Isoforsythiaside Attenuates Alzheimer’s Disease via Regulating Mitochondrial Function Through the PI3K/AKT Pathway
Abstract
:1. Introduction
2. Results
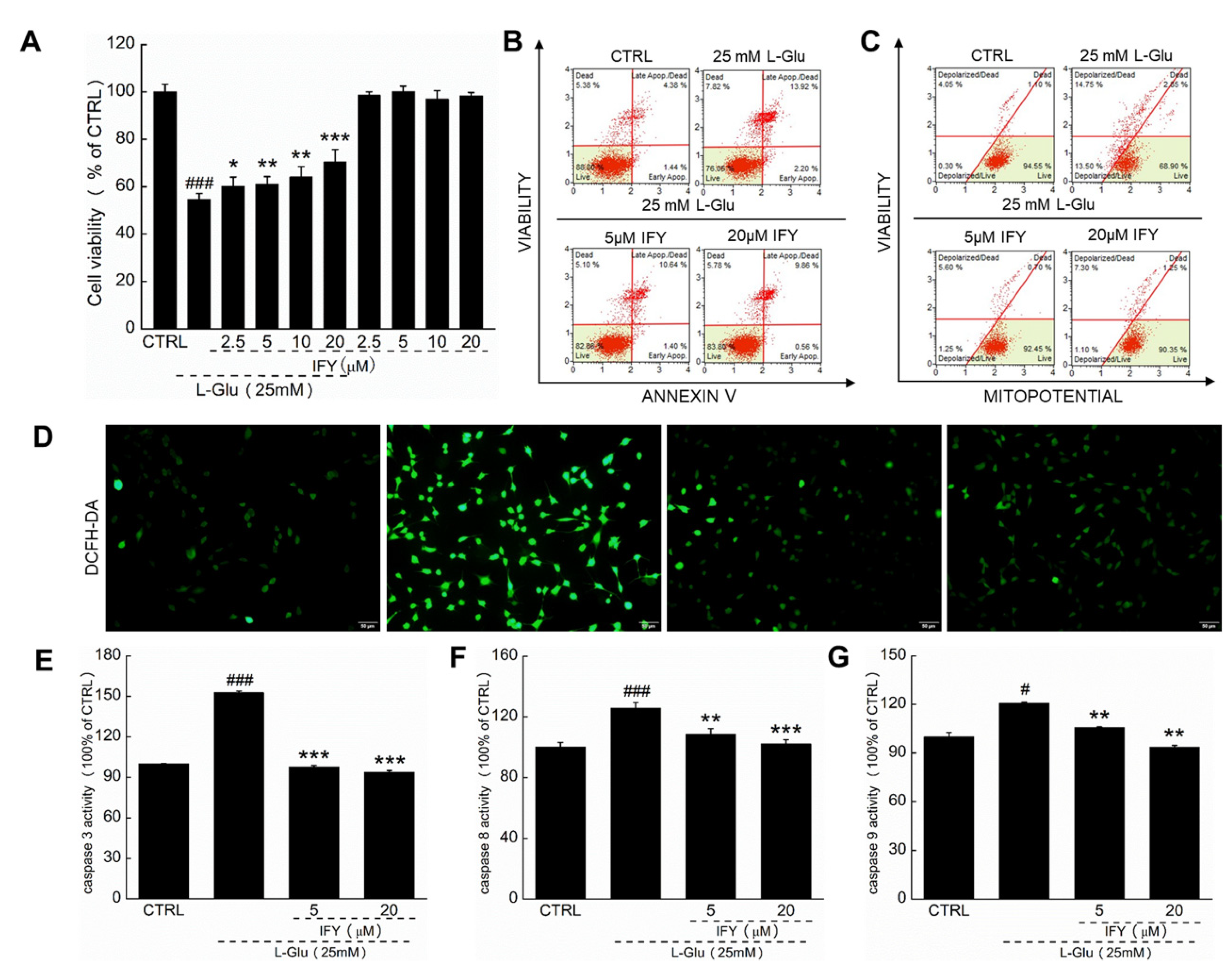
2.1. IFY Protected HT22 Cells against L-Glu Inducing Cell Apoptosis
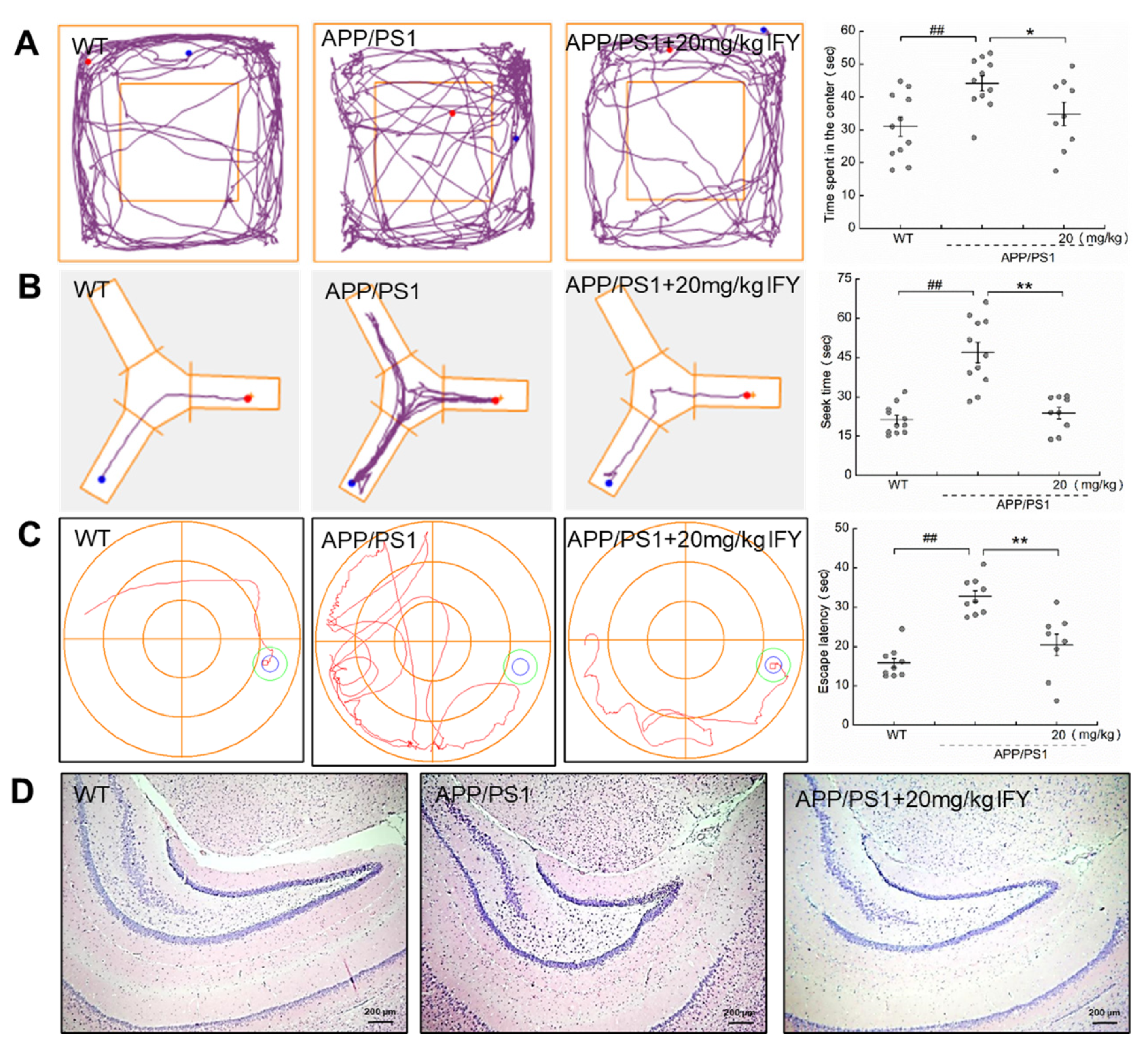
2.2. IFY Improved the Behavioral Cognitive Ability in APP/PS1 Mice
2.3. IFY Improved the Pathological Status of Brain and Hippocampus in APP/PS1 Mice
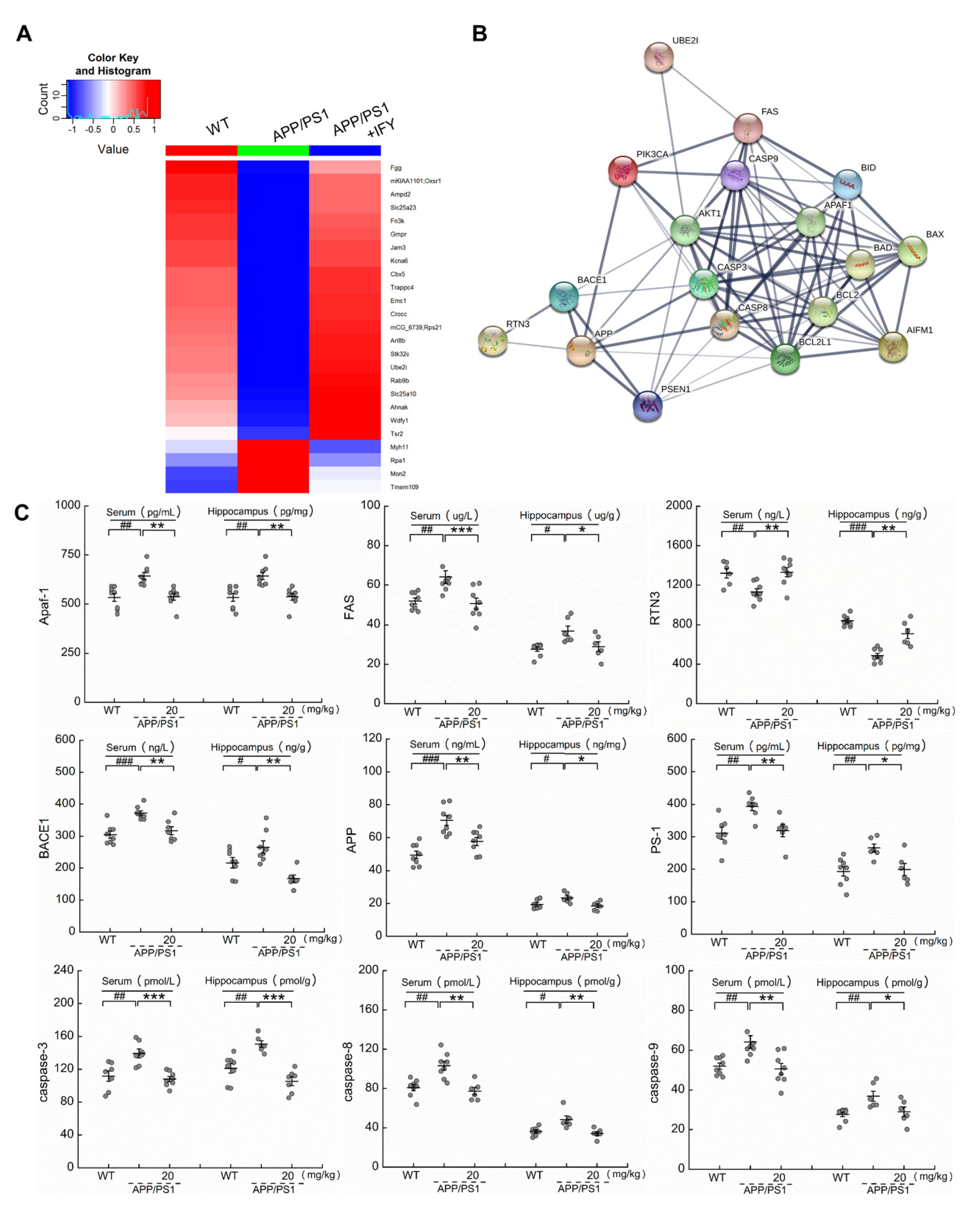
2.4. IFY Regulated the Levels of Apoptosis-Related Proteins
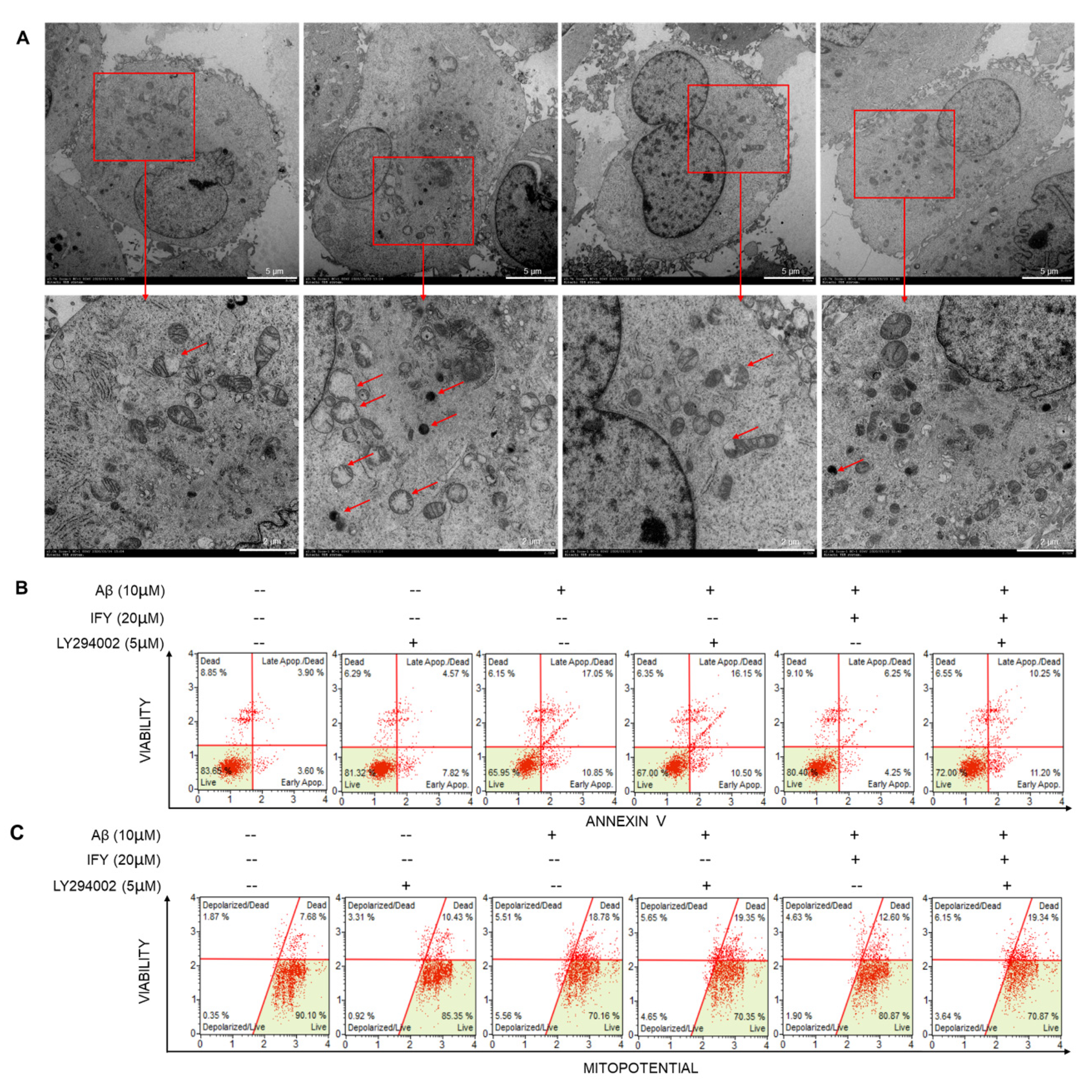
2.5. The Activation of PI3K/AKT Signaling Is Involved in IFY-Regulated Anti-Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Apoptosis and MMP Detection
4.5. ROS Fluorescence Detection
4.6. Caspase Detection
4.7. Transmission Electron Microscopy (TEM)
4.8. Animal Experimental Protocol
4.9. Behavioral Experiments
4.9.1. The Open Field Test
4.9.2. Y-Maze Test
4.9.3. MWM Test
4.10. Label-Free Quantitative Proteomics
4.11. ELISA
4.12. Hematoxylin-Eosin (H&E) Staining, Immunochemistry and Thioflavin S Staining
4.13. Western Blot
4.14. Statistical Analysis.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AKT | protein kinase B |
| Apaf-1 | apoptotic protease activating factor-1 |
| APP | amyloid precursor protein |
| APP/PS1 | APPswe/PSEN1dE9 transgenic |
| Aβ | beta amyloid |
| BACE1 | beta-site APP cleaving enzyme 1 |
| BAD | Bcl-associated death promoter |
| BAX | BCL-2-associated X protein |
| BCL-2 | B-cell lymphoma-2 |
| BCL-XL | B-cell lymphoma-extra-large |
| BID | BH3-interacting domain death agonist |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| ELISA | enzyme-linked immunosorbent assay |
| FAS | factor related apoptosis |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| HFIP | hexafluoro-2-propanol |
| H&E | hematoxylin-eosin |
| IFY | Isoforsythiaside |
| L-Glu | L-glutamate |
| MMP | mitochondrial membrane potential |
| MWM | Morris water maze |
| NFTs | neurofibrillary tangles |
| PBS | phosphate buffer saline |
| PI3K | phosphatidylinositol 3-kinase |
