Genistein Induces Adipogenic and Autophagic Effects in Rainbow Trout (Oncorhynchus mykiss) Adipose Tissue: In Vitro and In Vivo Models
Abstract
:1. Introduction
2. Results
2.1. In Vitro Evaluation of GE Effects on Primary Cultured Adipocytes
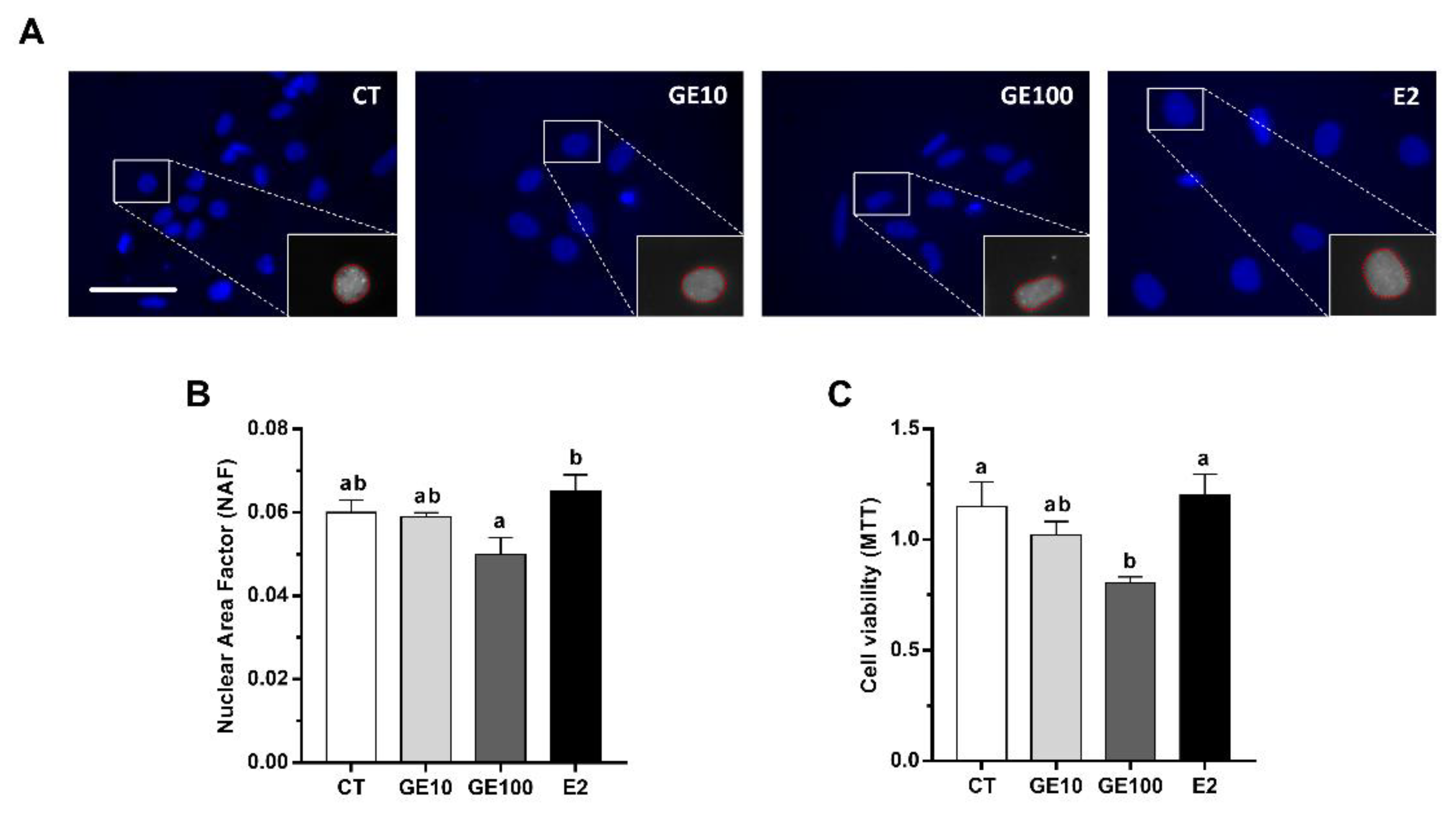
2.1.1. Adipocyte Cells Viability, Nuclear Morphology, and LC3-II Protein Expression
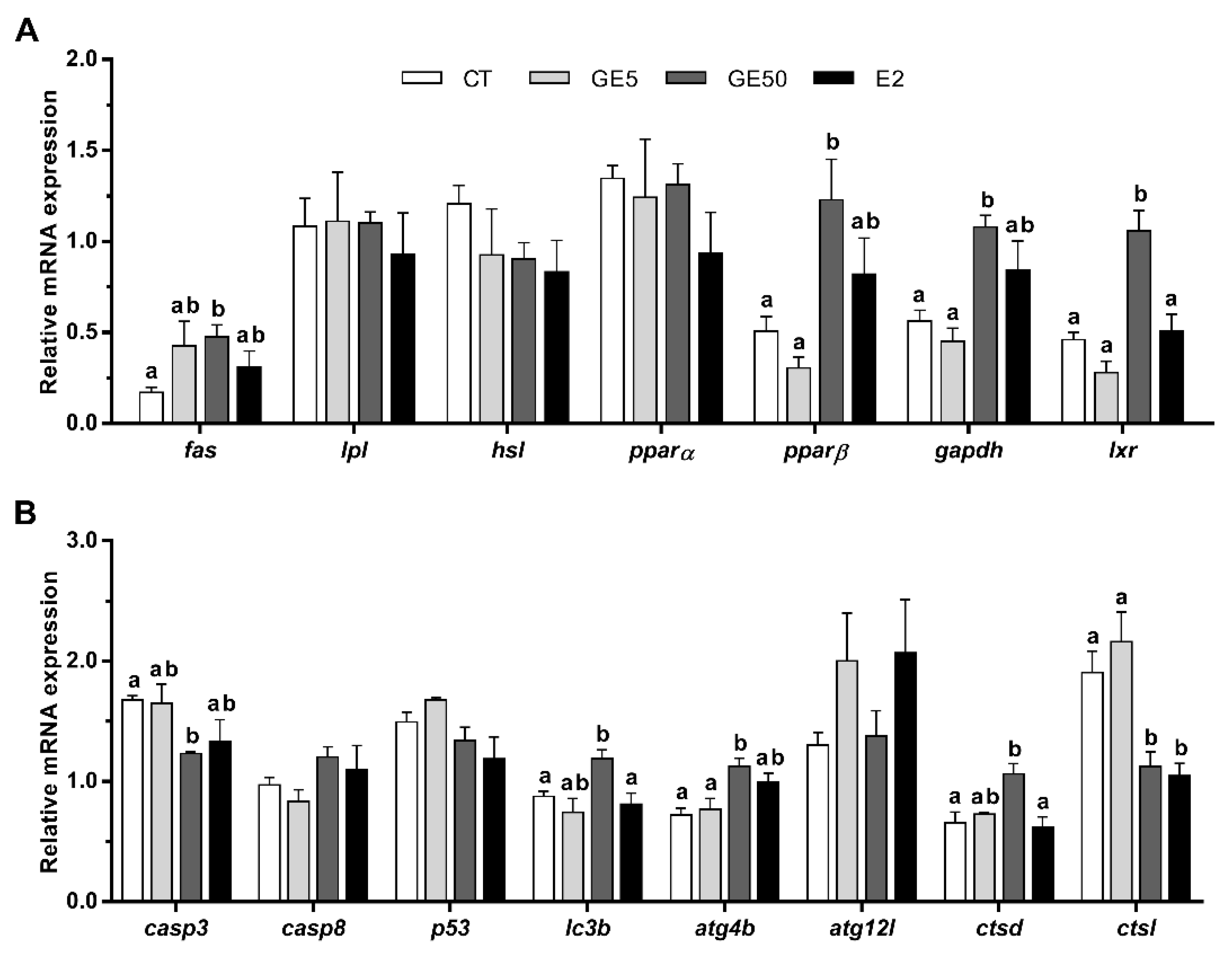
2.1.2. Adipocyte Gene Expression Related to Apoptosis and Autophagy
2.1.3. Adipocyte Differentiation, Lipid Accumulation, and Released Metabolites
2.2. In Vivo Evaluation of GE Effects after an Intraperitoneal Injection
2.2.1. Plasma Metabolites and Hepatic Gene Expression of a Biomarker of Estrogen’s Exposure
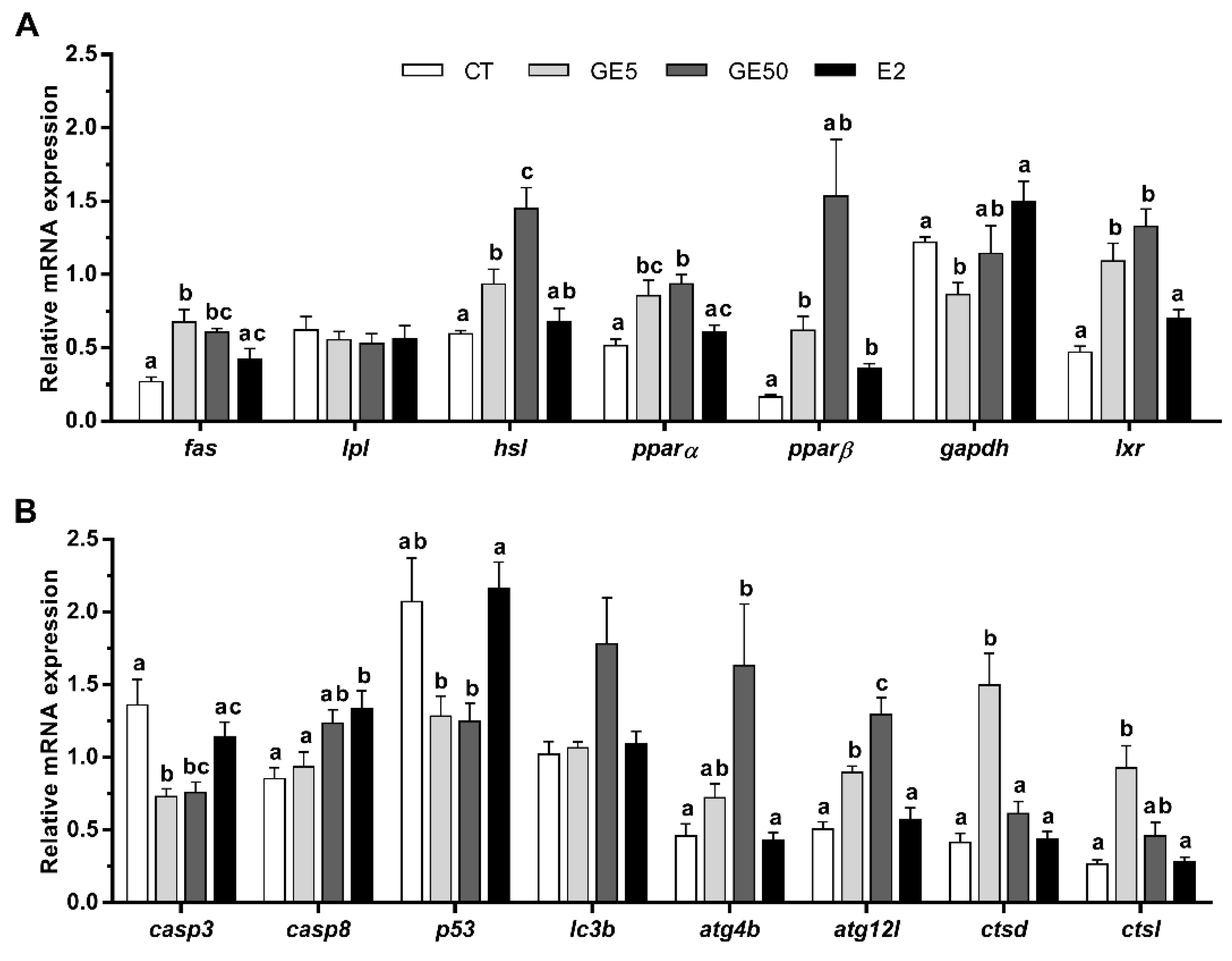
2.2.2. Gene Expression Related to Lipid Metabolism, Apoptosis, and Autophagy in Adipose Tissue
2.2.3. Gene Expression Related to Lipid Metabolism, Apoptosis, and Autophagy in Liver and White Muscle
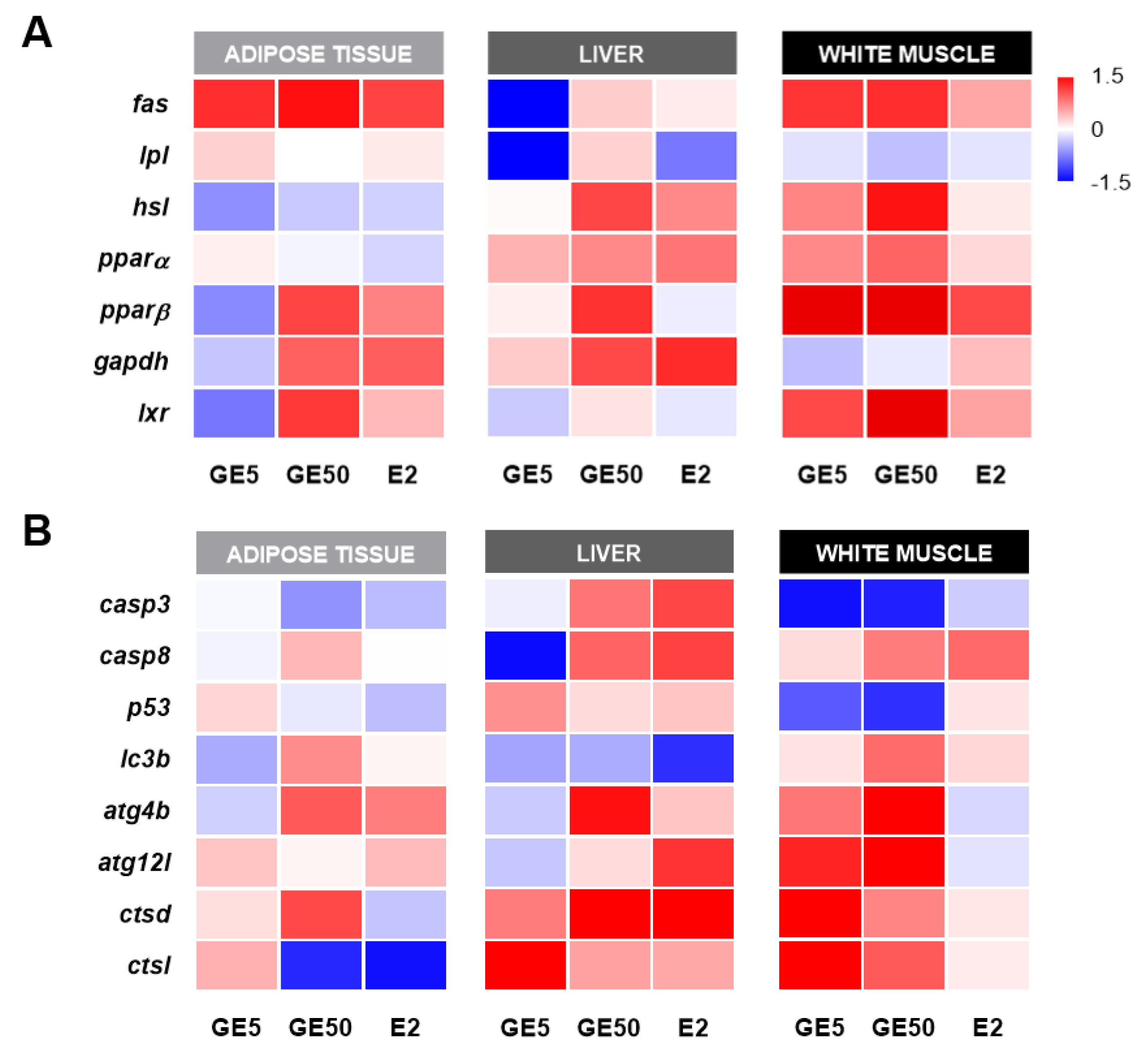
2.2.4. Comparative and Qualitative Gene Expression Analysis among Tissues
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Primary Culture of Preadipocytes and Experimental Treatments
4.3. Cell Viability Assay
4.4. Nuclear Morphology Analysis
4.5. Western Blot
4.6. Oil Red O Staining
4.7. Intraperitoneal Injection
4.8. Biochemical Analysis of Culture Media and Plasma Metabolites
4.9. Gene Expression Analyses
4.9.1. RNA Extraction and cDNA Synthesis
4.9.2. Real-Time Quantitative PCR (qPCR)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| atg | Autophagy-related gene |
| casp | Caspase |
| CT | Control |
| CMA | Chaperone-mediated autophagy |
| cts | Cathepsin |
| DMSO | Dimethyl sulfoxide |
| ef1α | Elongation factor 1 alpha |
| E2 | 17β-Estradiol |
| fas | Fatty acid synthase |
| FO | Fish oil |
| hsl | Hormone-sensitive lipase |
| gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| GE | Genistein |
| LC3b | Microtubule-associated protein-1 light chain 3b |
| lpl | Lipoprotein lipase |
| lxr | Liver x receptor |
| MTT | Methylthiazolyldiphenyl-tetrazolium bromide |
| NAF | Nuclear area factor |
| NEFA | Non-esterified fatty acids |
| ORO | Oil Red O staining |
| ppar | Peroxisome proliferator-activated receptor |
| p53 | Tumor protein p53 |
| TAG | Triglycerides |
| ubq | Ubiquitin |
| VO | Vegetable oil |
| vtg | Vitellogenin |
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture; General Fisheries Commission for the Mediterranean: Rome, Italy, 2020. [Google Scholar]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Betancor, M.B.; Sprague, M.; Montero, D.; Usher, S.; Sayanova, O.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Izquierdo, M.; Tocher, D.R. Replacement of marine fish oil with de novo omega-3 oils from transgenic camelina sativa in feeds for gilthead sea bream (Sparus aurata L.). Lipids 2016, 51, 1171–1191. [Google Scholar] [CrossRef] [PubMed]
