Heterologous Hydrogenase Overproduction Systems for Biotechnology—An Overview
Abstract
1. Introduction
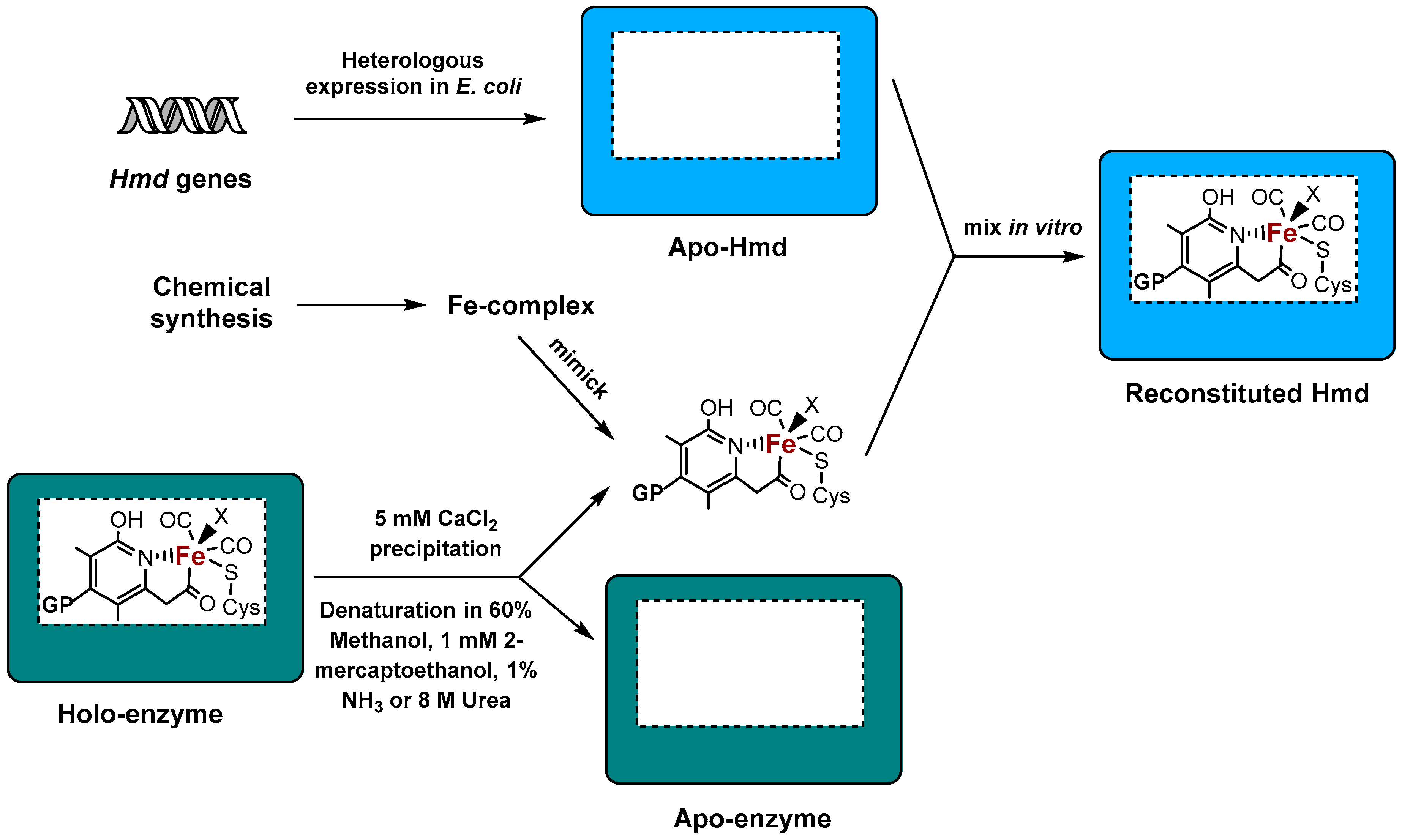
2. [Fe]-Hydrogenase Production Systems
3. [FeFe]-Hydrogenase Production Systems
3.1. Recombinant [FeFe] Hydrogenase Production in the Presence of the Maturases HydE, F and G
3.2. In Vitro Maturation Systems for [FeFe]-Hydrogenases
3.3. [FeFe]-Hydrogenase Production in Cyanobacteria and Microalgae
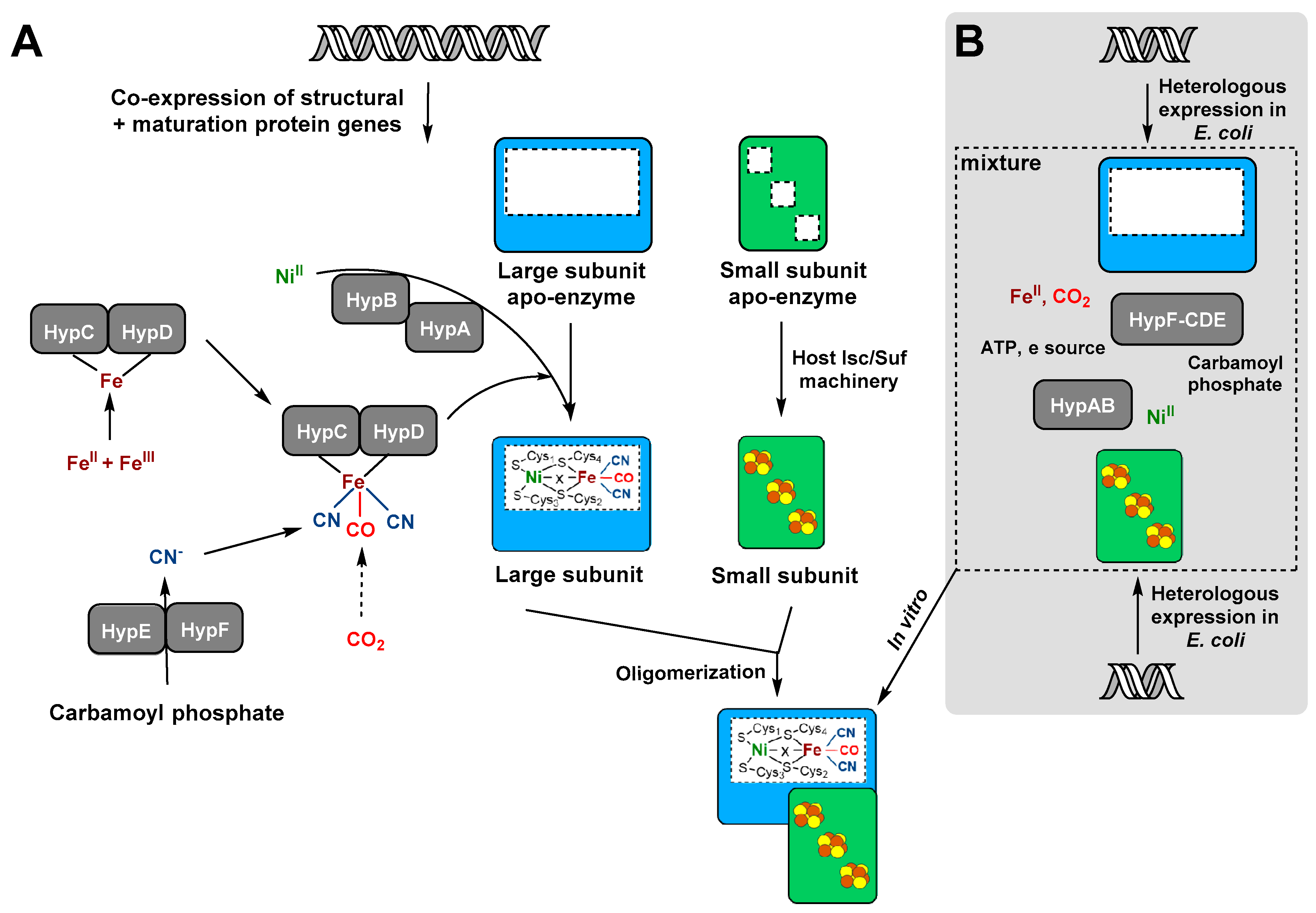
4. [NiFe]-Hydrogenase Production Systems
4.1. Heterologous Production of Hydrogenases in Hosts Encoding Closely Related Native Enzymes
4.2. Recombinant Hydrogenase Production in the Presence of Specific Accessory Proteins
4.3. In Vitro Reconstitution Systems for [NiFe]-Hydrogenases
5. Biohydrogen Production through Heterologous Gene Expression
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Shima, S.; Pilak, O.; Vogt, S.; Schick, M.; Stagni, M.S.; Meyer-Klaucke, W.; Warkentin, E.; Thauer, R.K.; Ermler, U. The crystal structure sof [Fe]-hydrogenase reveals the geometry of the active site. Science 2008, 321, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, G.W.; Dresselhaus, M.S. The hydrogen fuel alternative. MRS Bull. 2008, 33, 421–428. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef]
- Greening, C.; Biswas, A.; Carere, C.R.; Jackson, C.J.; Taylor, M.C.; Stott, M.B.; Cook, G.M.; Morales, S.E. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016, 10, 761–777. [Google Scholar] [CrossRef]
- Vignais, P.M.; Billoud, B. Occurrence, classification, and biological function of hydrogenases: An overview. Chem. Rev. 2007, 107, 4206–4272. [Google Scholar] [CrossRef]
- Kalia, V.C.; Lal, S.; Ghai, R.; Mandal, M.; Chauhan, A. Mining genomic databases to identify novel hydrogen producers. Trends Biotechnol. 2003, 21, 152–156. [Google Scholar] [CrossRef]
- Forzi, L.; Sawers, R.G. Maturation of [NiFe]-hydrogenases in Escherichia coli. BioMetals 2007, 20, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Jugder, B.-E.; Welch, J.; Aguey-Zinsou, K.-F.; Marquis, C.P. Fundamentals and electrochemical applications of [Ni–Fe]-uptake hydrogenases. RSC Adv. 2013, 3, 8142. [Google Scholar] [CrossRef]
- Edwards, E.H.; Bren, K.L. Light-driven catalysis with engineered enzymes and biomimetic systems. Biotechnol. Appl. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Krassen, H.; Schwarze, A.; Friedrich, B.; Ataka, K.; Lenz, O.; Heberle, J. Photosynthetic Hydrogen Production by a Hybrid Complex of Photosystem I and [NiFe]-Hydrogenase. ACS Nano 2009, 3, 4055–4061. [Google Scholar] [CrossRef]
- Wei, W.; Sun, P.; Li, Z.; Song, K.; Su, W.; Wang, B.; Liu, Y.; Zhao, J. A surface-display biohybrid approach to light-driven hydrogen production in air. Sci. Adv. 2018, 4, eaap9253. [Google Scholar] [CrossRef]
- Chenevier, P.; Mugherli, L.; Darbe, S.; Darchy, L.; DiManno, S.; Tran, P.D.; Valentino, F.; Iannello, M.; Volbeda, A.; Cavazza, C.; et al. Hydrogenase enzymes: Application in biofuel cells and inspiration for the design of noble-metal free catalysts for H2 oxidation. C. R. Chim. 2013, 16, 491–505. [Google Scholar] [CrossRef]
- Mertens, R.; Greiner, L.; van den Ban, E.C.; Haaker, H.B.C.M.; Liese, A. Practical applications of hydrogenase I from Pyrococcus furiosus for NADPH generation and regeneration. J. Mol. Catal. B Enzym. 2003, 24–25, 39–52. [Google Scholar] [CrossRef]
