Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review
Abstract
:1. Introduction
2. Definition and Diagnosis of Sarcopenia
3. Epidemiology of Sarcopenia in Autoimmune and Rheumatic Disease Patients
3.1. Rheumatoid Arthritis
3.2. Spondyloarthritis
3.3. Systemic Lupus Erythematosus
3.4. Systemic Sclerosis
3.5. Inflammatory Bowel Disease
3.6. Other Autoimmune Diseases
4. Rheumatoid Arthritis and Sarcopenia
4.1. Associated Factors
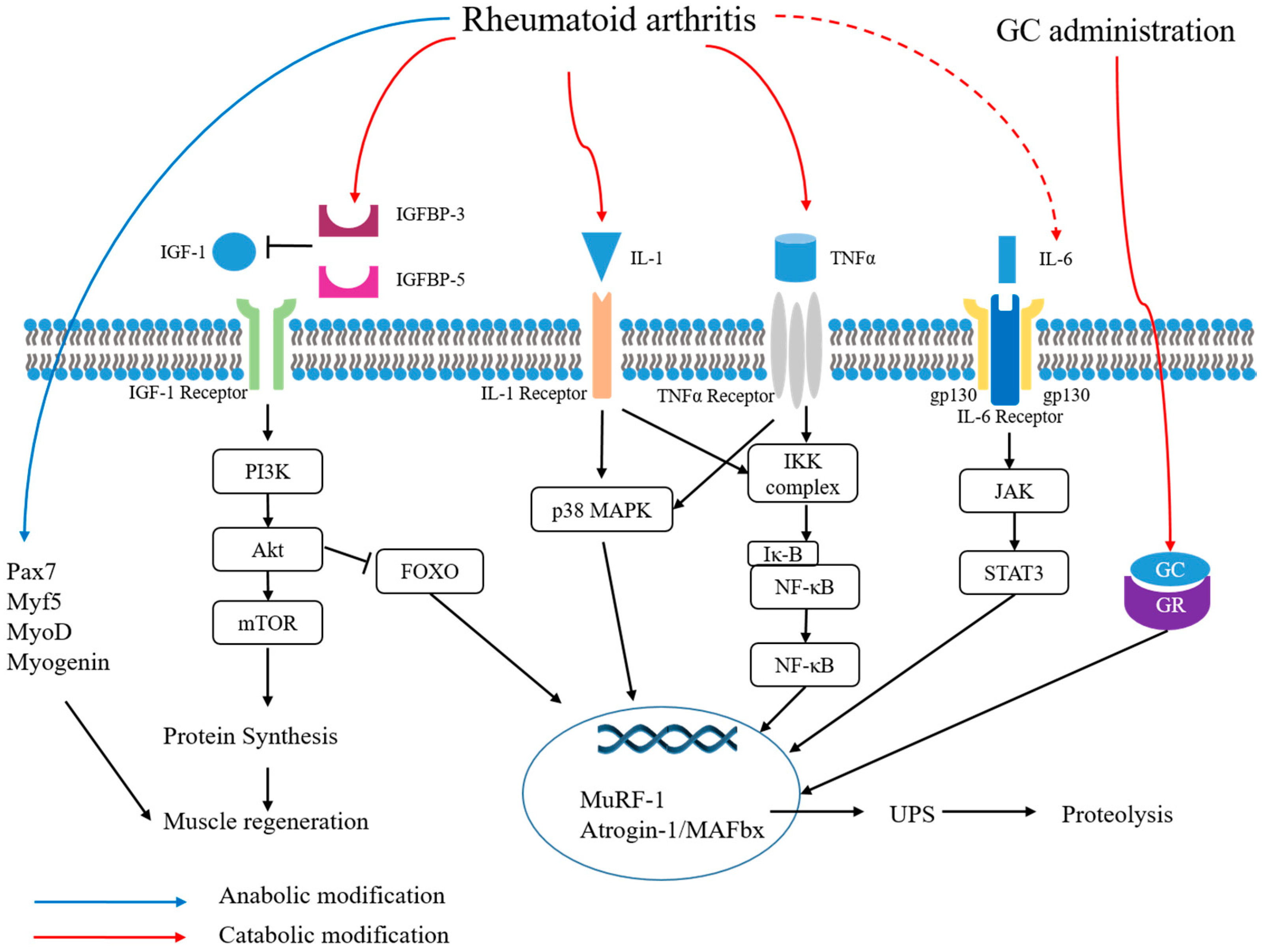
4.2. Pathogenesis
4.3. Treatments
5. Other Rheumatic Diseases and Sarcopenia
5.1. Spondyloarthritis
5.2. Systemic Sclerosis
| Spondyloarthritis |
| BASDAI (in AS and male SpA) [41,86] BASFI (in male SpA) [86] Bone mineral density (in AS) [41] Osteoporosis (in PsA) [24] |
| Systemic Sclerosis |
| Lung involvement (Medsger severity score) [42] Skin involvement (mRSS, Medsger severity score) [42,44] Microvascular involvement (capillaroscopy score) [44] Esophageal involvement [44] Overactive bladder [87] Disease duration [42,44,89] DLCO [42,44] Malnutrition [44] ESR [44] |
6. Inflammatory Bowel Disease and Sarcopenia
7. Autoimmune Diabetes and Sarcopenia
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ICD | International Classification of Diseases |
| OR | Odds ratio |
| RA | Rheumatoid arthritis |
| SMI | Skeletal muscle mass index |
| ASM | Appendicular skeletal muscle mass |
| EWGSOP | The European Working Group on Sarcopenia in Older People |
| FNIH | The Foundation of the National Institute of Health |
| IWGS | The International Working Group on Sarcopenia |
| ESPEN SIG | The European Society on Clinician Nutrition and Metabolism special interest groups |
| AWGS | The Asian Working Group for Sarcopenia |
| EWGSOP2 | The European Working Group on Sarcopenia in Older People 2 |
| FFMI | Free fat mass index |
| PsA | Psoriatic arthritis |
| TUG | Timed up and go |
| SpA | Spondyloarthritis |
| AS | Ankylosing spondylitis |
| SLE | Systemic lupus erythematosus |
| SSc | Systemic sclerosis |
| IBD | Inflammatory bowel disease |
| CT | Computed tomography |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| T1DM | Type 1 diabetes mellitus |
| LADA | Latent autoimmune diabetes in adults |
| HAQ-DI | Health Assessment Questionnaire Disability Index |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| AIA | Adjuvant-induced arthritis |
| MuRF-1 | Muscle RING-finger 1 |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Pax-7 | Paired box 7 |
| DMARD | Disease-modifying antirheumatic drug |
| GC | Glucocorticoid |
| BASDAI | Bath Ankylosing Spondylitis Disease Activity Index |
| BASFI | Bath Ankylosing Spondylitis Functional Index |
| IMCL | Intramyocellular lipid |
| WWP1 | WW domain containing E3 ubiquitin protein ligase 1 |
| KLF15 | Krüppel-like factor 15 |
| IGF-1 | Insulin-like growth factor-1 |
| IGFBP | Insulin-like growth factor binding protein |
| T2DM | Type 2 diabetes mellitus |
References
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, I.H. Summary comments. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexia. Sarcopenia Muscle 2016, 290–298. [Google Scholar] [CrossRef]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology—Update 2014. J. Cachexia. Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- Sousa, A.S.; Guerra, R.S.; Fonseca, I.; Pichel, F.; Ferreira, S.; Amaral, T.F. Financial impact of sarcopenia on hospitalization costs. Eur. J. Clin. Nutr. 2016, 70, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia. Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Jones, G.; Pilling, L.C.; Kuo, C.-L.; Kuchel, G.; Ferrucci, L.; Melzer, D. Sarcopenia and Variation in the Human Leukocyte Antigen Complex. J. Gerontol. A. Biol. Sci. Med. Sci. 2020, 75, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymstleld, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am J Epidemiol 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A. Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “ cachexia-anorexia in chronic wasting diseases” and “ nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, with the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef]