- Calduch-Giner, J.A.; Sitjà-Bobadilla, A.; Davey, G.C.; Cairns, M.T.; Kaushik, S.; Pérez-Sánchez, J. Dietary vegetable oils do not alter the intestine transcriptome of gilthead sea bream (Sparus aurata), but modulate the transcriptomic response to infection with Enteromyxum leei. BMC Genom. 2012, 13, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, J.A.; Rodríguez, C.; Bolaños, A.; Cejas, J.R.; Lorenzo, A. Beef tallow as an alternative to fish oil in diets for gilthead sea bream (Sparus aurata) juveniles: Effects on fish performance, tissue fatty acid composition, health and flesh nutritional value. Eur. J. Lipid Sci. Technol. 2014, 116, 571–583. [Google Scholar] [CrossRef]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2020, 519. [Google Scholar] [CrossRef]
- Godoy, A.C.; Santos, O.O.; Oxford, J.H.; de Amorim Melo, I.W.; Rodrigues, R.B.; Neu, D.; Vianna Nunes, R.; Boscolo, W.R. Soybean oil for Nile tilapia (Oreochromis niloticus) in finishing diets: Economic, zootechnical and nutritional meat improvements. Aquaculture 2019, 512, 734324. [Google Scholar] [CrossRef]
- Lazzari, R.; Emanuelli, T.; Maschio, D.; Ferreira, C.C.; Battisti, E.K.; Radünz-Neto, J. The inclusion of soybean oil in the diets of silver catfish (Rhamdia quelen) in relation to growth quality and fillet acceptability. Lat. Am. J. Aquat. Res. 2016, 44, 39–45. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, G.; Bacchetti, T.; Menanno, F.; Curatola, G. Effect of genistein against copper-induced lipid peroxidation of human high density lipoproteins (HDL). Atherosclerosis 2004, 172, 55–61. [Google Scholar] [CrossRef]
- Foti, P.; Erba, D.; Riso, P.; Spadafranca, A.; Criscuoli, F.; Testolin, G. Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Arch. Biochem. Biophys. 2005, 433, 421–427. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavese, J.M.; Farmer, R.L.; Bergan, R.C. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010, 29, 465–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behloul, N.; Wu, G. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013, 698, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Nef, S. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Nogowski, L. Genistein-A dietary compound inducing hormonal and metabolic changes. J. Steroid Biochem. Mol. Biol. 2007, 105, 37–45. [Google Scholar] [CrossRef]
- Hwang, J.T.; Park, I.J.; Shin, J.I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef]
- Park, H.J.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Ambati, S.; Baile, C.A. Genistein inhibits differentiation of primary human adipocytes. J. Nutr. Biochem. 2009, 20, 140–148. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, F.; Wang, R.; Ma, W.; Song, Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem. Biol. Interact. 2019, 310, 108665. [Google Scholar] [CrossRef]
- Grossini, E.; Farruggio, S.; Raina, G.; Mary, D.; Deiro, G.; Gentilli, S. Effects of genistein on differentiation and viability of human visceral adipocytes. Nutrients 2018, 10, 978. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Choi, J.S.; Kim, M.H.; Jung, M.H.; Lee, Y.S.; Song, J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition 2006, 22, 956–964. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in development and differentiation. Adv. Exp. Med. Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef]