- Ratzka, J.; Lauterbach, L.; Lenz, O.; Ansorge-Schumacher, M.B. Systematic evaluation of the dihydrogen-oxidising and NAD + -reducing soluble [NiFe]-hydrogenase from Ralstonia eutropha H16 as a cofactor regeneration catalyst. Biocatal. Biotransform. 2011, 29, 246–252. [Google Scholar] [CrossRef]
- Lauterbach, L.; Lenz, O.; Vincent, K.A. H2-driven cofactor regeneration with NAD (P) +-reducing hydrogenases. FEBS J. 2013, 280, 3058–3068. [Google Scholar] [CrossRef]
- Atomi, H.; Sato, T.; Kanai, T. Application of hyperthermophiles and their enzymes. Curr. Opin. Biotechnol. 2011, 22, 618–626. [Google Scholar] [CrossRef]
- Lojou, E. Hydrogenases as catalysts for fuel cells: Strategies for efficient immobilization at electrode interfaces. Electrochim. Acta 2011, 56, 10385–10397. [Google Scholar] [CrossRef]
- Sargent, F. The model [NiFe]-hydrogenases of Escherichia coli. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 68, pp. 433–507. [Google Scholar]
- Mulder, D.W.; Shepard, E.M.; Meuser, J.E.; Joshi, N.; King, P.W.; Posewitz, M.C.; Broderick, J.B.; Peters, J.W. Insights into [FeFe]-hydrogenase structure, mechanism, and maturation. Structure 2011, 19, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, M.J.; Zamble, D.B. [NiFe]-hydrogenase maturation. Biochemistry 2016, 55, 1689–1701. [Google Scholar] [PubMed]
- English, C.M.; Eckert, C.; Brown, K.; Seibert, M.; King, P.W. Recombinant and in vitro expression systems for hydrogenases: New frontiers in basic and applied studies for biological and synthetic H2 production. Dalt. Trans. 2009, 9970–9978. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wagner, T.; Ermler, U.; Shima, S. Methanogenesis involves direct hydride transfer from H2 to an organic substrate. Nat. Rev. Chem. 2020, 4, 213–221. [Google Scholar] [CrossRef]
- Zirngibl, C.; van Dongen, W.; Schwörer, B.; von Bünau, R.; Richter, M.; Klein, A.; Thauer, R.K. H2-forming methylenetetrahydromethanopterin dehydrogenase, a novel type of hydrogenase withaout iron-sulfur clusters in methanogenic archaea. Eur. J. Biochem. 1992, 208, 511–520. [Google Scholar] [CrossRef]
- Hiromoto, T.; Ataka, K.; Pilak, O.; Vogt, S.; Stagni, M.S.; Meyer-Klaucke, W.; Warkentin, E.; Thauer, R.K.; Shima, S.; Ermler, U. The crystal structure of C176A mutated [Fe]-hydrogenase suggests an acyl-iron ligation in the active site iron complex. FEBS Lett. 2009, 583, 585–590. [Google Scholar] [CrossRef]
- Korbas, M.; Vogt, S.; Meyer-Klaucke, W.; Bill, E.; Lyon, E.J.; Thauer, R.K.; Shima, S. The iron-sulfurcluster-free hydrogenase (Hmd) is a metalloenzyme with a novel iron binding motif. J. Biol. Chem. 2006, 281, 30804–30813. [Google Scholar] [CrossRef]
- Schick, M.; Xie, X.; Ataka, K.; Kahnt, J.; Linne, U.; Shima, S. Biosynthesis of the iron-guanylylpyridinol cofactor of [Fe]-hydrogenase in methanogenic archaea as elucidated by stable-isotope labeling. J. Am. Chem. Soc. 2012, 134, 3271–3280. [Google Scholar] [CrossRef]
- Buurman, G.; Shima, S.; Thauer, R.K. The metal-free hydrogenase from methanogenic archaea: Evidence for a bound cofactor. FEBS Lett. 2000, 485, 200–204. [Google Scholar] [CrossRef]
- Ma, K.; Zirngibl, C.; Linder, D.; Stetter, K.O.; Thauer, R.K. N5, N10-methylenetetrahydromethanopterin dehydrogenase (H2-forming) from the extreme thermophile Methanopyrus kandleri. Arch. Microbiol. 1991, 156, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wagner, T.; Ermler, U.; Bill, E.; Ataka, K.; Shima, S. Dioxygen Sensitivity of [Fe]-Hydrogenase in the Presence of Reducing Substrates. Angew. Chem. Int. Ed. 2018, 57, 4917–4920. [Google Scholar] [CrossRef] [PubMed]