- Ibrahim, K.; May, C.; Patel, H.P.; Baxter, M.; Sayer, A.A.; Roberts, H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): Study protocol. Pilot Feasibility Stud. 2016, 2, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Schaap, L.A.; Koster, A.; Visser, M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol. Rev. 2013, 35, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewska-Wlodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W. Changes in body composition and bone mineral density in postmenopausal women with psoriatic arthritis. Reumatologia 2017, 55, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Dao, H.H.; Do, Q.T.; Sakamoto, J. Abnormal body composition phenotypes in Vietnamese women with early rheumatoid arthritis. Rheumatology (Oxford) 2011, 50, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Hull, H.R.; Thornton, J.; Wang, J.; Pierson, R.N.; Kaleem, Z.; Pi-Sunyer, X.; Heymsfield, S.; Albu, J.; Fernandez, J.R.; Vanitallie, T.B.; et al. Fat-free mass index: Changes and race/ethnic differences in adulthood. Int. J. Obes. 2011, 35, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.J.; Vinagre, F.; Canas Da Silva, J.; Gil, V.; Fonseca, J.E. Body composition phenotypes in systemic lupus erythematosus and rheumatoid arthritis: A comparative study of Caucasian female patients. Clin. Exp. Rheumatol. 2011, 29, 470–476. [Google Scholar]
- Schutz, Y.; Kyle, U.U.G.; Pichard, C. Fat-free mass index and fat mass index percentiles in caucasians aged 189–8 y. Int. J. Obes. 2002, 26, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Giles, J.T.; Ling, S.M.; Ferrucci, L.; Bartlett, S.J.; Andersen, R.E.; Towns, M.; Muller, D.; Fontaine, K.R.; Bathon, J.M. Abnormal body composition phenotypes in older rheumatoid arthritis patients: Association with disease characteristics and pharmacotherapies. Arthritis Care Res. 2008, 59, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Ceyhan Dogan, S.; Hizmetli, S.; Hayta, E.; Kaptanoglu, E.; Erselcan, T.; Guler, E. Sarcopenia in women with rheumatoid arthritis. Eur. J. Rheumatol. 2015, 2, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Pereira, B.; Dutheil, F.; Giraud, C.; Courteix, D.; Sapin, V.; Frayssac, T.; Mathieu, S.; Malochet-Guinamand, S.; Soubrier, M. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J. Cachexia. Sarcopenia Muscle 2017, 8, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Liang, J.J.; Da Ma, J.; Li, Q.H.; Mo, Y.Q.; Cheng, W.M.; He, X.L.; Li, N.; Cao, M.H.; Xu, D.; et al. Myopenia is associated with joint damage in rheumatoid arthritis: A cross-sectional study. J. Cachexia. Sarcopenia Muscle 2019, 10, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Ngeuleu, A.; Allali, F.; Medrare, L.; Madhi, A.; Rkain, H.; Hajjaj-Hassouni, N. Sarcopenia in rheumatoid arthritis: Prevalence, influence of disease activity and associated factors. Rheumatol. Int. 2017, 37, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Yamada, Y.; Mandai, K.; Hidaka, N. Matrix metalloprotease 3 is associated with sarcopenia in rheumatoid arthritis—Results from the CHIKARA study. Int. J. Rheum. Dis. 2018, 21, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Yano, K.; Ikari, K.; Okazaki, K. Sarcopenia-associated factors in Japanese patients with rheumatoid arthritis: A cross-sectional study. Geriatr. Gerontol. Int. 2019, 19, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Torii, M.; Hashimoto, M.; Hanai, A.; Fujii, T.; Furu, M.; Ito, H.; Uozumi, R.; Hamaguchi, M.; Terao, C.; Yamamoto, W.; et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod. Rheumatol. 