Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease
Abstract
:1. Introduction
2. Biomarkers for Alzheimer’s Disease
3. Amyloid Cascade Hypothesis
4. Proteolytic Fragments of Amyloid Precursor Protein—CTF99 and sAPPα
5. Crosstalk of Aβ and Tau
6. Therapeutic Targeting of Aβ in Alzheimer’s Pathogenesis
6.1. Decreasing Aβ Production
6.1.1. α-Secretase Activators
6.1.2. β-Secretase Inhibitors
6.1.3. γ-Secretase Inhibitors
6.2. Blocking Aβ Aggregation
6.2.1. Anti-Amyloid Aggregators
6.2.2. Metal Chelators
6.3. Increasing Aβ Clearance
6.3.1. Aβ Vaccination
Active Immunization
Passive Immunization
6.3.2. Receptor-Mediated Clearance
6.4. Altering the Interplay of APP, Aβ, and Tauopathy
6.5. Altering the Interplay of Aβ and Neuroinflammation
7. Aβ Targeting Drugs
8. Promising New Opportunities for Alzheimer’s Disease
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koudinov, A.R.; Berezov, T.T. Alzheimer’s amyloid-beta (A beta) is an essential synaptic protein, not neurotoxic junk. Acta Neurobiol. Exp. (Wars) 2004, 64, 71–79. [Google Scholar]
- Sharma, P.; Sharma, A.; Fayaz, F.; Wakode, S.; Pottoo, F.H. Biological Signatures of Alzheimer’s Disease. Curr. Top. Med. Chem. 2020, 20, 770–781. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Asaduzzaman, M.; Hosn, F.; Abu Sufian, M.; Takeda, S.; Herrera-Calderon, O.; Abdel-Daim, M.M.; Uddin, G.M.S.; Noor, M.A.A.; et al. Spectrum of Disease and Prescription Pattern for Outpatients with Neurological Disorders: An Empirical Pilot Study in Bangladesh. Ann. Neurosci. 2018, 25, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, A.; Ghanbarzadeh, S.; Mahmoudi, J.; Nayebi, A.M.; Maleki-Dizaji, N.; Garjani, A. Investigation of the memory impairment in rats fed with oxidized-cholesterol-rich diet employing passive avoidance test. Drug Res. (Stuttg) 2015, 65, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Grienberger, C.; Rochefort, N.L.; Adelsberger, H.; Henning, H.A.; Hill, D.N.; Reichwald, J.; Staufenbiel, M.; Konnerth, A. Staged decline of neuronal function in vivo in an animal model of Alzheimer’s disease. Nat. Commun. 2012, 3, 774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009, 11, 111–128. [Google Scholar]
- Götz, J.; Ittner, L.M. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008, 9, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, A. Amyloid plaque imaging in vivo: Current achievement and future prospects. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Jakaria, M.; Sobarzo-Sánchez, E.; Barreto, G.E.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Exploring the potential of neuroproteomics in Alzheimer’s disease. Curr. Top. Med. Chem. 2020, 20. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Amyloid β-Peptide(1-42) Contributes to the Oxidative Stress and Neurodegeneration Found in Alzheimer Disease Brain. Brain Pathol. 2006, 14, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-β toxicity in alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Al Mamun, A.; Rahman, M.A.; Behl, T.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Emerging proof of protein misfolding and interactions in multifactorial Alzheimer’s disease. Curr. Top. Med. Chem. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Begum, M.M.; Thangapandiyan, S.; Rahman, M.S.; Aleya, L.; Mathew, B.; Ahmed, M.; Ashraf, G.M.; Barreto, G.E. Cholinesterase Inhibitors for Alzheimer’s Disease: Multitargeting Strategy based on Anti-Alzheimer’s Drugs Repositioning. Curr. Pharm. Des. 2019, 25, 3519–3535. [Google Scholar] [CrossRef]
- Kabir, M.T.; Abu Sufian, M.; Uddin, M.S.; Begum, M.M.; Akhter, S.; Islam, A.; Mathew, B.; Islam, M.S.; Amran, M.S.; Md Ashraf, G. NMDA Receptor Antagonists: Repositioning of Memantine as Multitargeting Agent for Alzheimer’s Therapy. Curr. Pharm. Des. 2019, 25, 3506–3518. [Google Scholar] [CrossRef]
- Butterfield, D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.R.; Zaman, T.; Uddin, M.S.; Islam, R.; Abdel-Daim, M.M.; Rhim, H. Emerging Potential of Naturally Occurring Autophagy Modulator against Neurodegeneration. Curr. Pharm. Des. 2020, 26, 772–779. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Jakaria, M.; Thangapandiyan, S.; Ahmad, J.; Rahman, M.A.; Mathew, B.; Abdel-Daim, M.M.; Aleya, L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020, 707, 135624. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Pottoo, F.H.; Dahiya, E.S.; Khan, F.A.; Kumar, J.S. Neuron-Glia interaction: Molecular basis of Alzheimer’s Disease and Applications of Neuroproteomics. Eur. J. Neurosci. 2020, 52, 2931–2943. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Wicklund, L.; Lacor, P.N.; Klein, W.L.; Nordberg, A.; Marutle, A. Different β-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol. Aging 2012, 33, 825.e1–825.e13. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Al Mamun, A.; Mathew, B.; Aleya, L.; Barreto, G.E.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Revisiting the role of brain and peripheral Aβ in the pathogenesis of Alzheimer’s disease. J. Neurol. Sci. 2020, 416, 116974. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.E.; DeVos, S.L.; Dujardin, S.; Corjuc, B.; Gor, R.; Gonzalez, J.; Roe, A.D.; Frosch, M.P.; Pitstick, R.; Carlson, G.A.; et al. Enhanced Tau Aggregation in the Presence of Amyloid β. Am. J. Pathol. 2017, 187, 1601–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, B.; Stancu, I.C.; Buist, A.; Bird, M.; Wang, P.; Vanoosthuyse, A.; Van Kolen, K.; Verheyen, A.; Kienlen-Campard, P.; Octave, J.N.; et al. Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. 2016, 131, 549–569. [Google Scholar] [CrossRef] [Green Version]
- Götz, J.; Chen, F.; Van Dorpe, J.; Nitsch, R.M. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science 2001, 293, 1491–1495. [Google Scholar] [CrossRef]
- Bolmont, T.; Clavaguera, F.; Meyer-Luehmann, M.; Herzig, M.C.; Radde, R.; Staufenbiel, M.; Lewis, J.; Hutton, M.; Tolnay, M.; Jucker, M. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP x tau transgenic mice. Am. J. Pathol. 2007, 171, 2012–2020. [Google Scholar] [CrossRef] [Green Version]
- Pooler, A.M.; Polydoro, M.; Maury, E.A.; Nicholls, S.B.; Reddy, S.M.; Wegmann, S.; William, C.; Saqran, L.; Cagsal-Getkin, O.; Pitstick, R.; et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun. 2015, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.; Dickson, D.W.; Lin, W.L.; Chisholm, L.; Corral, A.; Jones, G.; Yen, S.H.; Sahara, N.; Skipper, L.; Yager, D.; et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 2001, 293, 1487–1491. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Guo, J.L.; McBride, J.D.; Narasimhan, S.; Kim, H.; Changolkar, L.; Zhang, B.; Gathagan, R.J.; Yue, C.; Dengler, C.; et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 2018, 24, 29–38. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K.; Hanse, E. Amyloid β and APP as biomarkers for Alzheimer’s disease. Exp. Gerontol. 2010, 45, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, A.; Bernardini, S.; Panella, M.; Pierantozzi, M.; Nuccetelli, M.; Koch, G.; Urbani, A.; Giordano, A.; Martorana, A.; Orlacchio, A.; et al. AD with subcortical white matter lesions and vascular dementia: CSF markers for differential diagnosis. J. Neurol. Sci. 2005, 237, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Yoshita, M.; Matsumoto, Y.; Ono, K.; Iwasa, K.; Yamada, M. Decreased β-amyloid peptide42 in cerebrospinal fluid of patients with progressive supranuclear palsy and corticobasal degeneration. J. Neurol. Sci. 2005, 237, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Cedazo-Minguez, A.; Winblad, B. Biomarkers for Alzheimer’s disease and other forms of dementia: Clinical needs, limitations and future aspects. Exp. Gerontol. 2010, 45, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Sunderland, T.; Mirza, N.; Putnam, K.T.; Linker, G.; Bhupali, D.; Durham, R.; Soares, H.; Kimmel, L.; Friedman, D.; Bergeson, J.; et al. Cerebrospinal fluid β-amyloid 1-42 and tau in control subjects at risk for Alzheimer’s disease: The effect of APOE ε4 allele. Biol. Psychiatry 2004, 56, 670–676. [Google Scholar] [CrossRef]
- Schoonenboom, N.S.; Mulder, C.; Van Kamp, G.J.; Mehta, S.P.; Scheltens, P.; Blankenstein, M.A.; Mehta, P.D. Amyloid β 38, 40, and 42 species in cerebrospinal fluid: More of the same? Ann. Neurol. 2005, 58, 139–142. [Google Scholar] [CrossRef]
- Xiao, Z.; Prieto, D.; Conrads, T.P.; Veenstra, T.D.; Issaq, H.J. Proteomic patterns: Their potential for disease diagnosis. Mol. Cell. Endocrinol. 2005, 230, 95–106. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and Tau. JAMA Neurol. 2014, 71, 505. [Google Scholar] [CrossRef] [Green Version]
- Al Mamun, A.; Uddin, M.S.; Kabir, M.T.; Khanum, S.; Sarwar, M.S.; Mathew, B.; Rauf, A.; Ahmed, M.; Ashraf, G.M. Exploring the Promise of Targeting Ubiquitin-Proteasome System to Combat Alzheimer’s Disease. Neurotox. Res. 2020, 38, 8–17. [Google Scholar] [CrossRef]
- Sjögren, M.; Vanderstichele, H.; Agren, H.; Zachrisson, O.; Edsbagge, M.; Wikkelsø, C.; Skoog, I.; Wallin, A.; Wahlund, L.O.; Marcusson, J.; et al. Tau and Abeta42 in Cerebrospinal Fluid From Healthy Adults 21–93 Years of Age: Establishment of Reference Values. Clin. Chem. 2001, 47, 1776–1781. [Google Scholar]