| PMSF | phenylmethanesulfonyl fluoride |
| PS-1 | Presenilin-1 |
| RIPA | radio immunoprecipitation assay |
| ROS | reactive oxygen species |
| RTN3 | reticulon 3 |
| SDC | sodium deoxycholate |
| TEM | transmission electron microscopy |
| WT | wild type |
| 4-HNE | 4-hydroxynonenal |
References
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2020, 16, 391–460. [CrossRef]
- Geng, J.; Liu, W.; Xiong, Y.Y.; Ding, H.Q.; Jiang, C.H.; Yang, X.L.; Li, X.; Elgehama, A.; Sun, Y.; Xu, Q.; et al. Andrographolide sulfonate improves Alzheimer-associated phenotypes and mitochondrial dysfunction in APP/PS1 transgenic mice. Biomed. Pharmacother. 2018, 97, 1032–1039. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatton, W.; Chen, D.; Chalmers-Redman, R.; Wheeler, L.; Nixon, R.; Tatton, N. Hypothesis for a common basis for neuroprotection in glaucoma and Alzheimer’s disease: Anti-apoptosis by alpha-2-adrenergic receptor activation. Surv. Ophthalmol. 2003, 48, S25–S37. [Google Scholar] [CrossRef]
- Su, J.H.; Anderson, A.J.; Cummings, B.J.; Cotman, C.W. Immunohistochemical evidence for apoptosis in Alzheimer’s disease. NeuroReport 1994, 5, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Frey, W.H. Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion 2007, 7, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Yu, Q.L.; Han, L.; Ma, X.L.; Song, R.D.; Zhao, S.N.; Zhang, W.H. Study on the effect of reactive oxygen species-mediated oxidative stress on the activation of mitochondrial apoptosis and the tenderness of yak meat. Food Chem. 2018, 244, 394–402. [Google Scholar] [CrossRef]
- Crouch, P.J.; Blake, R.; Duce, J.A.; Ciccotosto, G.D.; Li, Q.X.; Barnham, K.J.; Curtain, C.C.; Cherny, R.A.; Cappai, R.; Dyrks, T.; et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta(1-42). J. Neurosci. 2005, 25, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, J.F.; Wei, Y.J.; Li, J.M. Effects of piceatannol and pterostilbene against beta-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct. 2016, 7, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Wang, X.M.; Ko, H.; Kwon, H.C.; Cha, J.W.; Yang, H.O. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2011, 672, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Wang, D.C.; Chen, S.S. Amyloid-beta Interrupts the PI3K-Akt-mTOR Signaling Pathway That Could Be Involved in Brain-Derived Neurotrophic Factor-Induced Arc Expression in Rat Cortical Neurons. J. Neurosci. Res. 2009, 87, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Sun, G.Q.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.Q.; Chu, X.K.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Qu, H.H.; Zhang, Y.M.; Chai, X.Y.; Sun, W.J. Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated from Forsythia suspensa. Bioorganic Chem. 2012, 40, 87–91. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xia, Q.; Liu, X.; Liu, W.X.; Huang, W.Z.; Mei, X.; Luo, J.; Shon, M.X.; Lin, R.C.; Zou, D.X.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef]
- Pan, L.; Ma, X.K.; Zhao, P.F.; Shang, Q.H.; Long, S.F.; Wu, Y.; Piao, X.S. Forsythia suspensa extract attenuates breast muscle oxidative injury induced by transport stress in broilers. Poult. Sci. 2018, 97, 1554–1563. [Google Scholar] [CrossRef]
- Wang, H.M.; Wang, L.W.; Liu, X.M.; Li, C.L.; Xu, S.P.; Farooq, A.D. Neuroprotective effects of forsythiaside on learning and memory deficits in senescence-accelerated mouse prone (SAMP8) mice. Pharmacol. Biochem. Behav. 2013, 105, 134–141. [Google Scholar] [CrossRef]
- Yan, X.J.; Chen, T.G.; Zhang, L.W.; Du, H.Z. Protective effects of Forsythoside A on amyloid beta-induced apoptosis in PC12 cells by downregulating acetylcholinesterase. Eur. J. Pharmacol. 2017, 810, 141–148. [Google Scholar] [CrossRef]
- Chen, L.Q.; Yan, Y.; Chen, T.G.; Zhang, L.W.; Gao, X.X.; Du, C.H.; Du, H.Z. Forsythiaside prevents beta-amyloid-induced hippocampal slice injury by upregulating 2-arachidonoylglycerol via cannabinoid receptor 1-dependent NF-kappa B pathway. Neurochem. Int. 2019, 125, 57–66. [Google Scholar] [CrossRef]