- Lyon, E.J.; Shima, S.; Buurman, G.; Chowdhuri, S.; Batschauer, A.; Steinbach, K.; Thauer, R.K. UV-A/blue-light inactivation of the “metal-free” hydrogenase (Hmd) from methanogenic archaea. Eur. J. Biochem. 2004, 271, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Wagner, T.; Huang, G.; Kahnt, J.; Ataka, K.; Ermler, U.; Shima, S. The bacterial [Fe]-hydrogenase paralog HmdII uses tetrahydrofolate derivatives as substrates. Angew. Chem. Int. Ed. 2019, 58, 3506–3510. [Google Scholar] [CrossRef]
- Shima, S.; Schick, M.; Tamura, H. Preparation of [Fe]-hydrogenase from methanogenic archaea. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2011; Volume 494, pp. 119–137. [Google Scholar]
- Pan, H.J.; Huang, G.; Wodrich, M.D.; Tirani, F.F.; Ataka, K.; Shima, S.; Hu, X. A catalytically active [Mn]-hydrogenase incorporating a non-native metal cofactor. Nat. Chem. 2019, 11, 669–675. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Goenrich, M.; Schick, M.; Hiromoto, T.; Shima, S. Hydrogenases from methanogenic archaea, nickel, a novel cofactor, and H2 Storage. Annu. Rev. Biochem. 2010, 79, 507–536. [Google Scholar] [CrossRef]
- Lie, T.J.; Costa, K.C.; Pak, D.; Sakesan, V.; Leigh, J.A. Phenotypic evidence that the function of the [Fe]-hydrogenase Hmd in Methanococcus maripaludis requires seven hcg (hmd co-occurring genes) but not hmdII. FEMS Microbiol. Lett. 2013, 343, 156–160. [Google Scholar] [CrossRef]
- Fujishiro, T.; Tamura, H.; Schick, M.; Kahnt, J.; Xie, X.; Ermler, U.; Shima, S. Identification of the HcgB Enzyme in [Fe]-Hydrogenase-Cofactor Biosynthesis. Angew. Chem. 2013, 125, 12787–12790. [Google Scholar] [CrossRef]
- Fujishiro, T.; Bai, L.; Xu, T.; Xie, X.; Schick, M.; Kahnt, J.; Rother, M.; Hu, X.; Ermler, U.; Shima, S. Identification of HcgC as a SAM-Dependent Pyridinol Methyltransferase in [Fe]-Hydrogenase Cofactor Biosynthesis. Angew. Chem. 2016, 128, 9800–9803. [Google Scholar] [CrossRef]
- Bai, L.; Fujishiro, T.; Huang, G.; Koch, J.; Takabayashi, A.; Yokono, M.; Tanaka, A.; Xu, T.; Hu, X.; Ermler, U.; et al. Towards artificial methanogenesis: Biosynthesis of the [Fe]-hydrogenase cofactor and characterization of the semi-synthetic hydrogenase. Faraday Discuss. 2017, 198, 37–58. [Google Scholar] [CrossRef]
- Fujishiro, T.; Ermler, U.; Shima, S. A possible iron delivery function of the dinuclear iron center of HcgD in [Fe]-hydrogenase cofactor biosynthesis. FEBS Lett. 2014, 588, 2789–2793. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, T.; Kahnt, J.; Ermler, U.; Shima, S. Protein-pyridinol thioester precursor for biosynthesis of the organometallic acyl-iron ligand in [Fe]-hydrogenase cofactor. Nat. Commun. 2015, 6, 6895. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.D.; Leigh, J.A.; Samudrala, R. Comprehensive computational analysis of Hmd enzymes and paralogs in methanogenic Archaea. BMC Evol. Biol. 2009, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, T.; Ataka, K.; Ermler, U.; Shima, S. Towards a functional identification of catalytically inactive [Fe]-hydrogenase paralogs. FEBS J. 2015, 282, 3412–3423. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-J.; Hu, X. Biomimetic hydrogenation catalyzed by a manganese model of [Fe]-hydrogenase. Angew. Chem. Int. Ed. 2020, 59, 4942–4946. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hall, M.B. Monoiron Hydrogenase Catalysis: Hydrogen Activation with the Formation of a Dihydrogen, Fe−Hδ−···Hδ+−O, Bond and Methenyl-H4MPT+ Triggered Hydride Transfer. J. Am. Chem. Soc. 2009, 131, 10901–10908. [Google Scholar] [CrossRef]