- Blennow, K. CSF biomarkers for mild cognitive impairment. J. Intern. Med. 2004, 256, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K. CSF biomarkers for Alzheimer’s disease: Use in early diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 2005, 5, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.-A.; Lim, J.-H.; Sul, A.-R.; Lee, M.; Youn, Y.C.; Kim, H.-J. Cerebrospinal Fluid β-Amyloid1–42 Levels in the Differential Diagnosis of Alzheimer’s Disease—Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0116802. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Watts, K.D.; Shaw, L.M.; Howell, J.C.; Trojanowski, J.Q.; Basra, S.; Glass, J.D.; Lah, J.J.; Levey, A.I. CSF beta-amyloid 1-42—what are we measuring in Alzheimer’s disease? Ann. Clin. Transl. Neurol. 2015, 2, 131–139. [Google Scholar] [CrossRef]
- Quiroz, Y.T.; Sperling, R.A.; Norton, D.J.; Baena, A.; Arboleda-Velasquez, J.F.; Cosio, D.; Schultz, A.; Lapoint, M.; Guzman-Velez, E.; Miller, J.B.; et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol. 2018, 75, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Assini, A.; Cammarata, S.; Vitali, A.; Colucci, M.; Giliberto, L.; Borghi, R.; Inglese, M.L.; Volpe, S.; Ratto, S.; Dagna-Bricarelli, F.; et al. Plasma levels of amyloid β-protein 42 are increased in women with mild cognitive impairment. Neurology 2004, 63, 828–831. [Google Scholar] [CrossRef]
- Borroni, B.; Di Luca, M.; Padovani, A. Predicting Alzheimer dementia in mild cognitive impairment patients. Are biomarkers useful? Eur. J. Pharmacol. 2006, 545, 73–80. [Google Scholar] [CrossRef]
- Brettschneider, S.; Morgenthaler, N.G.; Teipel, S.J.; Fischer-Schulz, C.; Bürger, K.; Dodel, R.; Du, Y.; Möller, H.J.; Bergmann, A.; Hampel, H. Decreased serum amyloid β1-42 autoantibody levels in Alzheimer’s disease, determined by a newly developed immuno-precipitation assay with radiolabeled amyloid β1-42 peptide. Biol. Psychiatry 2005, 57, 813–816. [Google Scholar] [CrossRef]
- Gustaw-Rothenberg, K.A.; Siedlak, S.L.; Bonda, D.J.; Lerner, A.; Tabaton, M.; Perry, G.; Smith, M.A. Dissociated amyloid-β antibody levels as a serum biomarker for the progression of Alzheimer’s disease: A population-based study. Exp. Gerontol. 2010, 45, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Kabir, M.T.; Jeandet, P.; Mathew, B.; Ashraf, G.M.; Perveen, A.; Bin-Jumah, M.N.; Mousa, S.A.; Abdel-Daim, M.M. Novel Anti-Alzheimer’s Therapeutic Molecules Targeting Amyloid Precursor Protein Processing. Oxid. Med. Cell. Longev. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Weggen, S.; Beher, D. Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimer’s Res. Ther. 2012, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLeod, R.; Hillert, E.-K.; Cameron, R.T.; Baillie, G.S. The role and therapeutic targeting of α-, β- and γ-secretase in Alzheimer’s disease. Futur. Sci. OA 2015, 1, FSO11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citron, M.; Teplow, D.B.; Selkoe, D.J. Generation of amyloid β protein from its precursor is sequence specific. Neuron 1995, 14, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Citron, M.; Oltersdorf, T.; Haass, C.; McConlogue, L.; Hung, A.Y.; Seubert, P.; Vigo-Pelfrey, C.; Lieberburg, I.; Selkoe, D.J. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature 1992, 360, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Rahman, M.M.; Mathew, B.; Shah, M.A.; Ashraf, G.M. TV 3326 for Alzheimer’s dementia: A novel multimodal ChE and MAO inhibitors to mitigate Alzheimer’s-like neuropathology. J. Pharm. Pharmacol. 2020, 72, 1001–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyreuther, K.; Masters, C.L. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: Precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991, 1, 241–251. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Setu, J.R.; Ashraf, G.M.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Exploring the Role of PSEN mutations in the pathogenesis of Alzheimer’s disease. Neurotox. Res. 2020. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M.; Strooper, B. De The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Borchelt, D.R.; Thinakaran, G.; Eckman, C.B.; Lee, M.K.; Davenport, F.; Ratovitsky, T.; Prada, C.M.; Kim, G.; Seekins, S.; Yager, D.; et al. Familial Alzheimer’s disease-linked presenilin I variants elevate aβ1-42/1-40 ratio in vitro and in vivo. Neuron 1996, 17, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Ertekin-Taner, N. Genetics of Alzheimer’s Disease: A Centennial Review. Neurol. Clin. 2007, 25, 611–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jäggi, F.; Wolburg, H.; Gengler, S.; et al. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.B.; Lindholm, K.; Yan, R.; Citron, M.; Xia, W.; Yang, X.L.; Beach, T.; Sue, L.; Wong, P.; Price, D.; et al. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 2003, 9, 3–4. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef] [Green Version]
- Bennett, D.A.; Schneider, J.A.; Arvanitakis, Z.; Kelly, J.F.; Aggarwal, N.T.; Shah, R.C.; Wilson, R.S. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006, 66, 1837–1844. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.J.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA J. Am. Med. Assoc. 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Vos, S.J.B.; Xiong, C.; Visser, P.J.; Jasielec, M.S.; Hassenstab, J.; Grant, E.A.; Cairns, N.J.; Morris, J.C.; Holtzman, D.M.; Fagan, A.M. Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study. Lancet Neurol. 2013, 12, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Burnham, S.C.; Bourgeat, P.; Doré, V.; Savage, G.; Brown, B.; Laws, S.; Maruff, P.; Salvado, O.; Ames, D.; Martins, R.N.; et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: A longitudinal study. Lancet Neurol. 2016, 15, 1044–1053. [Google Scholar] [CrossRef]
- Petersen, R.C.; Wiste, H.J.; Weigand, S.D.; Rocca, W.A.; Roberts, R.O.; Mielke, M.M.; Lowe, V.J.; Knopman, D.S.; Pankratz, V.S.; Machulda, M.M.; et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016, 73, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.C.; Sperling, R.A.; Petersen, R.; Sun, C.K.; Weiner, M.; Aisen, P.S. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA J. Am. Med. Assoc. 2017, 317, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, I.; Pardossi-Piquard, R.; Bauer, C.; Brigham, E.; Abraham, J.D.; Ranaldi, S.; Fraser, P.; St-George-Hyslop, P.; Le Thuc, O.; Espin, V.; et al. The β-secretase-derived C-terminal fragment of βAPP, C99, but not Aβ, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J. Neurosci. 2012, 32, 16243–16255. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, I.; Pardossi-Piquard, R.; Bourgeois, A.; Pagnotta, S.; Biferi, M.G.; Barkats, M.; Lacor, P.; Klein, W.; Bauer, C.; Checler, F. Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta Neuropathol. 2016, 132, 257–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasternak, S.H.; Callahan, J.W.; Mahuran, D.J. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer’s disease: Reexamining the spatial paradox from a lysosomal perspective. J. Alzheimer’s Dis. 2004, 6, 53–65. [Google Scholar] [CrossRef]
- Schubert, D.; Behl, C. The expression of amyloid beta protein precursor protects nerve cells from β-amyloid and glutamate toxicity and alters their interaction with the extracellular matrix. Brain Res. 1993, 629, 275–282. [Google Scholar] [CrossRef]
- Furukawa, K.; Mattson, M.P. Secreted amyloid precursor protein a selectively suppresses N-methyl-D- aspartate currents in hippocampal neurons: Involvement of cyclic GMP. Neuroscience 1998, 83, 429–438. [Google Scholar] [CrossRef]
- Bell, K.F.S.; Zheng, L.; Fahrenholz, F.; Cuello, A.C. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol. Aging 2008, 29, 554–565. [Google Scholar] [CrossRef]
- Mattson, M.P. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997, 77, 1081–1132. [Google Scholar] [CrossRef] [Green Version]
- Caillé, I.; Allinquant, B.; Dupont, E.; Bouillot, C.; Langer, A.; Müller, U.; Prochiantz, A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 2004, 131, 2173–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obregon, D.; Hou, H.; Deng, J.; Giunta, B.; Tian, J.; Darlington, D.; Shahaduzzaman, M.; Zhu, Y.; Mori, T.; Mattson, M.P.; et al. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β 2 generation. Nat. Commun. 2012, 3, 777. [Google Scholar] [CrossRef] [PubMed]
- Peters-Libeu, C.; Campagna, J.; Mitsumori, M.; Poksay, K.S.; Spilman, P.; Sabogal, A.; Bredesen, D.E.; John, V. SAβPPα is a Potent Endogenous Inhibitor of BACE1. J. Alzheimer’s Dis. 2015, 47, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Habib, A.; Obregon, D.F.; Barger, S.W.; Giunta, B.; Wang, Y.J.; Hou, H.; Sawmiller, D.; Tan, J. Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3β signaling pathway. J. Neurochem. 2015, 135, 630–637. [Google Scholar] [CrossRef]
- Arriagada, P.V.; Growdon, J.H.; Hedley-Whyte, E.T.; Hyman, B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992, 42, 631–639. [Google Scholar] [CrossRef]
- Bierer, L.M.; Hof, P.R.; Purohit, D.P.; Carlin, L.; Schmeidler, J.; Davis, K.L.; Perl, D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 1995, 52, 81–88. [Google Scholar] [CrossRef]
- Mamun, A.A.; Uddin, M.S.; Mathew, B.; Ashraf, G.M. Toxic Tau: Structural Origins of Tau Aggregation in Alzheimer’s Disease. Neural Regen. Res. 2020, 15, 1417–1420. [Google Scholar]