- Yang, S.H.; Lee, D.K.; Shin, J.; Lee, S.; Baek, S.; Kim, J.; Jung, H.; Hah, J.M.; Kim, Y. Nec-1 alleviates cognitive impairment with reduction of Abeta and tau abnormalities in APP/PS1 mice. EMBO Mol. Med. 2017, 9, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qin, L.; Lu, H.L.; Li, P.J.; Song, Y.J.; Yang, R.L. Methylene blue improves streptozotocin-induced memory deficit by restoring mitochondrial function in rats. Brain Res. 2017, 1657, 208–214. [Google Scholar] [CrossRef]
- Trinh, H.V.; Grossmann, J.; Gehrig, P.; Roschitzki, B.; Schlapbach, R.; Greber, U.F.; Hemmi, S. iTRAQ-Based and Label-Free Proteomics Approaches for Studies of Human Adenovirus Infections. Int. J. Proteom. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihara, M.; Stein, P.; Schultz, R.M. UBE2I (URC9), a SUMO-Conjugating Enzyme, Localizes to Nuclear Speckles and Stimulates Transcription in Mouse Oocytes. Biol. Reprod. 2008, 79, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Q.; Sarge, K.D. Sumoylation of amyloid precursor protein negatively regulates A beta aggregate levels. Biochem. Biophys. Res. Commun. 2008, 374, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Krajnak, K.; Dahl, R. Small molecule SUMOylation activators are novel neuroprotective agents. Bioorg. Med. Chem. Lett. 2018, 28, 405–409. [Google Scholar] [CrossRef]
- Dong, M.; Pang, X.Y.; Xu, Y.; Wen, F.; Zhang, Y. Ubiquitin-Conjugating Enzyme 9 Promotes Epithelial Ovarian Cancer Cell Proliferation in Vitro. Int. J. Mol. Sci. 2013, 14, 11061–11071. [Google Scholar] [CrossRef]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and function in cell death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef]
- Zhu, J.; Su, J.; Liu, R.; Yang, J. Relationship between the FAS gene A-670G polymorphism and Alzheimer’s disease: A meta-analysis. Aging Clin. Exp. Res. 2015, 27, 563–571. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Nam, G.; Kang, J.; Lee, H.J.; Lee, M.; Lim, M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef]