- Cleveland, B.M. In vitro and in vivo effects of phytoestrogens on protein turnover in rainbow trout (Oncorhynchus mykiss) white muscle. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 165, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Skjærven, K.H.; Søfteland, L. Metabolic signatures of bisphenol A and genistein in Atlantic salmon liver cells. Chemosphere 2017, 189, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, B.M.; Manor, M.L. Effects of phytoestrogens on growth-related and lipogenic genes in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 170, 28–37. [Google Scholar] [CrossRef]
- Pinto, P.I.S.; Estêvão, M.D.; Andrade, A.; Santos, S.; Power, D.M. Tissue responsiveness to estradiol and genistein in the sea bass liver and scale. J. Steroid Biochem. Mol. Biol. 2016, 158, 127–137. [Google Scholar] [CrossRef]
- Sarasquete, C.; Úbeda-Manzanaro, M.; Ortiz-Delgado, J.B. Effects of the isoflavone genistein in early life stages of the Senegalese sole, Solea senegalensis: Role of the Survivin and proliferation versus apoptosis pathways. BMC Vet. Res. 2018, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lutfi, E.; Riera-Heredia, N.; Córdoba, M.; Porte, C.; Gutiérrez, J.; Capilla, E.; Navarro, I. Tributyltin and triphenyltin exposure promotes in vitro adipogenic differentiation but alters the adipocyte phenotype in rainbow trout. Aquat. Toxicol. 2017, 188, 148–158. [Google Scholar] [CrossRef]
- Figueiredo-Silva, A.; Rocha, E.; Dias, J.; Silva, P.; Rema, P.; Gomes, E.; Valente, L.M.P. Partial replacement of fish oil by soybean oil on lipid distribution and liver histology in European sea bass (Dicentrarchus labrax) and rainbow trout (Oncorhynchus mykiss) juveniles. Aquac. Nutr. 2005, 11, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Mørkøre, T.; Netteberg, C.; Johnsson, L.; Pickova, J. Impact of dietary oil source on product quality of farmed Atlantic cod, Gadus morhua. Aquaculture 2007, 267, 236–247. [Google Scholar] [CrossRef]
- Peng, S.; Chen, L.; Qin, J.G.; Hou, J.; Yu, N.; Long, Z.; Ye, J.; Sun, X. Effects of replacement of dietary fish oil by soybean oil on growth performance and liver biochemical composition in juvenile black seabream, Acanthopagrus schlegeli. Aquaculture 2008, 276, 154–161. [Google Scholar] [CrossRef]
- Rosenlund, G.; Oach, A.; Sandberg, M.G.; Standal, H.; Tveit, K. Effect of alternative lipid sources on long-term growth performance and quality of Atlantic salmon (Salmo salar L.). Aquac. Res. 2001, 32, 323–328. [Google Scholar] [CrossRef]
- Ruyter, B.; Moya-Falcón, C.; Rosenlund, G.; Vegusdal, A. Fat content and morphology of liver and intestine of Atlantic salmon (Salmo salar): Effects of temperature and dietary soybean oil. Aquaculture 2006, 252, 441–452. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell. Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.; DeCoster, M.A. Quantification of sPLA2-induced early and late apoptosis changes in neuronal cell cultures using combined TUNEL and DAPI staining. Brain Res. Protoc. 2004, 13, 144–150. [Google Scholar] [CrossRef]