2019, 29, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Vlietstra, L.; Stebbings, S.; Meredith-Jones, K.; Haxby Abbott, J.; Treharne, G.J.; Waters, D.L. Sarcopenia in osteoarthritis and rheumatoid arthritis: The association with self-reported fatigue, physical function and obesity. PLoS ONE 2019, 14, e0217462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, M.; Viggiani, M.; Anelli, M.; Fanizzi, R.; Lorusso, O.; Lopalco, G.; Cantarini, L.; Di Leo, A.; Lapadula, G.; Iannone, F. Sarcopenia in Patients with Rheumatic Diseases: Prevalence and Associated Risk Factors. J. Clin. Med. 2018, 7, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Ebo’O, F.B.; Sadni, S.; Majjad, A.; Hamza, T.; Mounach, A. Is there a relation between pre-sarcopenia, sarcopenia, cachexia and osteoporosis in patients with ankylosing spondylitis? BMC Musculoskelet. Disord. 2016, 17, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caimmi, C.; Caramaschi, P.; Venturini, A.; Bertoldo, E.; Vantaggiato, E.; Viapiana, O.; Ferrari, M.; Lippi, G.; Frulloni, L.; Rossini, M. Malnutrition and sarcopenia in a large cohort of patients with systemic sclerosis. Clin. Rheumatol. 2018, 37, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Siegert, E.; March, C.; Otten, L.; Makowka, A.; Preis, E.; Buttgereit, F.; Riemekasten, G.; Müller-Werdan, U.; Norman, K. Prevalence of sarcopenia in systemic sclerosis: Assessing body composition and functional disability in patients with systemic sclerosis. Nutrition 2018, 55–56, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Corallo, C.; Fioravanti, A.; Tenti, S.; Pecetti, G.; Nuti, R.; Giordano, N. Sarcopenia in systemic sclerosis: The impact of nutritional, clinical, and laboratory features. Rheumatol. Int. 2019, 39, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, C.; Xie, T.; Yang, J.; Dai, X.; Lv, T.; Li, Y.; Gu, L.; Wei, Y.; Gong, J.; et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin. Nutr. 2017, 36, 1586–1592. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Cushing, K.C.; Kordbacheh, H.; Gee, M.S.; Kambadakone, A.; Ananthakrishnan, A.N. Sarcopenia is a novel predictor of the need for rescue therapy in hospitalized ulcerative colitis patients. J. Crohn’s Colitis 2018, 12, 1036–1041. [Google Scholar] [CrossRef]
- Mager, D.R.; Carroll, M.W.; Wine, E.; Siminoski, K.; MacDonald, K.; Kluthe, C.L.; Medvedev, P.; Chen, M.; Wu, J.; Turner, J.M.; et al. Vitamin D status and risk for sarcopenia in youth with inflammatory bowel diseases. Eur. J. Clin. Nutr. 2018, 72, 623–626. [Google Scholar] [CrossRef]
- Webber, C.E.; Barr, R.D. Age and gender-dependent values of skeletal muscle mass in healthy children and adolescents. J. Cachexia. Sarcopenia Muscle 2012, 3, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Bamba, S.; Sasaki, M.; Takaoka, A.; Takahashi, K.; Imaeda, H.; Nishida, A.; Inatomi, O.; Sugimoto, M.; Andoh, A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS ONE 2017, 12, e0180036. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.W.; Gurwara, S.; Silver, H.J.; Horst, S.N.; Beaulieu, D.B.; Schwartz, D.A.; Seidner, D.L. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflamm. Bowel Dis. 2017, 23, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, L.; Cao, T.; Yang, J.; Gong, J.; Zhu, W.; Li, N.; Li, J. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients with Crohn’s Disease Undergoing Bowel Resection. J. Parenter. Enter. Nutr. 2017, 41, 592–600. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Kavanagh, R.G.; Carey, B.W.; Maher, M.M.; O’Connor, O.J.; Andrews, E.J. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. Eur. Radiol. Exp. 2018, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Thiberge, C.; Charpentier, C.; Gillibert, A.; Modzelewski, R.; Dacher, J.-N.; Savoye, G.; Savoye-Collet, C. Lower Subcutaneous or Visceral Adiposity Assessed by Abdominal Computed Tomography Could Predict Adverse Outcome in Patients With Crohn’s Disease. J. Crohns. Colitis 2018, 12, 1429–1437. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Lee, C.H.; Yoon, H.; Oh, D.J.; Lee, J.M.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn’s disease. Intest. Res. 2020, 18, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Lee, Y.; Chung, Y.S.; Lee, D.-J.; Joo, N.S.; Hong, D.; Song, G.E.; Kim, H.J.; Choi, Y.J.; Kim, K.M. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J. Gerontol. A. Biol. Sci. Med. Sci. 2012, 67, 1107–1113. [Google Scholar] [CrossRef]