- Martin-Maestro, P.; Gargini, R.; Garcia, E.; Simon, D.; Avila, J.; Garcia-Escudero, V. Mitophagy Failure in APP and Tau Overexpression Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2019, 70, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.; Wong, L.R.; Pee, H.N.; Yang, S.L.; Ho, P.C.L. Reverting Metabolic Dysfunction in Cortex and Cerebellum of APP/PS1 Mice, a Model for Alzheimer’s Disease by Pioglitazone, a Peroxisome Proliferator-Activated Receptor Gamma (PPAR gamma) Agonist. Mol. Neurobiol. 2019, 56, 7267–7283. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Biswas, S.C. Amyloid-beta induced astrocytosis and astrocyte death: Implication of FoxO3a-Bim-caspase3 death signaling. Mol. Cell. Neurosci. 2015, 68, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Rehker, J.; Rodhe, J.; Nesbitt, R.R.; Boyle, E.A.; Martin, B.K.; Lord, J.; Karaca, I.; Naj, A.; Jessen, F.; Helisalmi, S.; et al. Caspase-8, association with Alzheimer’s Disease and functional analysis of rare variants. PLoS ONE 2017, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohn, T.T.; Rissman, R.A.; Davis, M.C.; Kim, Y.E.; Cotman, C.W.; Head, E. Caspase-9 Activation and Caspase Cleavage of tau in the Alzheimer’s Disease Brain. Neurobiol. Dis. 2002, 11, 341–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharoar, M.G.; Yan, R. Effects of altered RTN3 expression on BACE1 activity and Alzheimer’s neuritic plaques. Rev. Neurosci. 2017, 28, 145–154. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhanen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Manczak, M.; Anekonda, T.S.; Henson, E.; Park, B.S.; Quinn, J.; Reddy, P.H. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006, 15, 1437–1449. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.T.; Sun, Y.; Xu, Z.; Wang, Y.; Yao, S.Y.; Yao, W.B.; Gao, X.D. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer’s disease. Redox Biol. 2019, 22, 9. [Google Scholar] [CrossRef]
- Mancuso, M.; Coppede, F.; Migliore, L.; Siciliano, G.; Murri, L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J. Alzheimer’s Dis. 2006, 10, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Chung, H.W. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem. Biophys. Res. Commun. 2007, 363, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Koito, K.; Okada, F.; Kudo, N.; Yamamoto, K.; Fujimoto, K.; Nishida, M.; Ichimura, T.; Hori, M.; Satoh, T.; et al. High-intensity focused ultrasound induced apoptosis with caspase 3, 8, and 9/6 activation in rat hepatoma. J. Med. Ultrason. 