- Mizushima, N. Methods for monitoring autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2491–2502. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Jin, S. Autophagy in adipose tissue biology. Pharmacol. Res. 2012, 66, 505–512. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [Green Version]
- Gossner, G.; Choi, M.; Tan, L.; Fogoros, S.; Griffith, K.A.; Kuenker, M.; Liu, J.R. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol. Oncol. 2007, 105, 23–30. [Google Scholar] [CrossRef]
- Prietsch, R.F.; Monte, L.G.; Da Silva, F.A.; Beira, F.T.; Del Pino, F.A.B.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef]
- Kim, D.J.; Seok, S.H.; Baek, M.W.; Lee, H.Y.; Na, Y.R.; Park, S.H.; Lee, H.K.; Dutta, N.K.; Kawakami, K.; Park, J.H. Developmental toxicity and brain aromatase induction by high genistein concentrations in zebrafish embryos. Toxicol. Mech. Methods 2009, 19, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasquete, C.; Úbeda-Manzanaro, M.; Ortiz-Delgado, J.B. Toxicity and non-harmful effects of the soya isoflavones, genistein and daidzein, in embryos of the zebrafish, Danio rerio. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 211, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sassi-Messai, S.; Gibert, Y.; Bernard, L.; Nishio, S.I.; Ferri Lagneau, K.F.; Molina, J.; Andersson-Lendahl, M.; Benoit, G.; Balaguer, P.; Laudet, V. The phytoestrogen genistein affects zebrafish development through two different pathways. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stienstra, R.; Haim, Y.; Riahi, Y.; Netea, M.; Rudich, A.; Leibowitz, G. Autophagy in adipose tissue and the beta cell: Implications for obesity and diabetes. Diabetologia 2014, 57, 1505–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmerón, C. Adipogenesis in fish. J. Exp. Biol. 2018, 121, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; He, Y.; Okutsu, M.; Ong, L.C.; Jin, Y.; Zheng, L.; Chow, P.; Yu, S.; Zhang, M.; Yan, Z. Autophagy is involved in adipogenic differentiation by repressesing proteasome-dependent PPARγ2 degradation. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Bou, M.; Montfort, J.; Le Cam, A.; Rallière, C.; Lebret, V.; Gabillard, J.C.; Weil, C.; Gutiérrez, J.; Rescan, P.Y.; Capilla, E.; et al. Gene expression profile during proliferation and differentiation of rainbow trout adipocyte precursor cells. BMC Genom. 2017, 18, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Rayalam, S.; Della-Fera, M.A.; Yang, J.-Y.; Park, H.J.; Ambati, S.; Baile, C.A. Resveratrol potentiates genistein’s antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J. Nutr. 2007, 137, 2668–2673. [Google Scholar] [CrossRef] [Green Version]