- Cravo, M.L.; Velho, S.; Torres, J.; Costa Santos, M.P.; Palmela, C.; Cruz, R.; Strecht, J.; Maio, R.; Baracos, V. Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn’s disease: An exploratory study. Clin. Nutr. ESPEN 2017, 21, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.; Viana, C.; Marques, I.; Costa, C.; Martins, S.F. Sarcopenia is associated with Postoperative Outcome in Patients with Crohn’s Disease Undergoing Bowel Resection. Gastrointest. Disord. 2019, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Kuroda, A.; Araki, M.; Suzuki, R.; Taniguchi, S.; Tamaki, M.; Akehi, Y.; Matsuhisa, M. Advanced glycation end-products are a risk for muscle weakness in Japanese patients with type 1 diabetes. J. Diabetes Investig. 2017, 8, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Association of sarcopenia with both latent autoimmune diabetes in adults and type 2 diabetes: A cross-sectional study. J. Diabetes Complicat. 2017, 31, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Angulo, P.; Meza-Junco, J.; Prado, C.M.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia. Sarcopenia Muscle 2016, 7, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Roubenoff, R.A.; Cannon, J.G.; Kehayias, J.J.; Zhuang, H.; Dawson-Hughes, B.; Dinarello, C.A.; Rosenberg, I.H. Rheumatoid cachexia: Cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J. Clin. Investig. 1994, 93, 2379–2386. [Google Scholar] [CrossRef]
- Santo, R.C.E.; Fernandes, K.Z.; Lora, P.S.; Filippin, L.I.; Xavier, R.M. Prevalence of rheumatoid cachexia in rheumatoid arthritis: A systematic review and meta-analysis. J. Cachexia. Sarcopenia Muscle 2018, 9, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Munro, R.; Capell, H. Prevalence of low body mass in rheumatoid arthritis: Association with the acute phase response. Ann. Rheum. Dis. 1997, 56, 326–329. [Google Scholar] [CrossRef]
- Kasher, M.; Gabdulina, G.; Beissebayeva, A.; Mussabaeva, D.; Tokarev, A.; Sarssenbayeva, M.; Omarova, K.; Mominova, G.; Livshits, G. Rheumatoid arthritis is associated with exacerbated body composition deterioration in Kazakh females. Nutrition 2019, 66, 219–226. [Google Scholar] [CrossRef]
- Delgado-Frías, E.; González-Gay, M.A.; Muñiz-Montes, J.R.; Gómez Rodríguez-Bethencourt, M.A.; González-Díaz, A.; Díaz-González, F.; Ferraz-Amaro, I. Relationship of abdominal adiposity and body composition with endothelial dysfunction in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2015, 33, 516–523. [Google Scholar]
- Bruce, B.; Fries, J.F. The Stanford Health Assessment Questionnaire: Dimensions and practical applications. Health Qual. Life Outcomes 2003, 1, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.; Kull, M.; Põlluste, K.; Valner, A.; Lember, M.; Kallikorm, R. Factors associated with low lean mass in early rheumatoid arthritis: A cross-sectional study. Med. 2019, 55, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkan Melıkoğlu, M. Presarcopenia and its impact on disability in female patients with rheumatoid arthritis. Arch. Rheumatol. 2017, 32, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beenakker, K.G.M.; Ling, C.H.; Meskers, C.G.M.; de Craen, A.J.M.; Stijnen, T.; Westendorp, R.G.J.; Maier, A.B. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res. Rev. 2010, 9, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Fenton, C.G.; Webster, J.M.; Martin, C.S.; Fareed, S.; Wehmeyer, C.; MacKie, H.; Jones, R.; Seabright, A.P.; Lewis, J.W.; Lai, Y.C.; et al. Therapeutic glucocorticoids prevent bone loss but drive muscle wasting when administered in chronic polyarthritis. Arthritis Res. Ther. 2019, 21, 182. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Tada, M.; Mandai, K.; Hidaka, N.; Inui, K.; Nakamura, H. Glucocorticoid use is an independent risk factor for developing sarcopenia in patients with rheumatoid arthritis: From the CHIKARA study. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef]