- Hedegård, E.D.; Kongsted, J.; Ryde, U. Multiscale Modeling of the Active Site of [Fe] Hydrogenase: The H2 Binding Site in Open and Closed Protein Conformations. Angew. Chem. Int. Ed. 2015, 54, 6246–6250. [Google Scholar] [CrossRef]
- Huang, G.; Wagner, T.; Wodrich, M.D.; Ataka, K.; Bill, E.; Ermler, U.; Hu, X.; Shima, S. The atomic-resolution crystal structure of activated [Fe]-hydrogenase. Nat. Catal. 2019, 2, 537–543. [Google Scholar] [CrossRef]
- Peters, J.W.; Schut, G.J.; Boyd, E.S.; Mulder, D.W.; Shepard, E.M.; Broderick, J.B.; King, P.W.; Adams, M.W. [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim. Biophys. Acta—Mol. Cell Res. 2015, 1853, 1350–1369. [Google Scholar] [CrossRef]
- Horner, D.S.; Heil, B.; Happe, T.; Embley, T.M. Iron hydrogenases—Ancient enzymes in modern eukaryotes. Trends Biochem. Sci. 2002, 27, 148–153. [Google Scholar] [CrossRef]
- van der Giezen, M.; Tovar, J.; Clark, C.G. Mitochondrion-derived organelles in protists and fungi. Int. Rev. Cytol. 2005, 244, 175–225. [Google Scholar]
- Melis, A.; Seibert, M.; Happe, T. Genomics of green algal hydrogen research. Photosynth. Res. 2004, 82, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. [FeFe] hydrogenases and their evolution: A genomic perspective. Cell. Mol. Life Sci. 2007, 64, 1063–1084. [Google Scholar] [CrossRef]
- Peters, J.W.; Lanzilotta, W.N.; Lemon, B.J.; Seefeldt, L.C. X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 1998, 282, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Vignais, P. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev. 2001, 25, 455–501. [Google Scholar] [CrossRef]
- Peters, J.W. Structure and mechanism of iron-only hydrogenases. Curr. Opin. Struct. Biol. 1999, 9, 670–676. [Google Scholar] [CrossRef]
- Nicolet, Y.; Cavazza, C.; Fontecilla-Camps, J.C. Fe-only hydrogenases: Structure, function and evolution. J. Inorg. Biochem. 2002, 91, 1–8. [Google Scholar] [CrossRef]
- Bortolus, M.; Costantini, P.; Doni, D.; Carbonera, D. Overview of the Maturation Machinery of the H-Cluster of [FeFe]-Hydrogenases with a Focus on HydF. Int. J. Mol. Sci. 2018, 19, 3118. [Google Scholar] [CrossRef]
- Voordouw, G.; Hagen, W.R.; Krüse-Wolters, M.; van Berkel-Arts, A.; Veeger, C. Purification and characterization of Desulfovibrio vulgaris (Hildenborough) hydrogenase expressed in Escherichia coli. Eur. J. Biochem. 1987, 162, 31–36. [Google Scholar] [CrossRef]
- van Dongen, W.; Hagen Wilfred, H.; van den Berg, W.; Cees, V. Evidence for an unusual mechanism of membrane translocation of the periplasmic hydrogenase of Desulfovibrio vulgaris (Hildenborough), as derived from expression in Escherichia coli. FEMS Microbiol. Lett. 1988, 50, 5–9. [Google Scholar]
- Demuez, M.; Cournac, L.; Guerrini, O.; Soucaille, P.; Girbal, L. Complete activity profile of Clostridium acetobutylicum [FeFe]-hydrogenase and kinetic parameters for endogenous redox partners. FEMS Microbiol. Lett. 2007, 275, 113–121. [Google Scholar] [CrossRef] [PubMed]