- Gómez-Isla, T.; Hollister, R.; West, H.; Mui, S.; Growdon, J.H.; Petersen, R.C.; Parisi, J.E.; Hyman, B.T. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 1997, 41, 17–24. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Abdeen, A.; Ashraf, G.M.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Evidence linking protein misfolding to quality control in progressive neurodegenerative diseases. Curr. Top. Med. Chem. 2020, 20. [Google Scholar] [CrossRef]
- Bennett, D.A.; Schneider, J.A.; Wilson, R.S.; Bienias, J.L.; Arnold, S.E. Neurofibrillary Tangles Mediate the Association of Amyloid Load with Clinical Alzheimer Disease and Level of Cognitive Function. Arch. Neurol. 2004, 61, 378–384. [Google Scholar] [CrossRef]
- Wang, L.; Benzinger, T.L.; Su, Y.; Christensen, J.; Friedrichsen, K.; Aldea, P.; McConathy, J.; Cairns, N.J.; Fagan, A.M.; Morris, J.C.; et al. Evaluation of Tau imaging in staging Alzheimer disease and revealing interactions between β-Amyloid and tauopathy. JAMA Neurol. 2016, 73, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Sutphen, C.L.; McCue, L.; Herries, E.M.; Xiong, C.; Ladenson, J.H.; Holtzman, D.M.; Fagan, A.M. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 869–879. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.; Wang, G.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.S.; Buckles, V.; Fagan, A.M.; Holtzman, D.M.; Cairns, N.J.; Goate, A.M.; et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 2018, 91, E1295–E1306. [Google Scholar] [CrossRef]
- Sato, C.; Barthélemy, N.R.; Mawuenyega, K.G.; Patterson, B.W.; Gordon, B.A.; Jockel-Balsarotti, J.; Sullivan, M.; Crisp, M.J.; Kasten, T.; Kirmess, K.M.; et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 2018, 97, 1284–1298. [Google Scholar] [CrossRef] [Green Version]
- Hansson, O.; Zetterberg, H.; Buchhave, P.; Londos, E.; Blennow, K.; Minthon, L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006, 5, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Davidson, Y.S.; Robinson, A.; Prasher, V.P.; Mann, D.M.A. The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer’s disease in individuals with Down syndrome. Acta Neuropathol. Commun. 2018, 6, 56. [Google Scholar] [CrossRef]
- Lee, G.; Newman, S.T.; Gard, D.L.; Band, H.; Panchamoorthy, G. Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 1998, 111, 3167–3177. [Google Scholar]
- Nakazawa, T.; Komai, S.; Tezuka, T.; Hisatsune, C.; Umemori, H.; Semba, K.; Mishina, M.; Manabe, T.; Yamamoto, T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRε2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2001, 276, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.; Lu, X.; Bernard, A.; Khrestchatisky, M.; Baudry, M. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J. Neurochem. 2001, 79, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezuka, T.; Umemori, H.; Akiyama, T.; Nakanishi, S.; Yamamoto, T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc. Natl. Acad. Sci. USA 1999, 96, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salter, M.W.; Kalia, L.V. SRC kinases: A hub for NMDA receptor regulation. Nat. Rev. Neurosci. 2004, 5, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Liu, Y.; Liu, L.; Besshoh, S.; Arundine, M.; Gurd, J.W.; Wang, Y.T.; Salter, M.W.; Tymianski, M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 2002, 298, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.; Palop, J.J.; Puoliväli, J.; Massaro, C.; Bien-Ly, N.; Gerstein, H.; Scearce-Levie, K.; Masliah, E.; Mucke, L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2005, 25, 9694–9703. [Google Scholar] [CrossRef] [Green Version]
- Chin, J.; Palop, J.J.; Yu, G.Q.; Kojima, N.; Masliah, E.; Mucke, L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J. Neurosci. 2004, 24, 4692–4697. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, E.-M.; Lee, J.-P.; Park, C.H.; Kim, S.; Seo, J.-H.; Chang, K.-A.; Yu, E.; Jeong, S.-J.; Chong, Y.H.; et al. C-terminal fragments of amyloid precursor protein exert neurotoxicity by inducing glycogen synthase kinase-3β expression. FASEB J. 2003, 17, 1–28. [Google Scholar] [CrossRef]
- Kuhn, P.-H.; Wang, H.; Dislich, B.; Colombo, A.; Zeitschel, U.; Ellwart, J.W.; Kremmer, E.; Roßner, S.; Lichtenthaler, S.F. ADAM10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010, 29, 3020–3032. [Google Scholar] [CrossRef] [Green Version]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, S.; Goldstein, L.; Lahiri, D.; Rogers, J. Role of the APP Non-Amyloidogenic Signaling Pathway and Targeting α-Secretase as an Alternative Drug Target for Treatment of Alzheimers Disease. Curr. Med. Chem. 2007, 14, 2848–2864. [Google Scholar] [CrossRef] [PubMed]
- Hong-Qi, Y.; Zhi-Kun, S.; Sheng-Di, C. Current advances in the treatment of Alzheimer’s disease: Focused on considerations targeting Aβ and tau. Transl. Neurodegener. 2012, 1, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.Q.; Sun, Z.K.; Ba, M.W.; Xu, J.; Xing, Y. Involvement of protein trafficking in deprenyl-induced α-secretase activity regulation in PC12 cells. Eur. J. Pharmacol. 2009, 610, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Filip, V.; Kolibás, E. Selegiline in the treatment of Alzheimer’s disease: A long-term randomized placebo-controlled trial. Czech and Slovak Senile Dementia of Alzheimer Type Study Group. J. Psychiatry Neurosci. 1999, 24, 234–243. [Google Scholar] [PubMed]
- Zamrini, E.; McGwin, G.; Roseman, J.M. Association between Statin Use and Alzheimer’s Disease. Neuroepidemiology 2004, 23, 94–98. [Google Scholar] [CrossRef]
- Parvathy, S.; Ehrlich, M.; Pedrini, S.; Diaz, N.; Refolo, L.; Buxbaum, J.D.; Bogush, A.; Petanceska, S.; Gandy, S. Atorvastatin-induced activation of Alzheimer’s alpha secretase is resistant to standard inhibitors of protein phosphorylation-regulated ectodomain shedding. J. Neurochem. 2004, 90, 1005–1010. [Google Scholar] [CrossRef]
- Feldman, H.H.; Doody, R.S.; Kivipelto, M.; Sparks, D.L.; Waters, D.D.; Jones, R.W.; Schwam, E.; Schindler, R.; Hey-Hadavi, J.; Demicco, D.A.; et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010, 74, 956–964. [Google Scholar] [CrossRef]
- Vellas, B.; Sol, O.; Snyder, P.J.; Ousset, P.-J.; Haddad, R.; Maurin, M.; Lemarie, J.-C.; Desire, L.; Pando, M.P. EHT0202 in Alzheimers Disease: A 3-Month, Randomized, Placebo- Controlled, Double-Blind Study. Curr. Alzheimer Res. 2012, 8, 203–212. [Google Scholar] [CrossRef]
- Marcade, M.; Bourdin, J.; Loiseau, N.; Peillon, H.; Rayer, A.; Drouin, D.; Schweighoffer, F.; Désiré, L. Etazolate, a neuroprotective drug linking GABAA receptor pharmacology to amyloid precursor protein processing. J. Neurochem. 2008, 106, 392–404. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of PRX-03140 Monotherapy in Subjects with Alzheimer’s Sisease. Available online: https://clinicaltrials.gov/ct2/show/NCT00693004 (accessed on 18 June 2020).
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D.; et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [Green Version]
- Obregon, D.F.; Rezai-Zadeh, K.; Bai, Y.; Sun, N.; Hou, H.; Ehrhart, J.; Zeng, J.; Mori, T.; Arendash, G.W.; Shytle, D.; et al. ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallate-induced α-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 2006, 281, 16419–16427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Sunphenon EGCg (Epigallocatechin-Gallate) in the Early Stage of Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00951834 (accessed on 18 June 2020).
- Farlow, M.R.; Thompson, R.E.; Wei, L.J.; Tuchman, A.J.; Grenier, E.; Crockford, D.; Wilke, S.; Benison, J.; Alkon, D.L.; Moreira, P. A randomized, double-blind, placebo-controlled, phase II study assessing safety, tolerability, and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 67, 555–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.; Tory, M.C.; Pauley, A.M.; Brashler, J.R.; Stratman, N.C.; Mathews, W.R.; Buhl, A.E.; et al. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 1999, 402, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Roberds, S.L.; Anderson, J.; Basi, G.; Bienkowski, M.J.; Branstetter, D.G.; Chen, K.S.; Freedman, S.B.; Frigon, N.L.; Games, D.; Hu, K.; et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 2001, 10, 1317–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Bolon, B.; Kahn, S.; Bennett, B.D.; Babu-Khan, S.; Denis, P.; Fan, W.; Kha, H.; Zhang, J.; Gong, Y.; et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat. Neurosci. 2001, 4, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Chang, L.; Tseng, W.; Oakley, H.; Citron, M.; Klein, W.L.; Vassar, R.; Disterhoft, J.F. Temporal memory deficits in Alzheimer’s mouse models: Rescue by genetic deletion of BACE1. Eur. J. Neurosci. 2006, 23, 251–260. [Google Scholar] [CrossRef]
- Ohno, M.; Sametsky, E.A.; Younkin, L.H.; Oakley, H.; Younkin, S.G.; Citron, M.; Vassar, R.; Disterhoft, J.F. BACE1 Deficiency Rescues Memory Deficits and Cholinergic Dysfunction in a Mouse Model of Alzheimer’s Disease. Neuron 2004, 41, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Landreth, G.; Jiang, Q.; Mandrekar, S.; Heneka, M. PPARγ agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics 2008, 5, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Craft, S. The Role of Metabolic Disorders in Alzheimer Disease and Vascular Dementia. Arch. Neurol. 2009, 66, 300–305. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Biomarker Qualification for Risk of Mild Cognitive Impairment (MCI) Due to Alzheimer’s disease (AD) and Safety and Efficacy Evaluation of Pioglitazone in Delaying Its Onset. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01931566 (accessed on 18 June 2020).