- Gómez-SanMiguel, A.B.; Gomez-Moreira, C.; Nieto-Bona, M.P.; Fernández-Galaz, C.; Villanúa, M.Á.; Martín, A.I.; López-Calderón, A. Formoterol decreases muscle wasting as well as inflammation in the rat model of rheumatoid arthritis. Am. J. Physiol.—Endocrinol. Metab. 2016, 310, E925–E937. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Abe, M.; Lee, J.; Tatebayashi, D.; Himori, K.; Kanzaki, K.; Wada, M.; Bruton, J.D.; Westerblad, H.; Lanner, J.T. Muscle dysfunction associated with adjuvant-induced arthritis is prevented by antioxidant treatment. Skelet. Muscle 2015, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Himori, K.; Tatebayashi, D.; Kanzaki, K.; Wada, M.; Westerblad, H.; Lanner, J.T.; Yamada, T. Neuromuscular electrical stimulation prevents skeletal muscle dysfunction in adjuvant-induced arthritis rat. PLoS ONE 2017, 12, e0179925. [Google Scholar] [CrossRef]
- Little, R.D.; Prieto-Potin, I.; Pérez-Baos, S.; Villalvilla, A.; Gratal, P.; Cicuttini, F.; Largo, R.; Herrero-Beaumont, G. Compensatory anabolic signaling in the sarcopenia of experimental chronic arthritis. Sci. Rep. 2017, 7, 6311. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillero, E.; Martín, A.I.; López-Menduiña, M.; Granado, M.; Villanúa, M.Á.; López-Calderón, A. IGF-I system, atrogenes and myogenic regulatory factors in arthritis induced muscle wasting. Mol. Cell. Endocrinol. 2009, 309, 8–16. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Nunes Teixeira, V.; Filippin, L.I.; Viacava, P.R.; de Oliveira, P.G.; Xavier, R.M. Muscle wasting in collagen-induced arthritis and disuse atrophy. Exp. Biol. Med. 2013, 238, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moylan, J.S.; Chambers, M.A.; Smith, J.; Reid, M.B. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am. J. Physiol.—Cell Physiol. 2009, 297, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, N.; Van Den Bosch, F.; Deodhar, A. The concept of spondyloarthritis: Where are we now? Best Pract. Res. Clin. Rheumatol. 2014, 28, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.; Sequeira, J.; Meirinhos, T.; Ambrósio, C.; Barcelos, A. SARCOSPA-sarcopenia in spondyloarthritis patients. Acta Reumatol. Port. 2014, 2014, 322–326. [Google Scholar]
- Pacini, G.; Paolino, S.; C Trombetta, A.; Goegan, F.; Pizzorni, C.; Alessandri, E.; Patanè, M.; Gotelli, E.; Ferrari, G.; Cattelan, F.; et al. Lower urinary tract symptoms in systemic sclerosis: A detailed investigation. Rheumatology (Oxford) 2020, 59, 1315–1324. [Google Scholar] [CrossRef]
- Justo, A.C.; Guimarães, F.S.; Ferreira, A.S.; Soares, M.S.; Bunn, P.S.; Lopes, A.J. Muscle function in women with systemic sclerosis: Association with fatigue and general physical function. Clin. Biomech. 2017, 47, 33–39. [Google Scholar] [CrossRef]
- Marighela, T.F.; Genaro, P.D.S.; Pinheiro, M.M.; Szejnfeld, V.L.; Kayser, C. Risk factors for body composition abnormalities in systemic sclerosis. Clin. Rheumatol. 2013, 32, 1037–1044. [Google Scholar] [CrossRef]
- Ross, L.; Stevens, W.; Rabusa, C.; Wilson, M.; Ferdowsi, N.; Walker, J.; Sahhar, J.; Ngian, G.S.; Zochling, J.; Roddy, J.; et al. The role of inflammatory markers in assessment of disease activity in systemic sclerosis. Clin. Exp. Rheumatol. 2018, 36, S126–S134. [Google Scholar]