2009, 36, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Snigdha, S.; Smith, E.D.; Prieto, G.A.; Cotman, C.W. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci. Bull. 2012, 28, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Whitcomb, D.J.; Olsen, K.M.; Kerrigan, T.L.; Lo, S.C.; Bru-Mercier, G.; Dickinson, B.; Scullion, S.; Sheng, M.G.; Collingridge, G.; et al. A beta(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3 beta. Nat. Neurosci. 2011, 14, 545–547. [Google Scholar] [CrossRef]
- Fasulo, L.; Ugolini, G.; Cattaneo, A. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. J. Alzheimer’s Dis. 2005, 7, 3–13. [Google Scholar] [CrossRef]
- Tesco, G.; Koh, Y.H.; Kang, E.L.; Cameron, A.N.; Das, S.; Sena-Esteves, M.; Hiltunen, M.; Yang, S.H.; Zhong, Z.Y.; Shen, Y.; et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 2007, 54, 721–737. [Google Scholar] [CrossRef] [Green Version]
- Duyckaerts, C.; Potier, M.C.; Delatour, B. Alzheimer disease models and human neuropathology: Similarities and differences. Acta Neuropathol. 2008, 115, 5–38. [Google Scholar] [CrossRef] [Green Version]
- Tai, J.J.; Liu, W.Z.; Li, Y.W.; Li, L.; Holscher, C. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Brain Res. 2018, 1678, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Fu, W.; Waeg, G.; Uchida, K. 4-Hydroxynonenal, a product of lipid peroxidation, inhibits dephosphorylation of the microtubule-associated protein tau. NeuroReport 1997, 8, 2275–2281. [Google Scholar] [CrossRef]
- Sarge, K.D.; Park-Sarge, O.K. Sumoylation and human disease pathogenesis. Trends Biochem. Sci. 2009, 34, 200–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Prior, M.; He, W.X.; Tang, X.Y.; Hu, X.Y.; Yan, R.Q. Reduced Amyloid Deposition in Mice Overexpressing RTN3 Is Adversely Affected by Preformed Dystrophic Neurites. J. Neurosci. 