- Harrington, C.; Sawchak, S.; Chiang, C.; Davies, J.; Donovan, C.; Saunders, A.M.; Irizarry, M.; Jeter, B.; Zvartau-Hind, M.; van Dyck, C.H.; et al. Rosiglitazone Does Not Improve Cognition or Global Function when Used as Adjunctive Therapy to AChE Inhibitors in Mild-to-Moderate Alzheimers Disease: Two Phase 3 Studies. Curr. Alzheimer Res. 2011, 8, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.J.N. Beta-secretase as target for amyloid-reduction therapy. Alzheimer’s Dement. 2009, 5, 74. [Google Scholar] [CrossRef]
- Chang, W.-P.; Koelsch, G.; Wong, S.; Downs, D.; Da, H.; Weerasena, V.; Gordon, B.; Devasamudram, T.; Bilcer, G.; Ghosh, A.K.; et al. In vivo inhibition of Aβ production by memapsin 2 (β-secretase) inhibitors. J. Neurochem. 2004, 89, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Kumaragurubaran, N.; Hong, L.; Koelsh, G.; Tang, J. Memapsin 2 (beta-secretase) inhibitors: Drug development. Curr. Alzheimer Res. 2008, 5, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Alzforum. Keystone Drug News: CoMentis BACE Inhibitor Debuts. Available online: https://www.alzforum.org/news/conference-coverage/keystone-drug-news-comentis-bace-inhibitor-debuts (accessed on 3 August 2020).
- Ghosh, A.K.; Brindisi, M.; Tang, J. Developing β-secretase inhibitors for treatment of Alzheimer’s disease. J. Neurochem. 2012, 120, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Yan, R. Stepping closer to treating Alzheimer’s disease patients with BACE1 inhibitor drugs. Transl. Neurodegener. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Alzforum. Lilly Halts Phase 2 Trial of BACE Inhibitor Due to Liver Toxicity. Available online: https://www.alzforum.org/news/research-news/lilly-halts-phase-2-trial-bace-inhibitor-due-liver-toxicity (accessed on 3 August 2020).
- Kennedy, M.E.; Stamford, A.W.; Chen, X.; Cox, K.; Cumming, J.N.; Dockendorf, M.F.; Egan, M.; Ereshefsky, L.; Hodgson, R.A.; Hyde, L.A.; et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS b-Amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl. Med. 2016, 8, 363ra150. [Google Scholar] [CrossRef]
- Egan, M.F.; Kost, J.; Voss, T.; Mukai, Y.; Aisen, P.S.; Cummings, J.L.; Tariot, P.N.; Vellas, B.; Van Dyck, C.H.; Boada, M.; et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med. 2019, 380, 1408–1420. [Google Scholar] [CrossRef]
- Shoji, M.; Golde, T.E.; Ghiso, J.; Cheung, T.T.; Estus, S.; Shaffer, L.M.; Cai, X.D.; McKay, D.M.; Tintner, R.; Frangione, B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 1992, 258, 126–129. [Google Scholar] [CrossRef]
- Haass, C.; Schlossmacher, M.G.; Hung, A.Y.; Vigo-Pelfrey, C.; Mellon, A.; Ostaszewski, B.L.; Lieberburg, I.; Koo, E.H.; Schenk, D.; Teplow, D.B.; et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 1992, 359, 322–325. [Google Scholar] [CrossRef]
- De Strooper, B.; Annaert, W.; Cupers, P.; Saftig, P.; Craessaerts, K.; Mumm, J.S.; Schroeter, E.H.; Schrijvers, V.; Wolfe, M.S.; Ray, W.J.; et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 1999, 398, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Henley, D.B.; May, P.C.; Dean, R.A.; Siemers, E.R. Development of semagacestat (LY450139), a functional γ-secretase inhibitor, for the treatment of Alzheimer’s disease. Expert Opin. Pharmacother. 2009, 10, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Imbimbo, B.P. Therapeutic potential of gamma-secretase inhibitors and modulators. Curr. Top. Med. Chem. 2008, 8, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, A.S.; Raman, R.; Siemers, E.R.; Becerra, L.; Clark, C.M.; Dean, R.A.; Farlow, M.R.; Galvin, J.E.; Peskind, E.R.; Quinn, J.F.; et al. Phase 2 Safety Trial Targeting Amyloid β Production With a γ-Secretase Inhibitor in Alzheimer Disease. Arch. Neurol. 2008, 65, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Qian, S.; Soriano, S.; Wu, Y.; Fletcher, A.M.; Wang, X.J.; Koo, E.H.; Wu, X.; Zheng, H. Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 10863–10868. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, M.; Wolfer, A.; Raj, K.; Kummer, J.A.; Mill, P.; van Noort, M.; Hui, C.; Clevers, H.; Dotto, G.P.; Radtke, F. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 2003, 33, 416–421. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Datar, R.; Murtaugh, L.C.; Melton, D.A. Direct regulation of intestinal fate by Notch. Proc. Natl. Acad. Sci. USA 2005, 102, 12443–12448. [Google Scholar] [CrossRef] [Green Version]
- Maillard, I.; Adler, S.H.; Pear, W.S. Notch and the immune system. Immunity 2003, 19, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Bateman, R.J.; Siemers, E.R.; Mawuenyega, K.G.; Wen, G.; Browning, K.R.; Sigurdson, W.C.; Yarasheski, K.E.; Friedrich, S.W.; DeMattos, R.B.; May, P.C.; et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann. Neurol. 2009, 66, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013, 369, 341–350. [Google Scholar] [CrossRef]
- Borgegard, T.; Gustavsson, S.; Nilsson, C.; Parpal, S.; Klintenberg, R.; Berg, A.-L.; Rosqvist, S.; Serneels, L.; Svensson, S.; Olsson, F.; et al. Alzheimer’s Disease: Presenilin 2-Sparing-Secretase Inhibition Is a Tolerable A Peptide-Lowering Strategy. J. Neurosci. 2012, 32, 17297–17305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yu, M.; Neitzel, M.; Marugg, J.; Jagodzinski, J.; Lee, M.; Hu, K.; Schenk, D.; Yednock, T.; Basi, G. Identification of γ-Secretase Inhibitor Potency Determinants on Presenilin. J. Biol. Chem. 2008, 283, 2927–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serneels, L.; Van Biervliet, J.; Craessaerts, K.; Dejaegere, T.; Horre, K.; Van Houtvin, T.; Esselmann, H.; Paul, S.; Schafer, M.K.; Berezovska, O.; et al. gamma-Secretase Heterogeneity in the Aph1 Subunit: Relevance for Alzheimer’s Disease. Science 2009, 324, 639–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillman, K.W.; Starrett, J.E.; Parker, M.F.; Xie, K.; Bronson, J.J.; Marcin, L.R.; McElhone, K.E.; Bergstrom, C.P.; Mate, R.A.; Williams, R.; et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med. Chem. Lett. 2010, 1, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crump, C.J.; Castro, S.V.; Wang, F.; Pozdnyakov, N.; Ballard, T.E.; Sisodia, S.S.; Bales, K.R.; Johnson, D.S.; Li, Y.-M. BMS-708,163 Targets Presenilin and Lacks Notch-Sparing Activity. Biochemistry 2012, 51, 7209–7211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, C.R. ACS Chemical Neuroscience Molecule Spotlight on ELND006: Another γ-Secretase Inhibitor Fails in the Clinic. ACS Chem. Neurosci. 2011, 2, 279–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Global Efficacy Study of MPC-7869 to Treat Patients with Alzheimer’s. Available online: https://clinicaltrials.gov/ct2/show/NCT00322036 (accessed on 3 August 2020).
- Green, R.C.; Schneider, L.S.; Amato, D.A.; Beelen, A.P.; Wilcock, G.; Swabb, E.A.; Zavitz, K.H. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2009, 302, 2557–2564. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Open-label Treatment with MPC-7869 for Patients with Alzheimer’s Who Previously Participated in an MPC-7869 Protocol. Available online: https://clinicaltrials.gov/ct2/show/NCT00380276 (accessed on 3 August 2020).
- ClinicalTrials.gov. Evaluation of Safety & Tolerability of Multiple Dose Regimens of CHF 5074 (CT04). Available online: https://www.clinicaltrials.gov/ct2/show/NCT01303744 (accessed on 3 August 2020).
- Ereshefsky, L.; Jhee, S.; Yen, M.; Moran, S.; Pretorius, S.; Adams, J. Cerebrospinal fluid β-amyloid and dynabridging in Alzheimer’s disease drug development. Biomark. Med. 2009, 3, 711–721. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study Evaluating the Coadministration of Begacestat and Donepezil. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00959881 (accessed on 3 June 2020).
- ClinicalTrials.gov. Development of NIC5-15 in the Treatment of Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00470418 (accessed on 3 June 2020).
- ClinicalTrials.gov. A single Site, Randomized, Double-Blind, Placebo Controlled Trial of NIC5-15 in Subjects with Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT01928420 (accessed on 3 August 2020).
- Manzano, S.; Agüera, L.; Aguilar, M.; Olazarán, J. A Review on Tramiprosate (Homotaurine) in Alzheimer’s Disease and Other Neurocognitive Disorders. Front. Neurol. 2020, 11, 614. [Google Scholar] [CrossRef]
- Gervais, F.; Paquette, J.; Morissette, C.; Krzywkowski, P.; Yu, M.; Azzi, M.; Lacombe, D.; Kong, X.; Aman, A.; Laurin, J.; et al. Targeting soluble Aβ peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging 2007, 28, 537–547. [Google Scholar] [CrossRef]
- Abushakra, S.; Porsteinsson, A.; Vellas, B.; Cummings, J.; Gauthier, S.; Hey, J.A.; Power, A.; Hendrix, S.; Wang, P.; Shen, L.; et al. Clinical Benefits of Tramiprosate in Alzheimer’s Disease Are Associated with Higher Number of APOE4 Alleles: The “APOE4 Gene-Dose Effect”. J. Prev. Alzheimer’s Dis. 2016, 3, 219–228. [Google Scholar]
- Kocis, P.; Tolar, M.; Yu, J.; Sinko, W.; Ray, S.; Blennow, K.; Fillit, H.; Hey, J.A. Elucidating the Aβ42 Anti-Aggregation Mechanism of Action of Tramiprosate in Alzheimer’s Disease: Integrating Molecular Analytical Methods, Pharmacokinetic and Clinical Data. CNS Drugs 2017, 31, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Tolar, M.; Abushakra, S.; Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimer’s Dement. 2020. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, J.A.; Kierstead, M.E.; Brown, M.E.; Hawkes, C.A.; Lambermon, M.H.L.; Phinney, A.L.; Darabie, A.A.; Cousins, J.E.; French, J.E.; Lan, M.F.; et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006, 12, 801–808. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. ELND005 in Patients with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00568776 (accessed on 18 June 2020).