- Walker, U.A.; Clements, P.J.; Allanore, Y.; Distler, O.; Oddis, C.V.; Khanna, D.; Furst, D.E. Muscle involvement in systemic sclerosis: Points to consider in clinical trials. Rheumatology (Oxford) 2017, 56, v38–v44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, M.; Spira, D.; Demuth, I.; Steinhagen-Thiessen, E.; Norman, K. Polypharmacy as a Risk Factor for Clinically Relevant Sarcopenia: Results from the Berlin Aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Doerfler, B.; Allen, T.S.; Southwood, C.; Brenner, D.; Hirano, I.; Sheean, P. Medical Nutrition Therapy for Patients with Advanced Systemic Sclerosis (MNT PASS): A Pilot Intervention Study. J. Parenter. Enter. Nutr. 2017, 41, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Fiocchi, C. Inflammatory bowel disease: Autoimmune or immune-mediated pathogenesis? Clin. Dev. Immunol. 2004, 11, 195–204. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Bryant, R.V.; Schultz, C.G.; Ooi, S.; Goess, C.; Costello, S.P.; Vincent, A.D.; Schoeman, S.N.; Lim, A.; Bartholomeusz, F.D.; Travis, S.P.L.; et al. Obesity in inflammatory bowel disease: Gains in adiposity despite high prevalence of Myopenia and Osteopenia. Nutrients 2018, 10, 1192. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.; Cromwell, J.; Nau, P. Sarcopenia is a Predictor of Surgical Morbidity in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1867–1872. [Google Scholar] [CrossRef]
- Fujikawa, H.; Araki, T.; Okita, Y.; Kondo, S.; Kawamura, M.; Hiro, J.; Toiyama, Y.; Kobayashi, M.; Tanaka, K.; Inoue, Y.; et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg. Today 2017, 47, 92–98. [Google Scholar] [CrossRef]
- Ding, N.S.; Malietzis, G.; Lung, P.F.C.; Penez, L.; Yip, W.M.; Gabe, S.; Jenkins, J.T.; Hart, A. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment. Pharmacol. Ther. 2017, 46, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werkstetter, K.J.; Ullrich, J.; Schatz, S.B.; Prell, C.; Koletzko, B.; Koletzko, S. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J. Crohns. Colitis 2012, 6, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; de Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, K.; Fallon, K.; Ruut, T.; Lane, D.; McKay, R.; Shadbolt, B.; Ang, S.; Cook, M.; Platten, J.; Pavli, P.; et al. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 41, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 diabetes mellitus. Nat. Rev. Dis. Prim. 2017, 3, 17016. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Metter, E.J.; Egan, J.; Golden, S.H.; Ferrucci, L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 2015, 38, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Files, D.C.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Gregory, H.; Stone, J.; Lyles, M.F.; Dhar, S.; Marsh, A.P.; et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Ebeling, P.; Essén-Gustavsson, B.; Tuominen, J.A.; Koivisto, V.A. Intramuscular triglyceride content is increased in IDDM. Diabetologia 1998, 41, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Perseghin, G.; Lattuada, G.; Danna, M.; Sereni, L.P.; Maffi, P.; De Cobelli, F.; Battezzati, A.; Secchi, A.; Del Maschio, A.; Luzi, L. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am. J. Physiol.—Endocrinol. Metab. 2003, 285, E1174–E1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Lyons, T.J.; Mccance, D.R.; Baynes, J.W. Accumulation of Maillard Reaction Products in Skin Collagen in Diabetes and Aging. Ann. N. Y. Acad. Sci. 1992, 663, 421–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI insight 2019, 4, e124952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.I.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K.; et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Collier, B.; Roy, A. Hypothalamic-pituitary-adrenal axis dysregulation among diabetic outpatients. Psychiatry Res. 1990, 31, 31–37. [Google Scholar] [CrossRef]