2009, 29, 9163–9173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Q.W.; Kuang, E.; Dong, W.; Zhou, S.M.; Xu, H.; Qi, Y.P.; Liu, Y.L. Reticulon 3 mediates Bcl-2 accumulation in mitochondria in response to endoplasmic reticulum stress. Apoptosis 2007, 12, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.U.; D’Sa-Eipper, C.; Brenner, J.; Kuida, K.; Zheng, T.S.; Flavell, R.A.; Rakic, P.; Roth, K.A. Bcl-X-L-Caspase-9 interactions in the developing nervous system: Evidence for multiple death pathways. J. Neurosci. 2001, 21, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohn, T.T.; Vyas, V.; Hernandez-Estrada, T.; Nichol, K.E.; Christie, L.A.; Head, E. Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J. Neurosci. 2008, 28, 3051–3059. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lv, Y.G.; Du, Y.F.; Hu, M.; Reed, M.N.; Long, Y.; Suppiramaniam, V.; Hong, H.; Tang, S.S. Inhibitory effect of INT-777 on lipopolysaccharide-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 88, 360–374. [Google Scholar] [CrossRef]
- Kaufmann, T.; Strasser, A.; Jost, P.J. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012, 19, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Delavallee, L.; Cabon, L.; Galan-Malo, P.; Lorenzo, H.K.; Susin, S.A. AIF-mediated Caspase-independent Necroptosis: A New Chance for Targeted Therapeutics. IUBMB Life 2011, 63, 221–232. [Google Scholar] [CrossRef]
- De Zio, D.; Ferraro, E.; D’Amelio, M.; Simoni, V.; Bordi, M.; Soroldoni, D.; Berghella, L.; Meyer, B.I.; Cecconi, F. Faf1 is expressed during neurodevelopment and is involved in Apaf1-dependent caspase-3 activation in proneural cells. Cell. Mol. Life Sci. 2008, 65, 1780–1790. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Kong, Y.Y.; Yoshida, R.; Elia, A.J.; Hakem, A.; Hakem, R.; Penninger, J.M.; Mak, T.W. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 1998, 94, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3 pathway in the mouse hippocampus. J. Pineal Res. 2015, 59, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, Y.B.; Sun, J.Y.; Xiang, Y.; Yang, J.; Dai, S.Y.; Zhang, X. Neuroglobin Attenuates Beta Amyloid-Induced Apoptosis Through Inhibiting Caspases Activity by Activating PI3K/Akt Signaling Pathway. J. Mol. Neurosci. 