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Lopez Del Amo, J.M.; Fink, U.; Dasari, M.; Grelle, G.; Wanker, E.E.; Bieschke, J.; Reif, B. Structural properties of EGCG-induced, nontoxic Alzheimer’s disease Aβ oligomers. J. Mol. Biol. 2012, 421, 517–524. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Taguchi, H.; Yamamoto, A.; Shirai, N.; Hirao, K.; Tooyama, I. Curcuminoid binds to amyloid-β1-42 oligomer and fibril. J. Alzheimer’s Dis. 2011, 24, 33–42. [Google Scholar] [CrossRef]
- Liu, K.N.; Lai, C.M.; Lee, Y.T.; Wang, S.N.; Chen, R.P.Y.; Jan, J.S.; Liu, H.S.; Wang, S.S.S. Curcumin’s pre-incubation temperature affects its inhibitory potency toward amyloid fibrillation and fibril-induced cytotoxicity of lysozyme. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1774–1786. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [Green Version]
- Caesar, I.; Jonson, M.; Nilsson, K.P.R.; Thor, S.; Hammarström, P. Curcumin Promotes A-beta Fibrillation and Reduces Neurotoxicity in Transgenic Drosophila. PLoS ONE 2012, 7, e31424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 173412. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.Y.; Kwon, S.H.; Seong, Y.H.; Bae, K.; Hur, J.M.; Lee, Y.Y.; Suh, D.Y.; Song, K.S. β-secretase (BACE1)-inhibiting stilbenoids from Smilax Rhizoma. Phytomedicine 2007, 14, 403–408. [Google Scholar] [CrossRef]
- Huang, T.C.; Lu, K.T.; Wo, Y.Y.P.; Wu, Y.J.; Yang, Y.L. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE 2011, 6, e29102. [Google Scholar] [CrossRef] [Green Version]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 609–616. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 819–825. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.M.; Zheng, Y.L.; Hu, B.; Zhang, Z.F.; Shan, Q.; Zheng, Z.H.; Liu, C.M.; Wang, Y.J. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J. Pathol. 2010, 222, 199–212. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against Aβ(1-42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Pocernich, C.P.; Lange, M.L.B.; Sultana, R.; Butterfield, D.A. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 452–469. [Google Scholar] [CrossRef]
- Kim, H.; Park, B.S.; Lee, K.G.; Cheol, Y.C.; Sung, S.J.; Kim, Y.H.; Lee, S.E. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Multifunction of myricetin on Aβ: Neuroprotection via a conformational change of Aβ and reduction of Aβ via the interference of secretases. J. Neurosci. Res. 2008, 86, 368–377. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T. Oxidative Stress in Alzheimer’s Disease: Molecular Hallmarks of Underlying Vulnerability. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Springer: Singapore, 2019; pp. 91–115. [Google Scholar]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [Green Version]
- Bush, A.I. Drug development based on the metals hypothesis of Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 15, 223–240. [Google Scholar] [CrossRef] [Green Version]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- House, E.; Collingwood, J.; Khan, A.; Korchazkina, O.; Berthon, G.; Exley, C. Aluminium, iron, zinc and copper Influence the in vitro formation of amyloid fibrils of Aβ42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J. Alzheimer’s Dis. 2004, 6, 291–301. [Google Scholar] [CrossRef]
- Khan, A.; Dobson, J.P.; Exley, C. Redox cycling of iron by Aβ42. Free Radic. Biol. Med. 2006, 40, 557–569. [Google Scholar] [CrossRef]
- Adlard, P.A.; Cherny, R.A.; Finkelstein, D.I.; Gautier, E.; Robb, E.; Cortes, M.; Volitakis, I.; Liu, X.; Smith, J.P.; Perez, K.; et al. Rapid Restoration of Cognition in Alzheimer’s Transgenic Mice with 8-Hydroxy Quinoline Analogs is Associated with Decreased Interstitial Aβ. Neuron 2008, 59, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Faux, N.G.; Ritchie, C.W.; Gunn, A.; Rembach, A.; Tsatsanis, A.; Bedo, J.; Harrison, J.; Lannfelt, L.; Blennow, K.; Zetterberg, H.; et al. PBT2 rapidly improves cognition in alzheimer’s disease: Additional phase II analyses. J. Alzheimer’s Dis. 2010, 20, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [Green Version]
- Sayre, L.M.; Perry, G.; Harris, P.L.R.; Liu, Y.; Schubert, K.A.; Smith, M.A. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: A central role for bound transition metals. J. Neurochem. 2000, 74, 270–279. [Google Scholar] [CrossRef]
- Suh, S.W.; Jensen, K.B.; Jensen, M.S.; Silva, D.S.; Kesslak, P.J.; Danscher, G.; Frederickson, C.J. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000, 852, 274–278. [Google Scholar] [CrossRef]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal binding and oxidation of amyloid-β within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef]
- Smith, M.A.; Hirai, K.; Hsiao, K.; Pappolla, M.A.; Harris, P.L.R.; Siedlak, S.L.; Tabaton, M.; Perry, G. Amyloid-β Deposition in Alzheimer Transgenic Mice Is Associated with Oxidative Stress. J. Neurochem. 2002, 70, 2212–2215. [Google Scholar] [CrossRef]
- Lee, J.Y.; Mook-Jung, I.; Koh, J.Y. Histochemically reactive zinc in plaques of the Swedish mutant beta-amyloid precursor protein transgenic mice. J. Neurosci. 1999, 19, RC10. [Google Scholar] [CrossRef]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; Paradis, M.D.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef]
- Atwood, C.S.; Moir, R.D.; Huang, X.; Scarpa, R.C.; Bacarra, N.M.E.; Romano, D.M.; Hartshorn, M.A.; Tanzi, R.E.; Bush, A.I. Dramatic aggregation of alzheimer by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 1998, 273, 12817–12826. [Google Scholar] [CrossRef] [Green Version]
- Atwood, C.S.; Scarpa, R.C.; Huang, X.; Moir, R.D.; Jones, W.D.; Fairlie, D.P.; Tanzi, R.E.; Bush, A.I. Characterization of copper interactions with Alzheimer amyloid β peptides: Identification of an attomolar-affinity copper binding site on amyloid β1-42. J. Neurochem. 2000, 75, 1219–1233. [Google Scholar] [CrossRef]
- Curtain, C.C.; Ali, F.; Volitakis, I.; Cherny, R.A.; Norton, R.S.; Beyreuther, K.; Barrow, C.J.; Masters, C.L.; Bush, A.I.; Barnham, K.J. Alzheimer’s Disease Amyloid-β Binds Copper and Zinc to Generate an Allosterically Ordered Membrane-penetrating Structure Containing Superoxide Dismutase-like Subunits. J. Biol. Chem. 2001, 276, 20466–20473. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Cuajungco, M.P.; Atwood, C.S.; Hartshorn, M.A.; Tyndall, J.D.A.; Hanson, G.R.; Stokes, K.C.; Leopold, M.; Multhaup, G.; Goldstein, L.E.; et al. Cu(II) potentiation of Alzheimer aβ neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999, 274, 37111–37116. [Google Scholar] [CrossRef] [Green Version]
- Bayer, T.A.; Schäfer, S.; Simons, A.; Kemmling, A.; Kamer, T.; Tepest, R.; Eckert, A.; Schüssel, K.; Eikenberg, O.; Sturchler-Pierrat, C.; et al. Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Aβ production in APP23 transgenic mice. Proc. Natl. Acad. Sci. USA 2003, 100, 14187–14192. [Google Scholar] [CrossRef] [Green Version]
- Phinney, A.L.; Drisaldi, B.; Schmidt, S.D.; Lugowski, S.; Coronado, V.; Liang, Y.; Horne, P.; Yang, J.; Sekoulidis, J.; Coomaraswamy, J.; et al. In vivo reduction of amyloid-β by a mutant copper transporter. Proc. Natl. Acad. Sci. USA 2003, 100, 14193–14198. [Google Scholar] [CrossRef] [Green Version]
- Maynard, C.J.; Cappai, R.; Volitakis, I.; Cherny, R.A.; White, A.R.; Beyreuther, K.; Masters, C.L.; Bush, A.I.; Li, Q.X. Overexpression of Alzheimer’s disease amyloid-β opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002, 277, 44670–44676. [Google Scholar] [CrossRef] [Green Version]
- Borchardt, T.; Camakaris, J.; Cappai, R.; Masters, C.L.; Beyreuther, K.; Multhaup, G. Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor-protein secretion. Biochem. J. 1999, 344 Pt2, 461–467. [Google Scholar] [CrossRef]
- Bard, F.; Cannon, C.; Barbour, R.; Burke, R.-L.; Games, D.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000, 6, 916–919. [Google Scholar] [CrossRef] [Green Version]
- Vasilevko, V.; Xu, F.; Previti, M.L.; Van Nostrand, W.E.; Cribbs, D.H. Experimental Investigation of Antibody-Mediated Clearance Mechanisms of Amyloid- in CNS of Tg-SwDI Transgenic Mice. J. Neurosci. 2007, 27, 13376–13383. [Google Scholar] [CrossRef]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K.; et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999, 400, 173–177. [Google Scholar] [CrossRef]
- Weiner, H.L.; Lemere, C.A.; Maron, R.; Spooner, E.T.; Grenfell, T.J.; Mori, C.; Issazadeh, S.; Hancock, W.W.; Selkoe, D.J. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann. Neurol. 2000, 48, 567–579. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mathew, B.; Das, P.K.; Perveen, A.; Ashraf, G.M. Emerging Promise of Immunotherapy for Alzheimer’s Disease: A New Hope for the Development of Alzheimer’s Vaccine. Curr. Top. Med. Chem. 2020, 20, 1214–1234. [Google Scholar] [CrossRef]
- Lambracht-Washington, D.; Rosenberg, R.N. Advances in the development of vaccines for alzheimer’s disease. Discov. Med. 2013, 15, 319–326. [Google Scholar]
- Lemere, C.A.; Masliah, E. Can Alzheimer disease be prevented by amyloid-Β immunotherapy? Nat. Rev. Neurol. 2010, 6, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Wiessner, C.; Wiederhold, K.H.; Tissot, A.C.; Frey, P.; Danner, S.; Jacobson, L.H.; Jennings, G.T.; Lüönd, R.; Ortmann, R.; Reichwald, J.; et al. The second-generation active Aβ immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J. Neurosci. 2011, 31, 9323–9331. [Google Scholar] [CrossRef]
- Winblad, B.; Andreasen, N.; Minthon, L.; Floesser, A.; Imbert, G.; Dumortier, T.; Maguire, R.P.; Blennow, K.; Lundmark, J.; Staufenbiel, M.; et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: Randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012, 11, 597–604. [Google Scholar] [CrossRef]
- Winblad, B.; Minthon, L.; Floesser, A. Results of the first-in-man study with the active Aβimmunotherapy CAD106 in Alzheimer patients. Alzheimer’s Dement. 2009, 5, P113–P114. [Google Scholar] [CrossRef]
- Schneeberger, A.; Mandler, M.; Otawa, O.; Zauner, W.; Mattner, F.; Schmidt, W. Development of AFFITOPE vaccines for Alzheimer’s disease (AD)—From concept to clinical testing. J. Nutr. Health Aging 2009, 13, 264–267. [Google Scholar] [CrossRef]
- Khandelwal, P.J.; Herman, A.M.; Moussa, C.E.-H. Inflammation in the early stages of neurodegenerative pathology. J. Neuroimmunol. 2011, 238, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Winblad, B.; Graf, A.; Riviere, M.-E.; Andreasen, N.; Ryan, J. Active immunotherapy options for Alzheimer’s disease. Alzheimers. Res. Ther. 2014, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Schneeberger, A.; Hendrix, S.; Mandler, M.; Ellison, N.; Bürger, V.; Brunner, M.; Frölich, L.; Mimica, N.; Hort, J.; Rainer, M.; et al. Results from a Phase II Study to Assess the Clinical and Immunological Activity of AFFITOPE® AD02 in Patients with Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2015, 2, 103–114. [Google Scholar]
- Alzforum. Vanutide Cridificar. Available online: https://www.alzforum.org/therapeutics/vanutide-cridificar (accessed on 3 August 2020).