- Harmon, A.W.; Harp, J.B. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Dang, Z.C. Dose-dependent effects of soy phyto-oestrogen genistein on adipocytes: Mechanisms of action. Obes. Rev. 2009, 10, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Xue, H.G.; Chen, J.Y.; Chai, W.; Ni, M. Genistein induces adipogenic differentiation in human bone marrow mesenchymal stem cells and suppresses their osteogenic potential by upregulating PPARγ. Exp. Ther. Med. 2016, 11, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Haux, C.; Norberg, B. The influence of estradiol-17β on the liver content of protein, lipids, glycogen and nucleic acids in juvenile rainbow trout, Salmo gairdnerii. Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 81, 275–279. [Google Scholar] [CrossRef]
- Shen, M.; Shi, H. Sex hormones and their receptors regulate liver energy homeostasis. Int. J. Endocrinol. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- He, A.Y.; Ning, L.J.; Chen, L.Q.; Chen, Y.L.; Xing, Q.; Li, J.M.; Qiao, F.; Li, D.L.; Zhang, M.L.; Du, Z.Y. Systemic adaptation of lipid metabolism in response to low- and high-fat diet in Nile tilapia (Oreochromis niloticus). Physiol. Rep. 2015, 3, 1–18. [Google Scholar] [CrossRef]
- Davis, L.K.; Fox, B.K.; Lim, C.; Hiramatsu, N.; Sullivan, C.V.; Hirano, T.; Grau, E.G. Induction of vitellogenin production in male tilapia (Oreochromis mossambicus) by commercial fish diets. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 249–254. [Google Scholar] [CrossRef]
- Andreassen, T.K.; Skjoedt, K.; Korsgaard, B. Upregulation of estrogen receptor α and vitellogenin in eelpout (Zoarces viviparus) by waterborne exposure to 4-tert-octylphenol and 17β-estradiol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 340–346. [Google Scholar] [CrossRef]
- Bowman, C.J.; Kroll, K.J.; Gross, T.G.; Denslow, N.D. Estradiol-induced gene expression in largemouth bass (Micropterus salmoides). Mol. Cell. Endocrinol. 2002, 196, 67–77. [Google Scholar] [CrossRef]
- Leaños-Castañeda, O.; Van Der Kraak, G. Functional characterization of estrogen receptor subtypes, ERα and ERβ, mediating vitellogenin production in the liver of rainbow trout. Toxicol. Appl. Pharmacol. 2007, 224, 116–125. [Google Scholar] [CrossRef]
- Ishibashi, H.; Tachibana, K.; Tsuchimoto, M.; Soyano, K.; Tatarazako, N.; Matsumura, N.; Tomiyasu, Y.; Tominaga, N.; Arizono, K. Effects of nonylphenol and phytoestrogen-enriched diet on plasma vitellogenin, steroid hormone, hepatic cytochrome P450 1A, and glutathione-S-transferase values in Goldfish (Carassius auratus). Comp. Med. 2004, 54, 54–62. [Google Scholar] [PubMed]
- Cruz-Garcia, L.; Sánchez-Gurmaches, J.; Gutiérrez, J.; Navarro, I. Role of LXR in trout adipocytes: Target genes, hormonal regulation, adipocyte differentiation and relation to lipolysis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, C.; Riera-Heredia, N.; Gutiérrez, J.; Navarro, I.; Capilla, E. Adipogenic gene expression in gilthead sea bream mesenchymal stem cells from different origin. Front. Endocrinol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Chen, C.; Hu, Y.Y.; Feng, Q. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). Biomed. Pharmacother. 2019, 117, 109047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Upadhyay, S.; Bhuiyan, M.; Sarkar, F.H. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene 1999, 18, 3166–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Li, W.; Liu, F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004, 215, 159–166. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, B.H. Evidence for genistein mediated cytotoxicity and apoptosis in rat brain. Life Sci. 2004, 75, 499–509. [Google Scholar] [CrossRef]
- Kim, H.-K.; Nelson-Dooley, C.; Della-Fera, M.A.; Yang, J.-Y.; Zhang, W.; Duan, J.; Hartzell, D.L.; Hamrick, M.W.; Baile, C.A. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J. Nutr. 2006, 136, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Della-Fera, M.A.; Qian, H.; Baile, C.A. Adipocyte apoptosis in the regulation of body fat mass by leptin. Diabetes, Obes. Metab. 2001, 3, 299–310. [Google Scholar] [CrossRef]
- Gullicksen, P.S.; Hausman, D.B.; Dean, R.G.; Hartzell, D.L.; Baile, C.A. Adipose tissue cellularity and apoptosis after intracerebroventricular injections of leptin and 21 days of recovery in rats. Int. J. Obes. 2003, 27, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.Y.; Roh, K.H.; Jeong, Y.J.; Kim, S.H.; Lee, E.J.; Kim, M.S.; Oh, W.M.; Oh, H.K.; Kim, W.J. Estradiol protects PC12 cells against CoCl2-induced apoptosis. Brain Res. Bull. 2008, 76, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.D.; Pourati, I.; Aronovitz, M.J.; Baur, J.; Celestin, F.; Chen, X.; Michael, A.; Haq, S.; Nuedling, S.; Grohe, C.; et al. 17β-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of Phospho-inositide-3 kinase/Akt signaling. Circ. Res. 2004, 95, 692–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, R.; Kang, Y.; Li, X.; Roife, D.; Zhang, R.; Fleming, J.B. Genistein potentiates the antitumor effect of 5-fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014, 34, 4685–4692. [Google Scholar]
- Pierzynowska, K.; Gaffke, L.; Hać, A.; Mantej, J.; Niedziałek, N.; Brokowska, J.; Węgrzyn, G. Correction of Huntington’s disease phenotype by genistein-induced autophagy in the cellular model. Neuromol. Med. 2018, 20, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Zhang, T.; Chi, Y.; Liu, M.; Liu, Y. Genistein and myd88 activate autophagy in high glucose-induced renal podocytes in vitro. Med. Sci. Monit. 2018, 24, 4823–4831. [Google Scholar] [CrossRef]
- Lescat, L.; Herpin, A.; Mourot, B.; Véron, V.; Guiguen, Y.; Bobe, J.; Seiliez, I. CMA restricted to mammals and birds: Myth or reality? Autophagy 2018, 14, 1267–1270. [Google Scholar] [CrossRef]
- Lescat, L.; Véron, V.; Mourot, B.; Péron, S.; Chenais, N.; Dias, K.; Riera-Heredia, N.; Beaumatin, F.; Pinel, K.; Priault, M.; et al. Chaperone-mediated autophagy in the light of evolution: Insight from fish. Mol. Biol. Evol. 2020. [Google Scholar] [CrossRef]
- Jansen, H.J.; Van Essen, P.; Koenen, T.; Joosten, L.A.B.; Netea, M.G.; Tack, C.J.; Stienstra, R. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology 2012, 153, 5866–5874. [Google Scholar] [CrossRef] [Green Version]
