockdown in cells with limited proliferative capacity. Mol. Biol. Rep. 2010, 37, 1987–1991. [Google Scholar] [CrossRef]

| Name | Sequence |
|---|---|
| 138top | 5′-TGCTGACTCCTTGCAGCATGACCATCGTTTTGGCCACTGACTGACGATGGTCACTGCAAGGAGT-3′ |
| 138bottom | 5′-CCTGACTCCTTGCAGTGACCATCGTCAGTCAGTGGCCAAAACGATGGTCATGCTGCAAGGAGTC-3′ |
| 3991top | 5′-TGCTGAGAAAGTGCTGTCCATTCACAGTTTTGGCCACTGACTGACTGTGAATGCAGCACTTTCT-3′ |
| 3991bottom | 5′-CCTGAGAAAGTGCTGCATTCACAGTCAGTCAGTGGCCAAAACTGTGAATGGACAGCACTTTCTC-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, E.-S.; Hsu, Y.-A.; Chang, C.-Y.; Lin, H.-J.; Chen, C.S.; Wan, L. Ablation of Galectin-12 Inhibits Atherosclerosis through Enhancement of M2 Macrophage Polarization. Int. J. Mol. Sci. 2020, 21, 5511. https://doi.org/10.3390/ijms21155511
Lin E-S, Hsu Y-A, Chang C-Y, Lin H-J, Chen CS, Wan L. Ablation of Galectin-12 Inhibits Atherosclerosis through Enhancement of M2 Macrophage Polarization. International Journal of Molecular Sciences. 2020; 21(15):5511. https://doi.org/10.3390/ijms21155511
Chicago/Turabian StyleLin, En-Shyh, Yu-An Hsu, Ching-Yao Chang, Hui-Ju Lin, Chih Sheng Chen, and Lei Wan. 2020. "Ablation of Galectin-12 Inhibits Atherosclerosis through Enhancement of M2 Macrophage Polarization" International Journal of Molecular Sciences 21, no. 15: 5511. https://doi.org/10.3390/ijms21155511
APA StyleLin, E.-S., Hsu, Y.-A., Chang, C.-Y., Lin, H.-J., Chen, C. S., & Wan, L. (2020). Ablation of Galectin-12 Inhibits Atherosclerosis through Enhancement of M2 Macrophage Polarization. International Journal of Molecular Sciences, 21(15), 5511. https://doi.org/10.3390/ijms21155511