- ClinicalTrials.gov. Long Term Extension Study Evaluating Safety, Tolerability and Immunogenicity of ACC-001 in Japanese Subjects with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT01238991 (accessed on 18 June 2020).
- ClinicalTrials.gov. Study Evaluating Safety, Tolerability, and Immunogenicity of ACC-001 in Subjects with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00479557 (accessed on 18 June 2020).
- ClinicalTrials.gov. Study Evaluating ACC-001 in Subjects with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00498602 (accessed on 18 June 2020).
- Wilcock, D.M.; Gharkholonarehe, N.; Van Nostrand, W.E.; Davis, J.; Vitek, M.P.; Colton, C.A. Amyloid Reduction by Amyloid- Vaccination Also Reduces Mouse Tau Pathology and Protects from Neuron Loss in Two Mouse Models of Alzheimer’s Disease. J. Neurosci. 2009, 29, 7957–7965. [Google Scholar] [CrossRef]
- Oddo, S.; Billings, L.; Kesslak, J.P.; Cribbs, D.H.; LaFerla, F.M. Aβ Immunotherapy Leads to Clearance of Early, but Not Late, Hyperphosphorylated Tau Aggregates via the Proteasome. Neuron 2004, 43, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Klyubin, I.; Walsh, D.M.; Lemere, C.A.; Cullen, W.K.; Shankar, G.M.; Betts, V.; Spooner, E.T.; Jiang, L.; Anwyl, R.; Selkoe, D.J.; et al. Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nat. Med. 2005, 11, 556–561. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two phase 3 trials of Bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef] [Green Version]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study Evaluating the Safety, Tolerability and Efficacy of PF-04360365 in Adults with Probable Cerebral Amyloid Angiopathy. Available online: https://clinicaltrials.gov/ct2/show/NCT01821118 (accessed on 3 August 2020).
- Samadi, H.; Sultzer, D. Solanezumab for Alzheimer’s disease. Expert Opin. Biol. Ther. 2011, 11, 787–798. [Google Scholar] [CrossRef]
- Bohrmann, B.; Baumann, K.; Benz, J.; Gerber, F.; Huber, W.; Knoflach, F.; Messer, J.; Oroszlan, K.; Rauchenberger, R.; Richter, W.F.; et al. Gantenerumab: A novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J. Alzheimer’s Dis. 2012, 28, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Ostrowitzki, S.; Deptula, D.; Thurfjell, L.; Barkhof, F.; Bohrmann, B.; Brooks, D.J.; Klunk, W.E.; Ashford, E.; Yoo, K.; Xu, Z.X.; et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch. Neurol. 2012, 69, 198–207. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia. A Study of Potential Disease Modifying Treatments in Individuals at Risk for or with a Type of Early Onset Alzheimer’s Disease Caused by a Genetic Mutation. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01760005 (accessed on 3 August 2020).
- Washington University School of Medicine. The Dominantly Inherited Alzheimer Network: Clinical Trial. Available online: https://dian.wustl.edu/our-research/clinical-trial/ (accessed on 3 August 2020).
- Alzheimer’s News Today. Solanezumab. Available online: https://alzheimersnewstoday.com/solanezumab/ (accessed on 3 August 2020).
- Adolfsson, O.; Pihlgren, M.; Toni, N.; Varisco, Y.; Buccarello, A.L.; Antoniello, K.; Lohmann, S.; Piorkowska, K.; Gafner, V.; Atwal, J.K.; et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 2012, 32, 9677–9689. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.B.; Brown, T.P.; Wallace, K.; Bales, K.R. Chronic administration of an aglycosylated murine antibody of ponezumab does not worsen microhemorrhages in Aged Tg2576 mice. Curr. Alzheimer Res. 2012, 9, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- La Porte, S.L.; Bollini, S.S.; Lanz, T.A.; Abdiche, Y.N.; Rusnak, A.S.; Ho, W.H.; Kobayashi, D.; Harrabi, O.; Pappas, D.; Mina, E.W.; et al. Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer’s disease. J. Mol. Biol. 2012, 421, 525–536. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Multiple Dose Study of Aducanumab (BIIB037) (Recombinant, Fully Human Anti-Aβ IgG1 mAb) in Participants with Prodromal or Mild Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT01677572 (accessed on 3 August 2020).
- ClinicalTrials.gov. 221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT02477800 (accessed on 3 August 2020).
- ClinicalTrials.gov. 221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02484547 (accessed on 3 August 2020).
- Biogen. About Aducanumab. Available online: https://biogenalzheimers.com/about-aducanumab/ (accessed on 3 August 2020).
- Biogen. Biogen Completes Submission of Biologics License Application to FDA for Aducanumab as a Treatment for Alzheimer’s Disease. Available online: https://investors.biogen.com/news-releases/news-release-details/biogen-completes-submission-biologics-license-application-fda (accessed on 3 August 2020).
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and tolerability of BAN2401—A clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimers. Res. Ther. 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, S.; Möller, C.; Tegerstedt, K.; Lord, A.; Laudon, H.; Sjödahl, J.; Söderberg, L.; Spens, E.; Sahlin, C.; Waara, E.R.; et al. The murine Version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe Mice. J. Alzheimer’s Dis. 2015, 43, 575–588. [Google Scholar] [CrossRef]
- Söllvander, S.; Nikitidou, E.; Gallasch, L.; Zyśk, M.; Söderberg, L.; Sehlin, D.; Lannfelt, L.; Erlandsson, A. The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death. J. Neuroinflammation 2018, 15, 98. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study to Confirm Safety and Efficacy of BAN2401 in Participants with Early Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT03887455 (accessed on 3 August 2020).