- King, P.W.; Posewitz, M.C.; Ghirardi, M.L.; Seibert, M. Functional studies of [FeFe] hydrogenase maturation in an Escherichia coli biosynthetic system. J. Bacteriol. 2006, 188, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.K.; Jones, P.R. Deletion of iscR stimulates recombinant clostridial Fe-Fe hydrogenase activity and H2-accumulation in Escherichia coli BL21 (DE3). Appl. Microbiol. Biotechnol. 2008, 78, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Sachdeva, G.; Silver, P.A. Rewiring hydrogenase-dependent redox circuits in cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, S.; Lal, B. Fermentative hydrogen production in recombinant Escherichia coli harboring a [FeFe]-hydrogenase gene isolated from Clostridium butyricum. Int. J. Hydrogen Energy 2011, 36, 14024–14030. [Google Scholar] [CrossRef]
- Adams, M.W.W.; Mortenson, L.E. The purification of hydrogenase II (uptake hydrogenase) from the anaerobic N2-fixing bacterium Clostridium pasteurianum. Biochim. Biophys. Acta—Bioenerg. 1984, 766, 51–61. [Google Scholar] [CrossRef]
- Boyer, M.E.; Stapleton, J.A.; Kuchenreuther, J.M.; Wang, C.; Swartz, J.R. Cell-free synthesis and maturation of [FeFe] hydrogenases. Biotechnol. Bioeng. 2008, 99, 59–67. [Google Scholar] [CrossRef]
- Kuchenreuther, J.M.; Grady-Smith, C.S.; Bingham, A.S.; George, S.J.; Cramer, S.P.; Swartz, J.R. High-yield expression of heterologous [FeFe] hydrogenases in Escherichia coli. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Bingham, A.S.; Smith, P.R.; Swartz, J.R. Evolution of an [FeFe] hydrogenase with decreased oxygen sensitivity. Int. J. Hydrogen Energy 2012, 37, 2965–2976. [Google Scholar] [CrossRef]
- Lampret, O.; Esselborn, J.; Haas, R.; Rutz, A.; Booth, R.L.; Kertess, L.; Wittkamp, F.; Megarity, C.F.; Armstrong, F.A.; Winkler, M.; et al. The final steps of [FeFe]-hydrogenase maturation. Proc. Natl. Acad. Sci. USA 2019, 116, 15802–15810. [Google Scholar] [CrossRef]
- Esselborn, J.; Lambertz, C.; Adamska-Venkatesh, A.; Simmons, T.; Berggren, G.; Noth, J.; Siebel, J.; Hemschemeier, A.; Artero, V.; Reijerse, E.; et al. Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic. Nat. Chem. Biol. 2013, 9, 607–609. [Google Scholar] [CrossRef]
- Asada, Y.; Koike, Y.; Schnackenberg, J.; Miyake, M.; Uemura, I.; Miyake, J. Heterologous expression of clostridial hydrogenase in the cyanobacterium Synechococcus PCC7942. Biochim. Biophys. Acta—Gene Struct. Expr. 2000, 1490, 269–278. [Google Scholar] [CrossRef]
- Kamp, C.; Silakov, A.; Winkler, M.; Reijerse, E.J.; Lubitz, W.; Happe, T. Isolation and first EPR characterization of the [FeFe]-hydrogenases from green algae. Biochim. Biophys. Acta—Bioenerg. 2008, 1777, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Girbal, L.; von Abendroth, G.; Winkler, M.; Benton, P.M.; Meynial-Salles, I.; Croux, C.; Peters, J.W.; Happe, T.; Soucaille, P. Homologous and heterologous overexpression in Clostridium acetobutylicum and characterization of purified clostridial and algal Fe-only hydrogenases with high specific activities. Appl. Environ. Microbiol. 2005, 71, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- von Abendroth, G.; Stripp, S.; Silakov, A.; Croux, C.; Soucaille, P.; Girbal, L.; Happe, T. Optimized over-expression of [FeFe] hydrogenases with high specific activity in Clostridium acetobutylicum. Int. J. Hydrogen Energy 2008, 33, 6076–6081. [Google Scholar] [CrossRef]
- Posewitz, M.C.; King, P.W.; Smolinski, S.L.; Zhang, L.; Seibert, M.; Ghirardi, M.L. Discovery of two novel radical S-adenosylmethionine proteins required for the assembly of an active [Fe] hydrogenase. J. Biol. Chem. 2004, 279, 25711–25720. [Google Scholar] [CrossRef] [PubMed]