- Galassetti, P.R.; Iwanaga, K.; Crisostomo, M.; Zaldivar, F.P.; Larson, J.; Pescatello, A. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatr. Diabetes 2006, 7, 16–24. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Wȩdrychowicz, A.; Dziatkowiak, H.; Nazim, J.; Sztefko, K. Insulin-like growth factor-1 and its binding proteins, IGFBP-1 and IGFBP-3, in adolescents with type-1 diabetes mellitus and microalbuminuria. Horm. Res. 2005, 63, 245–251. [Google Scholar] [CrossRef]
- Jehle, P.M.; Jehle, D.R.; Mohan, S.; Böhm, B.O. Serum levels of insulin-like growth factor system components and relationship to bone metabolism in type 1 and type 2 diabetes mellitus patients. J. Endocrinol. 1998, 159, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Purohit, S.; Sharma, S.; Bai, S.; Zhi, W.; Ponny, S.R.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; et al. IGF-binding proteins in type-1 diabetes are more severely altered in the presence of complications. Front. Endocrinol. (Lausanne) 2016, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Monaco, C.M.F.; Gingrich, M.A.; Hawke, T.J. Considering Type 1 Diabetes as a Form of Accelerated Muscle Aging. Exerc. Sport Sci. Rev. 2019, 47, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Laugesen, E.; Østergaard, J.A.; Leslie, R.D. Latent Autoimmune Diabetes of the Adult. Diabet. Med. 2015, 32, 843–852. [Google Scholar] [CrossRef] [PubMed]
| Author | Prevalence (%) | Patients (N) | Group Feature | p-Value | Definition of Sarcopenia (Cut-off) | |
|---|---|---|---|---|---|---|
| Rheumatoid Arthritis | ||||||
| Dao et al. [26] 1 | Purely sarcopenic | 18.1 | 105 | Vietnamese, female | 0.007 | FFMI (Hull et al. [27]) |
| Sarcopenic obesity 2 | 12.4 | 0.002 | ||||
| Total | 30.5 | - | ||||
| Santos et al. [28] 1 | Purely sarcopenic | 4.5 | 89 | Caucasian, Portuguese, female | >0.05 3 | FFMI z score ≤ −2 (Schutz et al. [29]) |
| Sarcopenic obesity 2 | 5.6 | 0.01 | ||||
| Total | 10.1 | - | ||||
| Giles et al. [30] | Male | 33.3 | 72 | American | 0.157 3 | SMI (Janssen et al. [14]) |
| Female | 21.4 | 117 | 0.004 | |||
| Total | 25.9 | 189 | - | |||
| Doğan et al. [31] | 43.3 | 30 | Female, Age 35–50 | 0.004 | SMI (Janssen et al. [14]) | |
| Tournadre et al. [32] | 28.6 | 21 | Active RA (DAS28 > 3.2) | <0.05 | SMI (Baumgartner et al. [13]) | |
| Lin et al. [33] | 45.1 | 457 | Chinese | <0.05 4 | SMI (AWGS [18]) | |
| Ngeuleu et al. [34] | 39.8 | 123 | Moroccan | - | SMI (Baumgartner et al. [13]) | |
| Tada et al. [35] | 28.0 | 100 | Japanese | - | AWGS [18] | |
| Mochizuki et al. [36] | 29.6 | 240 | Japanese, age ≥ 65 | - | AWGS [18] | |
| Torii et al. [37] | 37.1 | 388 | Japanese, female | - | EWGSOP [4], AWGS [18] | |
| Vlietstra et al. [38] | 17.1 | 82 | New Zealander | - | SMI (FNIH [15]) | |
| Barone et al. [39] | 21.0 | 76 | Caucasian, Italian, age 40–75 | - | SMI (Janssen et al. [14]), HS (Lauretani et al. [40]) | |
| Spondyloarthritis Ankylosing Spondylitis | ||||||
| Barone et al. [39] | 22.7 | 22 | Caucasian, Italian, age 40–75 | - | SMI (Janssen et al. [14]), HS (Lauretani et al. [40]) | |
| El Maghraoui et al. [41] | 34.3 | 67 | Moroccan, male | - | EWGSOP [4] | |
| Psoriatic Arthritis | ||||||
| Barone et al. [39] | 20.0 | 70 | Caucasian, Italian, age 40–75 | - | SMI (Janssen et al. [14]), HS (Lauretani et al. [40]) | |
| Krajewska-Włodarczyk et al. [24] | 13.7 | 51 | Polish, age 50–75, female | - | SMI (Baumgartner et al. [13]) | |
| 49.0 | SMI (Janssen et al. [25]) | |||||
| 43.1 | SMI(Janssen et al. [25]), TUG > 14s | |||||
| Systemic Lupus Erythematosus | ||||||
| Santos et al. [28] 1 | Purely sarcopenic | 10.9 | 92 | Caucasian, Portuguese, female | 0.01 | FFMI (Schutz et al. [29]) |
| Sarcopenic obesity 2 | 6.5 | 0.009 | ||||
| Total | 17.4 | - | ||||
| Systemic Sclerosis | ||||||
| Caimmi et al. [42] | 20.7 | 140 | Italian | - | SMI (Baumgartner et al. [13]) | |
| Siegert et al. [43] | 22.5 | 129 | German, 91.5% female | - | EWGSOP [4] | |
| Corallo et al. [44] | 41.9 | 62 | Caucasian, Italian | - | SMI (Baumgartner et al. [13]) | |