- Tanzi, R.E.; Moir, R.D.; Wagner, S.L. Clearance of Alzheimer’s Aβ Peptide. Neuron 2004, 43, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Deane, R.; Wu, Z.; Zlokovic, B. V RAGE (Yin) Versus LRP (Yang) Balance Regulates Alzheimer Amyloid -Peptide Clearance through Transport across the Blood-Brain Barrier. Stroke 2004, 35, 2628–2631. [Google Scholar] [CrossRef] [Green Version]
- Zlokovic, B.V. New therapeutic targets in the neurovascular pathway in Alzheimer’s disease. Neurotherapeutics 2008, 5, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Deane, R.; Zlokovic, B. V Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 191–197. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Zlokovic, B. V The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer’s disease. Curr. Pharm. Des. 2008, 14, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.A.; Kulstad, J.J.; Savard, C.E.; Green, P.S.; Lee, S.P.; Craft, S.; Watson, G.S.; Cook, D.G. Peripheral Amyloid-β Levels Regulate Amyloid-β Clearance from the Central Nervous System. J. Alzheimer’s Dis. 2009, 16, 325–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagare, A.; Deane, R.; Bell, R.D.; Johnson, B.; Hamm, K.; Pendu, R.; Marky, A.; Lenting, P.J.; Wu, Z.; Zarcone, T.; et al. Clearance of amyloid-β by circulating lipoprotein receptors. Nat. Med. 2007, 13, 1029–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaney, M.O.; Stine, W.B.; Kokjohn, T.A.; Kuo, Y.-M.; Esh, C.; Rahman, A.; Luehrs, D.C.; Schmidt, A.M.; Stern, D.; Du Yan, S.; et al. RAGE and amyloid beta interactions: Atomic force microscopy and molecular modeling. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1741, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Yan, S.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.M.; Du Yan, S.; Yan, S.F.; Schmidt, A.M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res. Rev. 2002, 1, 1–15. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Cerebrovascular transport of Alzheimer’s amyloid beta and apolipoproteins J and E: Possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996, 59, 1483–1497. [Google Scholar] [CrossRef]
- Wang, W.; Bodles-Brakhop, A.M.; Barger, S.W. A role for P-glycoprotein in clearance of Alzheimer amyloid β-peptide from the brain. Curr. Alzheimer Res. 2016, 13, 615–620. [Google Scholar] [CrossRef]
- Park, J.H.; Strittmatter, S.M. Nogo receptor interacts with brain APP and Abeta to reduce pathologic changes in Alzheimer’s transgenic mice. Curr. Alzheimer Res. 2007, 4, 568–570. [Google Scholar] [CrossRef]
- Tang, B.L.; Liou, Y.C. Novel modulators of amyloid-β precursor protein processing. J. Neurochem. 2007, 100, 314–323. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kanekiyo, T. Blood-brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef] [Green Version]
- Mao, P.; Manczak, M.; Calkins, M.J.; Truong, Q.; Reddy, T.P.; Reddy, A.P.; Shirendeb, U.; Lo, H.-H.; Rabinovitch, P.S.; Reddy, P.H. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid production and BACE1 in a mouse model of Alzheimer’s disease: Implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 2012, 21, 2973–2990. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Al Mamun, A.; Barreto, G.E.; Rashid, M.; Perveen, A.; Ashraf, G.M. Pharmacological approaches to mitigate neuroinflammation in Alzheimer’s disease. Int. Immunopharmacol. 2020, 84, 106479. [Google Scholar] [CrossRef]
- Carret-Rebillat, A.-S.; Pace, C.; Gourmaud, S.; Ravasi, L.; Montagne-Stora, S.; Longueville, S.; Tible, M.; Sudol, E.; Chang, R.C.-C.; Paquet, C.; et al. Neuroinflammation and Aβ accumulation linked to systemic inflammation are decreased by genetic PKR down-regulation. Sci. Rep. 2015, 5, 8489. [Google Scholar] [CrossRef]
- Cai, H.-Y.; Yang, J.-T.; Wang, Z.-J.; Zhang, J.; Yang, W.; Wu, M.-N.; Qi, J.-S. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2018, 495, 1034–1040. [Google Scholar] [CrossRef]
- Mullane, K.; Williams, M. Alzheimer’s disease (AD) therapeutics – 1: Repeated clinical failures continue to question the amyloid hypothesis of AD and the current understanding of AD causality. Biochem. Pharmacol. 2018, 158, 359–375. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Coric, V.; Salloway, S.; Van Dyck, C.H.; Dubois, B.; Andreasen, N.; Brody, M.; Curtis, C.; Soininen, H.; Thein, S.; Shiovitz, T.; et al. Targeting prodromal Alzheimer disease with avagacestat: A randomized clinical trial. J. Am. Med Assoc. Neurol. 2015, 72, 1324–1333. [Google Scholar] [CrossRef]
- Timmers, M.; Streffer, J.R.; Russu, A.; Tominaga, Y.; Shimizu, H.; Shiraishi, A.; Tatikola, K.; Smekens, P.; Börjesson-Hanson, A.; Andreasen, N.; et al. Pharmacodynamics of atabecestat (JNJ-54861911), an oral BACE1 inhibitor in patients with early Alzheimer’s disease: Randomized, double-blind, placebo-controlled study. Alzheimer’s Res. Ther. 2018, 10, 85. [Google Scholar] [CrossRef]
- FierceBiotech. Janssen Drops the BACE as Alzheimer’s Candidate Joins Fail List. Available online: https://www.fiercebiotech.com/biotech/janssen-drops-bace-as-alzheimer-s-candidate-joins-fail-list (accessed on 3 August 2020).
- Janssen. Update on Janssen’s BACE Inhibitor Program Regarding the Dominantly Inherited Alzheimer’s Network Trial (DIAN-TU). Available online: https://www.janssen.com/neuroscience/update-janssens-bace-inhibitor-program-regarding-DIAN-TU (accessed on 3 August 2020).
- ClinicalTrials.gov. A Study of Lanabecestat (LY3314814) in Early Alzheimer’s Disease Dementia. Available online: https://clinicaltrials.gov/ct2/show/NCT02972658 (accessed on 3 August 2020).
- Cebers, G.; Alexander, R.C.; Haeberlein, S.B.; Han, D.; Goldwater, R.; Ereshefsky, L.; Olsson, T.; Ye, N.; Rosen, L.; Russell, M.; et al. AZD3293: Pharmacokinetic and Pharmacodynamic Effects in Healthy Subjects and Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 55, 1039–1053. [Google Scholar] [CrossRef]
- Eketjäll, S.; Janson, J.; Kaspersson, K.; Bogstedt, A.; Jeppsson, F.; Fälting, J.; Haeberlein, S.B.; Kugler, A.R.; Alexander, R.C.; Cebers, G. AZD3293: A Novel, Orally Active BACE1 Inhibitor with High Potency and Permeability and Markedly Slow Off-Rate Kinetics. J. Alzheimer’s Dis. 2016, 50, 1109–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, M.F.; Kost, J.; Tariot, P.N.; Aisen, P.S.; Cummings, J.L.; Vellas, B.; Sur, C.; Mukai, Y.; Voss, T.; Furtek, C.; et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2018, 378, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Resolving controversies on the path to Alzheimer’s therapeutics. Nat. Med. 2011, 17, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N. Clinical Effects of Oral Tramiprosate in APOE4/4 Homozygous Patients with Mild Alzheimer’s Disease Suggest Disease Modification. J. Prev. Alzheimer’s Dis. 2017, 4, 136–137. [Google Scholar]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P.; et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Cohen, S.; Van Dyck, C.H.; Brody, M.; Curtis, C.; Cho, W.; Ward, M.; Friesenhahn, M.; Brunstein, F.; Quartino, A.; et al. A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 2018, 90, E1889–E1897. [Google Scholar] [CrossRef] [Green Version]
- Salloway, S.P.; Sperling, R.; Fox, N.C.; Sabbagh, M.N.; Honig, L.S.; Porsteinsson, A.P.; Rofael, H.; Ketter, N.; Wang, D.; Liu, E.; et al. Long-term follow up of patients with mild-to-moderate Alzheimer’s disease treated with bapineuzumab in a Phase III, open-label, extension study. J. Alzheimer’s Dis. 2018, 64, 689–707. [Google Scholar] [CrossRef]
- Ketter, N.; Brashear, H.R.; Bogert, J.; Di, J.; Miaux, Y.; Gass, A.; Purcell, D.D.; Barkhof, F.; Arrighi, H.M. Central Review of Amyloid-Related Imaging Abnormalities in Two Phase III Clinical Trials of Bapineuzumab in Mild-To-Moderate Alzheimer’s Disease Patients. J. Alzheimers. Dis. 2017, 57, 557–573. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. A Study to Evaluate Safety and Tolerability of Aducanumab in Participants with Alzheimer’s Disease Who Had Previously Participated in the Aducanumab Studies 221AD103, 221AD301, 221AD302 and 221AD205. Available online: https://clinicaltrials.gov/ct2/show/NCT04241068 (accessed on 30 July 2020).
- ClinicalTrials.gov. A Study of CAD106 and CNP520 Versus Placebo in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer’s disease. Available online: https://clinicaltrials.gov/ct2/show/NCT02565511 (accessed on 3 August 2020).
- ClinicalTrials.gov. Safety and Efficacy Study of Gantenerumab in Participants with Early Alzheimer’s Disease (AD). Available online: https://clinicaltrials.gov/ct2/show/NCT03443973 (accessed on 3 August 2020).
- ClinicalTrials.gov. Efficacy and Safety Study of Gantenerumab in Participants with Early Alzheimer’s Disease (AD). Available online: https://clinicaltrials.gov/ct2/show/NCT03444870 (accessed on 3 August 2020).
- ClinicalTrials.gov. Clinical Trial of Solanezumab for Older Individuals Who May Be at Risk for Memory Loss. Available online: https://clinicaltrials.gov/ct2/show/NCT02008357 (accessed on 3 August 2020).
- ClinicalTrials.gov. An Efficacy and Safety Study of Sodium Oligo-Mannurarate (GV-971) Capsule for the Treatment of Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT02293915 (accessed on 3 August 2020).
- ClinicalTrials.gov. A Study of Gantenerumab in Participants with Mild Alzheimer Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT02051608 (accessed on 3 August 2020).
- ClinicalTrials.gov. A Study to Evaluate Albumin and Immunoglobulin in Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT01561053 (accessed on 3 August 2020).