- Yacoby, I.; Tegler, L.T.; Pochekailov, S.; Zhang, S.; King, P.W. Optimized Expression and Purification for High-Activity Preparations of Algal [FeFe]-Hydrogenase. PLoS ONE 2012, 7, e35886. [Google Scholar] [CrossRef]
- Stapleton, J.A.; Swartz, J.R. A cell-free microtiter plate screen for improved [FeFe] hydrogenases. PLoS ONE 2010, 5, e10554. [Google Scholar] [CrossRef]
- Berggren, G.; Adamska, A.; Lambertz, C.; Simmons, T.R.; Esselborn, J.; Atta, M.; Gambarelli, S.; Mouesca, J.-M.; Reijerse, E.; Lubitz, W.; et al. Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 2013, 499, 66–69. [Google Scholar] [CrossRef]
- Sybirna, K.; Antoine, T.; Lindberg, P.; Fourmond, V.; Rousset, M.; Mejean, V.; Bottin, H. Shewanella oneidensis: A new and efficient System for Expression and Maturation of heterologous [Fe-Fe] Hydrogenase from Chlamydomonas reinhardtii. BMC Biotechnol. 2008, 8, 73. [Google Scholar] [CrossRef]
- Berto, P.; D’apos Adamo, S.; Bergantino, E.; Vallese, F.; Giacometti, G.M.; Costantini, P. The cyanobacterium Synechocystis sp. PCC 6803 is able to express an active [FeFe]-hydrogenase without additional maturation proteins. Biochem. Biophys. Res. Commun. 2011, 405, 678–683. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, S.E.; Ruebush, S.S.; Naumov, A.; Nagy, L.E.; Dubini, A.; King, P.W.; Broderick, J.B.; Posewitz, M.C.; Peters, J.W. In vitro activation of [FeFe] hydrogenase: New insights into hydrogenase maturation. J. Biol. Inorg. Chem. 2007, 12, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.L.; Pinske, C.; Murphy, B.J.; Parkin, A.; Armstrong, F.; Palmer, T.; Sargent, F. Integration of an [FeFe]-hydrogenase into the anaerobic metabolism of Escherichia coli. Biotechnol. Rep. 2015, 8, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xing, D.; Zhang, L.; Ren, N. Characterization and overexpression of a [FeFe]-hydrogenase gene of a novel hydrogen-producing bacterium Ethanoligenens harbinense. Int. J. Hydrogen Energy 2010, 35, 9598–9602. [Google Scholar] [CrossRef]
- Nixon, J.E.J.; Field, J.; McArthur, A.G.; Sogin, M.L. Iron-dependent Hydrogenases of Entamoeba histolytica and Giardia lamblia: Activity of the Recombinant Entamoebic enzyme and evidence for lateral gene transfer. Biol. Bull. 2003, 204, 1–9. [Google Scholar] [CrossRef]
- Inoue, J.I.; Saita, K.; Kudo, T.; Ui, S.; Ohkuma, M. Hydrogen production by termite gut protists: Characterization of iron hydrogenases of parabasalian symbionts of the termite Coptotermes formosanus. Eukaryot. Cell 2007, 6, 1925–1932. [Google Scholar] [CrossRef]
- Gärtner, K.; Lechno-Yossef, S.; Cornish, A.J.; Wolk, C.P.; Hegg, E.L. Expression of Shewanella oneidensis MR-1 [FeFe]-hydrogenase genes in Anabaena sp. strain PCC 7120. Appl. Environ. Microbiol. 2012, 78, 8579–8586. [Google Scholar] [CrossRef]
- Atta, M.; Meyer, J. Characterization of the gene encoding the [Fe]-hydrogenase from Megasphaera elsdenii. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 2000, 1476, 368–371. [Google Scholar] [CrossRef]
- Gorwa, M.F.; Croux, C.; Soucaille, P. Molecular characterization and transcriptional analysis of the putative hydrogenase gene of Clostridium acetobutylicum ATCC 824. J. Bacteriol. 1996, 178, 2668–2675. [Google Scholar] [CrossRef]
- Nagy, L.E.; Meuser, J.E.; Plummer, S.; Seibert, M.; Ghirardi, M.L.; King, P.W.; Ahmann, D.; Posewitz, M.C. Application of gene-shuffling for the rapid generation of novel [FeFe]-hydrogenase libraries. Biotechnol. Lett. 2007, 29, 421–430. [Google Scholar] [CrossRef]