- Kovsan, J.; Blüher, M.; Tarnovscki, T.; Klöting, N.; Kirshtein, B.; Madar, L.; Shai, I.; Golan, R.; Harman-Boehm, I.; Schön, M.R.; et al. Altered autophagy in human adipose tissues in obesity. J. Clin. Endocrinol. Metab. 2011, 96, 268–277. [Google Scholar] [CrossRef]
- Nuñez, C.E.; Rodrigues, V.S.; Gomes, F.S.; De Moura, R.F.; Victorio, S.C.; Bombassaro, B.; Chaim, E.A.; Pareja, J.C.; Geloneze, B.; Velloso, L.A.; et al. Defective regulation of adipose tissue autophagy in obesity. Int. J. Obes. 2013, 37, 1473–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öst, A.; Svensson, K.; Ruishalme, I.; Brännmark, C.; Franck, N.; Krook, H.; Sandström, P.; Kjolhede, P.; Strålfors, P. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol. Med. 2010, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.M.; Chiappetta, C.; Stancel, G.M. Interaction of human estrogen receptors α and β with the same naturally occurring estrogen response elements. Biochem. Pharmacol. 1999, 57, 597–601. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M. Effects of sex steroids on indices of protein turnover in rainbow trout (Oncorhynchus mykiss) white muscle. Gen. Comp. Endocrinol. 2011, 174, 132–142. [Google Scholar] [CrossRef]
- Readman, G.D.; Owen, S.F.; Knowles, T.G.; Murrell, J.C. Species specific anaesthetics for fish anaesthesia and euthanasia. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bouraoui, L.; Gutiérrez, J.; Navarro, I. Regulation of proliferation and differentiation of adipocyte precursor cells in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2008, 198, 459–469. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective assessment of changes in nuclear morphology and cell distribution following induction of apoptosis. Diagn. Pathol. 2014, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vélez, E.J.; Balbuena-Pecino, S.; Capilla, E.; Navarro, I.; Gutiérrez, J.; Riera-Codina, M. Effects of β2-adrenoceptor agonists on gilthead sea bream (Sparus aurata) cultured muscle cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 227, 179–193. [Google Scholar] [CrossRef]
- Riera-Heredia, N.; Lutfi, E.; Sánchez-Moya, A.; Gutiérrez, J.; Capilla, E.; Navarro, I. Short-term responses to fatty acids on lipid metabolism and adipogenesis in rainbow trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2020, 21, 1623. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]




| CT | GE 5 μg/g | GE 50 μg/g | E2 5 μg/g | |
|---|---|---|---|---|
| TAG (mM) | 8.44 ± 1.14 a | 9.12 ± 1.83 a | 3.45 ± 0.81 b | 11.89 ± 2.38 a |
| NEFA (mM) | 0.151 ± 0.013 a | 0.182 ± 0.012 a | 0.156 ± 0.006 a | 0.250 ± 0.038 b |
| Glucose (mM) | 6.08 ± 0.46 | 6.37 ± 0.50 | 4.39 ± 0.73 | 5.96 ± 0.85 |
| CT | GE 5 μg/g | GE 50 μg/g | E2 5 μg/g | |
|---|---|---|---|---|
| vtg | 0.196 ± 0.041 a | 0.168 ± 0.028 a | 0.653 ± 0.100 b | 1.362 ± 0.131 c |
| Gene | Primer Sequences (5′–3′) | Tm, °C | Accession Number |
|---|---|---|---|