- Doig, A.J.; Del Castillo-Frias, M.P.; Berthoumieu, O.; Tarus, B.; Nasica-Labouze, J.; Sterpone, F.; Nguyen, P.H.; Hooper, N.M.; Faller, P.; Derreumaux, P. Why Is Research on Amyloid-β Failing to Give New Drugs for Alzheimer’s Disease? ACS Chem. Neurosci. 2017, 8, 1435–1437. [Google Scholar] [CrossRef] [Green Version]
- Selkoe, D.J. Alzheimer disease and aducanumab: Adjusting our approach. Nat. Rev. Neurol. 2019, 15, 365–366. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Mielke, J.G.; Wang, Y.T. Insulin, synaptic function, and opportunities for neuroprotection. In Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2011; Volume 98, pp. 133–186. [Google Scholar]
- Chiu, S.L.; Chen, C.M.; Cline, H.T. Insulin Receptor Signaling Regulates Synapse Number, Dendritic Plasticity, and Circuit Function In Vivo. Neuron 2008, 58, 708–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, F.G.; Ferreira, S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer Disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarchoan, M.; Arnold, S.E. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes 2014, 63, 2253–2261. [Google Scholar] [CrossRef] [Green Version]
- Bruehl, H.; Sweat, V.; Hassenstab, J.; Polyakov, V.; Convit, A. Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. J. Clin. Exp. Neuropsychol. 2010, 32, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Benedict, C.; Grillo, C.A. Insulin resistance as a therapeutic target in the treatment of Alzheimer’s disease: A state-of-the-art review. Front. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Batista, A.F.; Forny-Germano, L.; Clarke, J.R.; Lyra e Silva, N.M.; Brito-Moreira, J.; Boehnke, S.E.; Winterborn, A.; Coe, B.C.; Lablans, A.; Vital, J.F.; et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J. Pathol. 2018, 245, 85–100. [Google Scholar] [CrossRef]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimer’s Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A. Is “friendly fire” in the brain provoking Alzheimer’s disease? Nature 2018, 556, 426–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.L.; Yao, X.Q.; Jiao, S.S.; Zeng, F.; Liu, Y.H.; Xiang, Y.; Liang, C.R.; Wang, Q.H.; Wang, X.; Cao, H.Y.; et al. A study on the association between infectious burden and Alzheimer’s disease. Eur. J. Neurol. 2015, 22, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Fani, L.; Wolters, F.J.; Ikram, M.K.; Bruno, M.J.; Hofman, A.; Koudstaal, P.J.; Darwish Murad, S.; Ikram, M.A. Helicobacter pylori and the risk of dementia: A population-based study. Alzheimer’s Dement. 2018, 14, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.E.; Abud, E.M.; Lakatos, A.; Karimzadeh, A.; Yeung, S.T.; Davtyan, H.; Fote, G.M.; Lau, L.; Weinger, J.G.; Lane, T.E.; et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. USA 2016, 113, E1316–E1325. [Google Scholar] [CrossRef] [Green Version]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
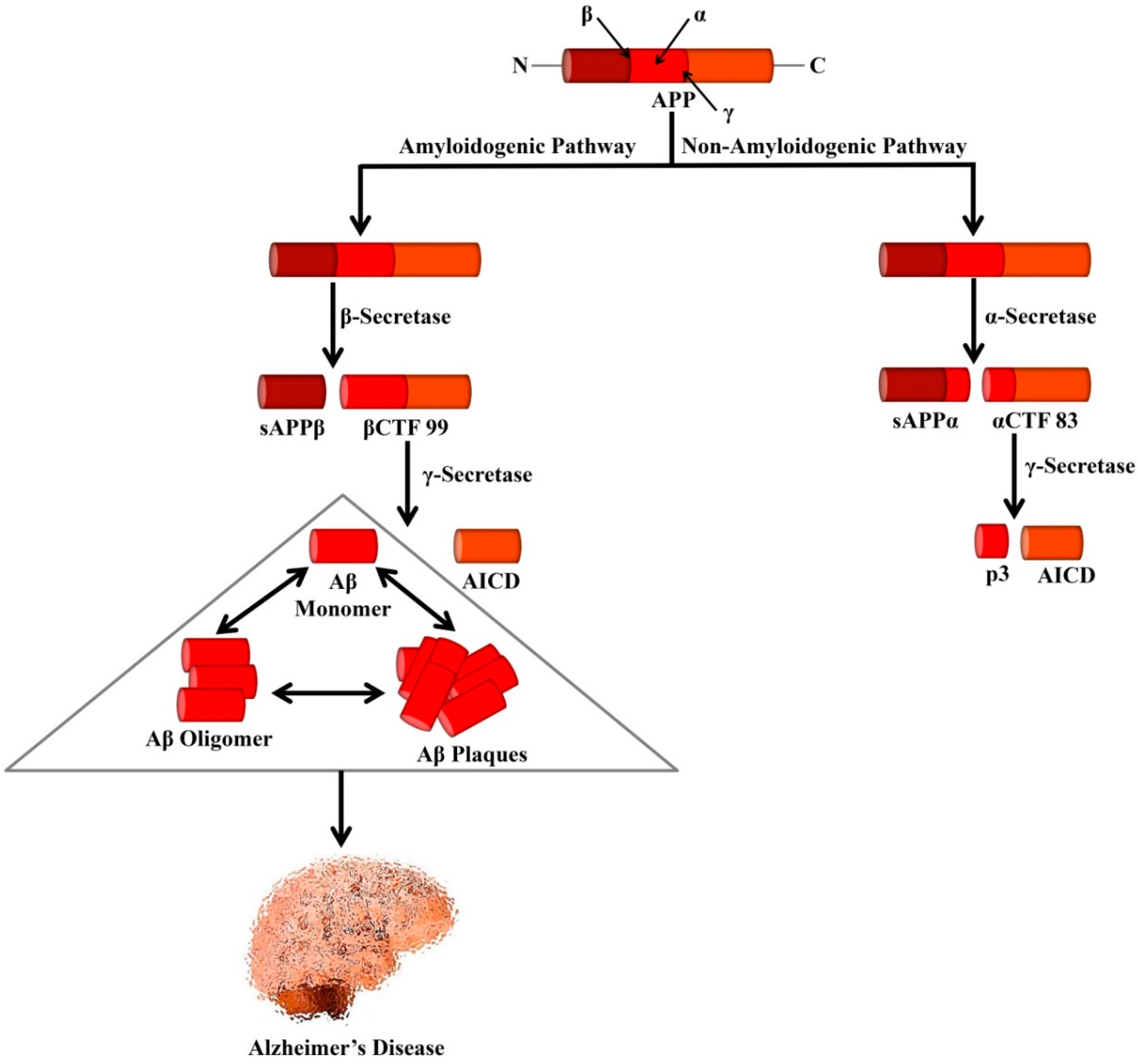
| Therapeutic Agent | Drug Class | Disease State of Participants | Clinical Trial Design | Reason of Failure | References |
|---|---|---|---|---|---|
| Avagacestat | γ-secretase inhibitor | Prodromal AD | Randomized, placebo-controlled phase II | No clinical efficacy; Adverse effects: Glycosuria, Weight loss | [285] |
| Atabecestat | BACE1 inhibitor | Early AD | Randomized, phase II/III | Adverse effects: Elevation of liver enzymes | [286,287,288] |
| Lanabecestat | BACE1 inhibitor | Mild-to-moderate AD | Randomized, phase III | Not likely to meet primary endpoint; terminated for futility | [289,290,291] |
| Verubecestat | BACE1 inhibitor | Mild-to-moderate AD | Randomized, placebo-controlled, double-blind phase III | No clinical efficacy; Adverse effects: rash, alterations in hair color, more chances of falls and injuries, weight loss, sleep disturbance, suicidal thoughts | [142,292] |
| Semagacestat | γ-secretase inhibitor | Probable AD | Double-blind, placebo-controlled phase III | No clinical efficacy, exacerbates cognitive functions at higher doses, increased incidence of skin cancer and infections | [142,155] |
| Tramiprosate | Aβ aggregation inhibitor | Mild-to-moderate AD | Phase III | No clinical efficacy | [173,293,294] |
| Gantenerumab | IgG1 humanized anti-Aβ mAbs | Prodromal AD | Randomized, placebo-controlled, double-blind phase III | Terminated owing to futility, no important differences were seen in primary and secondary endpoint | [295] |
| Crenezumab | IgG1 humanized anti-Aβ mAbs | Mild-to-moderate AD | Randomized, phase II | No clinical efficacy, Did not achieve primary and secondary endpoints. | [296] |
| Bapineuzumab | IgG1 humanized anti-Aβ mAbs | Mild-to-moderate AD | Placebo-controlled, double-blind phase III | No clinical efficacy | [297,298] |
| Therapeutic Agent | Drug Class | Number of Participants | Disease State of Participants | Study Design | Clinical Trial Phase | Current Status | References |
|---|---|---|---|---|---|---|---|
| Aducanumab | IgG1 humanized anti-Aβ mAbs | 2400 | AD patients who had participated in previous studies 221AD103, 221AD301, 221AD302 and 221AD205 | Open-label, multicenter trial | Phase III | Continuing | [299] |
| Gantenerumab and solanezumab | Monoclonal antibody | 149 | Early-onset AD caused by a genetic mutation | Randomized, placebo-controlled, double-blind trial | Phase II/III | Completed | [249] |
| BAN2401 | Monoclonal antibody | 1566 | Early AD | Placebo-controlled, double-blind, parallel-group study | Phase III | Continuing | [263] |
| CAD106 CNP520 | BACE inhibitor | 480 | Asymptomatic AD but carriers of homozygous APOE*ε4 | Randomized, placebo-controlled, double-blind trial | Phase II/III | Continuing | [300] |
| Gantenerumab | Monoclonal antibody | 1016 | Early-onset AD | Multicenter, randomized, placebo-controlled, double-blind trial | Phase III | Continuing | [301,302] |
| Solanezumab | Monoclonal antibody | 1150 | Asymptomatic AD | Randomized trial | Phase III | Continuing | [303] |
| Sodium oligomannurarate (GV-971) | Aβ aggregation inhibitor | 818 | Mild to moderate AD | Randomized trial | Phase III | Completed | [304] |
| Gantenerumab | Monoclonal antibody | 389 | Mild AD | Randomized, placebo-controlled, double-blind trial | Phase III | Continuing | [305] |
| Albumin and Immunoglobulin | Polyclonal antibodies | 347 | Mild to moderate AD | Multicenter, randomized, controlled trial | Phase II/III | Completed | [306] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, M.S.; Kabir, M.T.; Rahman, M.S.; Behl, T.; Jeandet, P.; Ashraf, G.M.; Najda, A.; Bin-Jumah, M.N.; El-Seedi, H.R.; Abdel-Daim, M.M. Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 5858. https://doi.org/10.3390/ijms21165858
Uddin MS, Kabir MT, Rahman MS, Behl T, Jeandet P, Ashraf GM, Najda A, Bin-Jumah MN, El-Seedi HR, Abdel-Daim MM. Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease. International Journal of Molecular Sciences. 2020; 21(16):5858. https://doi.org/10.3390/ijms21165858
Chicago/Turabian StyleUddin, Md. Sahab, Md. Tanvir Kabir, Md. Sohanur Rahman, Tapan Behl, Philippe Jeandet, Ghulam Md Ashraf, Agnieszka Najda, May N. Bin-Jumah, Hesham R. El-Seedi, and Mohamed M. Abdel-Daim. 2020. "Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease" International Journal of Molecular Sciences 21, no. 16: 5858. https://doi.org/10.3390/ijms21165858
APA StyleUddin, M. S., Kabir, M. T., Rahman, M. S., Behl, T., Jeandet, P., Ashraf, G. M., Najda, A., Bin-Jumah, M. N., El-Seedi, H. R., & Abdel-Daim, M. M. (2020). Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease. International Journal of Molecular Sciences, 21(16), 5858. https://doi.org/10.3390/ijms21165858