| 54.8 | HS (Male < 30, Female < 20) | |||||
| Inflammatory Bowel Disease Ulcerative colitis | ||||||
| Zhang et al. [45] | 27.3 | 99 | Chinese. | <0.05 | SMI (Fearon et al. [46]) | |
| Cushing et al. [47] | 69.5 | 82 | Admitted for ASUC | - | SMI (Fearon et al. [46]) | |
| Mager et al. [48] | 14.8 | 27 | Age 5–18 | - | SMM z score < −2 [49] | |
| Bamba et al. [50] | 48.3 | 29 | Japanese | - | SMI (Nishikawa et al. [51]) | |
| Adams et al. [52] | 50.0 | 14 | American | - | SMI (Prado et al. [53]) | |
| Crohn’s Disease | ||||||
| Zhang et al. [45] | 59.0 | 105 | Chinese | <0.05 | SMI (Fearon et al. [46]) | |
| Mager et al. [48] | 31.0 | 58 | Age 5–18 | - | SMM z score < −2 [49] | |
| Zhang et al. [54] | 61.4 | 114 | Chinese, required BR | - | SMI (Fearon et al. [46]) | |
| O’Brien et al. [55] | 39.0 | 77 | Retrospectively selected (BR) | - | SMI (Martin et al. [56]) | |
| Bamba et al. [50] | 37.2 | 43 | Japanese | - | SMI (Nishikawa et al. [51]) | |
| Thiberge et al. [57] | 33.6 | 149 | French | - | SMI (Mourtzakis et al. [58]) | |
| Adams et al. [52] | 44.7 | 76 | American | - | SMI (Prado et al. [53]) | |
| Lee et al. [59] | 50.6 | 79 | Korean | - | SMI (Kim et al. [60]) | |
| Cravo et al. [61] | 31.0 | 71 | Portuguese | - | SMI (Martin et al. [56]) | |
| Carvalho et al. [62] | 41.4 | 58 | Portuguese | - | SMI (Prado et al. [53]) | |
| Diabetes Type 1 Diabetes Mellitus | ||||||
| Mori et al. [63] | 16.6 | 36 | Japanese | - | AWGS [18] | |
| Latent Autoimmune Diabetes in Adults | ||||||
| Bouchi et al. [64] | 35.0 | 20 | Japanese | 0.022 | AWGS [18] | |
| Autoimmune Liver Disease (Autoimmune Hepatitis, Primary Biliary Cirrhosis, Primary Sclerosing Cholangitis) | ||||||
| Montano-Loza et al. [65] | 41.8 | 55 | Canadian, evaluated for LT | - | SMI (Martin et al. [56]) | |
| Associated Factors |
|---|
| Age [36,37] BMI [34,35,36] Body fat mass [35,38] Disease duration [37,74] Bone erosion and mineral density [34,36] Malnutrition and protein intake [37,72] Joint damage [30,33,37] Functional status (HAQ score) [26,30,33,72,73] CRP level [30,36,68,72] ESR [68,72] RF [26,30] MMP3 [35] Use of GC [38,72,75,76] |
| Treatment |
| IL-6 inhibitor (TCZ) [32] DMARDs [30,37] β2-adrenoceptor agonist (formoterol) [77] Antioxidant [78] Neuromuscular electrical stimulation [79] |
| Risk |
| Falls [37] Fractures [37] Low bone mineral density [37] Cardiometabolic risk [34] Endothelial dysfunction [70] |
| Cytokines/Pathways |
| IL-1β [66,80] IL-6 [81] TNF- α [66,81] NF-Κb [80] p38 MAPK [80] pSTAT3 [80] Pax7 [80] Myostatin [80] MyoD [80,82] Myogenin [80,82] IGFBP-5 [82] IGFBP-3 [82] atrogin-1 [80,82] MuRF-1 [80,82] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.J.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Nam, S.W.; Kim, J.S.; Yang, J.W.; Lee, J.Y.; Smith, L.; et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5678. https://doi.org/10.3390/ijms21165678
An HJ, Tizaoui K, Terrazzino S, Cargnin S, Lee KH, Nam SW, Kim JS, Yang JW, Lee JY, Smith L, et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. International Journal of Molecular Sciences. 2020; 21(16):5678. https://doi.org/10.3390/ijms21165678
Chicago/Turabian StyleAn, Hyo Jin, Kalthoum Tizaoui, Salvatore Terrazzino, Sarah Cargnin, Keum Hwa Lee, Seoung Wan Nam, Jae Seok Kim, Jae Won Yang, Jun Young Lee, Lee Smith, and et al. 2020. "Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review" International Journal of Molecular Sciences 21, no. 16: 5678. https://doi.org/10.3390/ijms21165678
APA StyleAn, H. J., Tizaoui, K., Terrazzino, S., Cargnin, S., Lee, K. H., Nam, S. W., Kim, J. S., Yang, J. W., Lee, J. Y., Smith, L., Koyanagi, A., Jacob, L., Li, H., Shin, J. I., & Kronbichler, A. (2020). Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. International Journal of Molecular Sciences, 21(16), 5678. https://doi.org/10.3390/ijms21165678