- Heidelberg, J.F.; Paulsen, I.T.; Nelson, K.E.; Gaidos, E.J.; Nelson, W.C.; Read, T.D.; Eisen, J.A.; Seshadri, R.; Ward, N.; Methe, B.; et al. Genome sequence of the dissimilatory metal ion–reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 2002, 20, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Birrell, J.A.; Rüdiger, O.; Reijerse, E.J.; Lubitz, W. Semisynthetic hydrogenases propel biological energy research into a new era. Joule 2017, 1, 61–76. [Google Scholar] [CrossRef]
- De Lacey, A.L.; Fernández, V.M.; Rousset, M.; Cammack, R. Activation and Inactivation of Hydrogenase Function and the Catalytic Cycle: Spectroelectrochemical Studies. Chem. Rev. 2007, 107, 4304–4330. [Google Scholar] [CrossRef] [PubMed]
- Goldet, G.; Brandmayr, C.; Stripp, S.T.; Happa, T.; Cavazza, C.; Fontecilla-Camps, J.C.; Armstrong, F.A. Electrochemical kinetic investigations of the reactions of [FeFe]-hydrogenases with carbon monoxide and oxygen: Comparing the importance of gas tunnels and active-site electronic/redox effects. J. Am. Chem. Soc. 2009, 131, 14979–14989. [Google Scholar] [CrossRef]
- Stripp, S.T.; Goldet, G.; Brandmayr, C.; Sanganas, O.; Vincent, K.A.; Haumann, M.; Armstong, F.A.; Happe, T. How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc. Natl. Acad. Sci. USA 2009, 106, 17331–17336. [Google Scholar] [CrossRef]
- Ghirardi, M.L.; Posewitz, M.C.; Maness, P.-C.; Dubini, A.; Yu, J.; Seibert, M. Hydrogenases and Hydrogen Photoproduction in Oxygenic Photosynthetic Organisms. Annu. Rev. Plant Biol. 2007, 58, 71–91. [Google Scholar] [CrossRef]
- Stripp, S.T.; Happe, T. How algae produce hydrogen—News from the photosynthetic hydrogenase. Dalt. Trans. 2009, 9960. [Google Scholar] [CrossRef]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Giannelli, L. Advances in the biotechnology of hydrogen production with the microalga Chlamydomonas reinhardtii. Crit. Rev. Biotechnol. 2015, 35, 485–496. [Google Scholar] [CrossRef]
- Giannelli, L.; Scoma, A.; Torzillo, G. Interplay between light intensity, chlorophyll concentration and culture mixing on the hydrogen production in sulfur-deprived Chlamydomonas reinhardtii cultures grown in laboratory photobioreactors. Biotechnol. Bioeng. 2009, 104, 76–90. [Google Scholar] [CrossRef]
- Cohen, J.; Kim, K.; King, P.; Seibert, M.; Schulten, K. Finding gas diffusion pathways inproteins: Application to O2 and H2 transport in CpI [FeFe]-hydrogenase and the role of packing defects. Structure 2005, 13, 1321–1329. [Google Scholar] [CrossRef]
- Cohen, J.; Kim, K.; Posewitz, M.; Ghirardi, M.L.; Schulten, K.; Seibert, M.; King, P. Molecular dynamics and experimental investigation of H2 and O2 diffusion in [Fe]-hydrogenase. Biochem. Soc. Trans. 2005, 33, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Hosler, J.P.; Johnson, A.M.; Jones, A.R.; Randolph-Anderson, B.L.; Robertson, D.; Klein, T.M.; Shark, K.B.; et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 1988, 240, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Blowers, A.D.; Bogorad, L.; Shark, K.B.; Sanford, J.C. Studies on Chlamydomonas chloroplast transformation: Foreign DNA can be stably maintained in the chromosome. Plant Cell 1989, 1, 123–132. [Google Scholar] [PubMed]
- Kindle, K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Methods Enzymol. 1998, 297, 27–38. [Google Scholar]
- Lumbreras, V.; Stevens, D.R.; Purton, S. Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J. 1998, 14, 441–447. [Google Scholar] [CrossRef]