| ubq | F: ACAACATCCAGAAAGAGTCCA R: AGGCGAGCGTAGCACTTG | 58 | NM_001124194.1 |
| ef1α | F: TCCTCTTGGTCGTTTCGCTG R: ACCCGAGGGACATCCTGTG | 58 | NM_001124339.1 |
| β-actin | F: ATCCTGACGGAGCGCGGTTACAGC R: TGCCCATCTCCTGCTCAAAGTCCA | 61 | AJ438158 |
| vtg | F: GAGCTAAGGTCCGCACAATTG R: GGGAAACAGGGAAAGCTTCAA | 58 | X92804 |
| fas | F: GAGACCTAGTGGAGGCTGTC R: TCTTGTTGATGGTGAGCTGT | 54 | tcaa0001c.m.06_5.1.om.4 |
| lpl | F: TAATTGGCTGCAGAAAACAC R: CGTCAGCAAACTCAAAGGT | 59 | AJ224693 |
| hsl | F: AGGGTCATGGTCATCGTCTC R: CTTGACGGAGGGACAGCTAC | 58 | TC172767 |
| pparα | F: CTGGAGCTGGATGACAGTGA R: GGCAAGTTTTTGCAGCAGAT | 54 | NM_001197211.1 |
| pparβ | F: CTGGAGCTGGATGACAGTA R: GTCAGCCATCTTGTTGAGCA | 60 | AY356399.1 |
| gapdh | F: TCTGGAAAGCTGTGGAGGGATGGA R: AACCTTCTTGATGGCATCATAGC | 61 | NM_001123561 |
| lxr | F: TGCAGCAGCCGTATGTGGA R: GCGGCGGGAGCTTCTTGTC | 62 | NM_001159338 |
| casp3 | F: TTTGGGAGTAGATTGCAGGG R: TGCACATCCACGATTTGATT | 57 | TC139042 |
| casp8 | F: CAGCATAGAGAAGCAAGGGG R: TGACTGAGGGGAGCTGAGTT | 56 | TC172513 |
| p53 | F: GTGGAATTTGATCCGAGTCTGT R: AGTGTCCAGGGTAGAATGGAG | 56 | - |
| lc3b | F: GAACAGTTTGACCTGCGTGAA R: TCTCTCAATGATGACCGGAATCT | 57 | CA350545 |
| atg4b | F: TATGCGCTTCCGAAAGTTGTC R: CAGGATCGTTGGGTTTCTGC | 58 | CA345181.s.om.10 |
| atg12l | F: GATGGAGGCCAATGAACAGC R: GCGTTTGAACTGAAAAGGGCTAA | 60 | CB490089.s.om.10 |
| ctsd | F: GCCTGTCATCACATTCAACT R: CCACTCAGGCAGATGGTCTTA | 55 | NM_001124711 |
| ctsl | F: TGAAGGAGAAGATGTGGATGG R: TTCCTGTCTTTGGCCATGTAG | 56 | NM_001124305 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balbuena-Pecino, S.; Lutfi, E.; Riera-Heredia, N.; Gasch-Navalón, E.; Vélez, E.J.; Gutiérrez, J.; Capilla, E.; Navarro, I. Genistein Induces Adipogenic and Autophagic Effects in Rainbow Trout (Oncorhynchus mykiss) Adipose Tissue: In Vitro and In Vivo Models. Int. J. Mol. Sci. 2020, 21, 5884. https://doi.org/10.3390/ijms21165884
Balbuena-Pecino S, Lutfi E, Riera-Heredia N, Gasch-Navalón E, Vélez EJ, Gutiérrez J, Capilla E, Navarro I. Genistein Induces Adipogenic and Autophagic Effects in Rainbow Trout (Oncorhynchus mykiss) Adipose Tissue: In Vitro and In Vivo Models. International Journal of Molecular Sciences. 2020; 21(16):5884. https://doi.org/10.3390/ijms21165884
Chicago/Turabian StyleBalbuena-Pecino, Sara, Esmail Lutfi, Natàlia Riera-Heredia, Esther Gasch-Navalón, Emilio J. Vélez, Joaquim Gutiérrez, Encarnación Capilla, and Isabel Navarro. 2020. "Genistein Induces Adipogenic and Autophagic Effects in Rainbow Trout (Oncorhynchus mykiss) Adipose Tissue: In Vitro and In Vivo Models" International Journal of Molecular Sciences 21, no. 16: 5884. https://doi.org/10.3390/ijms21165884
APA StyleBalbuena-Pecino, S., Lutfi, E., Riera-Heredia, N., Gasch-Navalón, E., Vélez, E. J., Gutiérrez, J., Capilla, E., & Navarro, I. (2020). Genistein Induces Adipogenic and Autophagic Effects in Rainbow Trout (Oncorhynchus mykiss) Adipose Tissue: In Vitro and In Vivo Models. International Journal of Molecular Sciences, 21(16), 5884. https://doi.org/10.3390/ijms21165884