2016, 58, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Murphy, A.N.; Brown, J.H. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008, 15, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resende, R.; Ferreiro, E.; Pereira, C.; De Oliveira, C.R. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1-42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience 2008, 155, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Nagakura, A.; Shitaka, Y.; Yarimizu, J.; Matsuoka, N. Characterization of cognitive deficits in a transgenic mouse model of Alzheimer’s disease and effects of donepezil and memantine. Eur. J. Pharmacol. 2013, 703, 53–61. [Google Scholar] [CrossRef]
- Stewart, S.; Cacucci, F.; Lever, C. Which Memory Task for My Mouse? A Systematic Review of Spatial Memory Performance in the Tg2576 Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2011, 26, 105–126. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Hao, J.; Liu, X.; Li, C.; Yuan, X.; Lee, R.J.; Bai, T.; Wang, D. Isoforsythiaside Attenuates Alzheimer’s Disease via Regulating Mitochondrial Function Through the PI3K/AKT Pathway. Int. J. Mol. Sci. 2020, 21, 5687. https://doi.org/10.3390/ijms21165687
Wang C, Hao J, Liu X, Li C, Yuan X, Lee RJ, Bai T, Wang D. Isoforsythiaside Attenuates Alzheimer’s Disease via Regulating Mitochondrial Function Through the PI3K/AKT Pathway. International Journal of Molecular Sciences. 2020; 21(16):5687. https://doi.org/10.3390/ijms21165687
Chicago/Turabian StyleWang, Chunyue, Jie Hao, Xin Liu, Chenliang Li, Xuyang Yuan, Robert J. Lee, Tian Bai, and Di Wang. 2020. "Isoforsythiaside Attenuates Alzheimer’s Disease via Regulating Mitochondrial Function Through the PI3K/AKT Pathway" International Journal of Molecular Sciences 21, no. 16: 5687. https://doi.org/10.3390/ijms21165687
APA StyleWang, C., Hao, J., Liu, X., Li, C., Yuan, X., Lee, R. J., Bai, T., & Wang, D. (2020). Isoforsythiaside Attenuates Alzheimer’s Disease via Regulating Mitochondrial Function Through the PI3K/AKT Pathway. International Journal of Molecular Sciences, 21(16), 5687. https://doi.org/10.3390/ijms21165687