- Fontecilla-Camps, J.C.; Amara, P.; Cavazza, C.; Nicolet, Y.; Volbeda, A. Structure-function relationships of anaerobic gas-processing metalloenzymes. Nature 2009, 460, 814–822. [Google Scholar] [CrossRef]
- Ogata, H.; Lubitz, W.; Higuchi, Y. [NiFe] hydrogenases: Structural and spectroscopic studies of the reaction mechanism. Dalt. Trans. 2009, 7577–7587. [Google Scholar] [CrossRef]
- Happe, R.P.; Roseboom, W.; Plerlk, A.J.; Albracht, S.P.J.; Bagley, K.A. Biological activition of hydrogen. Nature 1997, 385, 126. [Google Scholar] [CrossRef]
- Pierik, A.J.; Roseboom, W.; Happe, R.P.; Bagley, K.A.; Albracht, S.P.J. Carbon monoxide and cyanide as intrinsic ligands to iron in the active site of [NiFe]-hydrogenases. NiFe(CN)2CO, biology’s way to activate H2. J. Biol. Chem. 1999, 274, 3331–3337. [Google Scholar] [CrossRef]
- Böck, A.; King, P.W.; Blokesch, M.; Posewitz, M.C. Maturation of Hydrogenases. Adv. Microb. Physiol. 2006, 51, 1–71. [Google Scholar]
- Vargas, W.A.; Weyman, P.D.; Tong, Y.; Smith, H.O.; Xu, Q. [NiFe] Hydrogenase from Alteromonas macleodii with unusual stability in the presence of oxygen and high temperature. Appl. Environ. Microbiol. 2011, 77, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Weyman, P.D.; Vargas, W.A.; Chuang, R.Y.; Chang, Y.; Smith, H.O.; Xu, Q. Heterologous expression of Alteromonas macleodii and Thiocapsa roseopersicina [NiFe] hydrogenases in Escherichia coli. Microbiology 2011, 157, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Yonemoto, I.T.; Matteri, C.W.; Nguyen, T.A.; Smith, H.O.; Weyman, P.D. Dual organism design cycle reveals small subunit substitutions that improve [NiFe] hydrogenase hydrogen evolution. J. Biol. Eng. 2013, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maroti, G.; Tong, Y.; Yooseph, S.; Baden-Tillson, H.; Smith, H.O.; Kovacs, K.L.; Frazier, M.; Venter, J.C.; Xu, Q. Discovery of [NiFe] hydrogenase genes in metagenomic DNA: Cloning and heterologous expression in Thiocapsa roseopersicina. Appl. Environ. Microbiol. 2009, 75, 5821–5830. [Google Scholar] [CrossRef]
- Mura, G.M.; Pedroni, P.; Pratesi, C.; Galli, G.; Serbolisca, L.; Grandit, G. The [Ni-Fe] hydrogenase from the thermophilic bacterium Acetornicrobiurn flavidurn. Microbiology 1996, 1553, 829–836. [Google Scholar] [CrossRef]
- Hatchikian, E.C.; Bruschi, M.; Le Gall, J. Characterization of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 1978, 82, 451–461. [Google Scholar] [CrossRef]
- Rousset, M.; Magro, V.; Forget, N.; Guigliarelli, B.; Belaich, J.P.; Hatchikian, E.C. Heterologous expression of the Desulfovibrio gigas [NiFe] hydrogenase in Desulfovibrio fructosovorans MR400. J. Bacteriol. 1998, 180, 4982–4986. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, B.; Cha, H. Production of biohydrogen by recombinant expression of [NiFe]-hydrogenase 1 in Escherichia coli. Microb. Cell Fact. 2010, 9, 54. [Google Scholar] [CrossRef]
- Kim, J.Y.H.; Jo, B.H.; Cha, H.J. Production of biohydrogen by heterologous expression of oxygen-tolerant Hydrogenovibrio marinus [NiFe]-hydrogenase in Escherichia coli. J. Biotechnol. 2011, 155, 312–319. [Google Scholar] [CrossRef]
- Soboh, B.; Lindenstrauss, U.; Granish, C.; Javed, M.; Herzberg, M.; Thomas, C.; Stripp, S.T. [NiFe]-hydrogenase maturation in vitro: Analysis of the roles of the HybG and HypD accessory proteins. Biochem. J. 2014, 464, 169–177. [Google Scholar] [CrossRef]
- Maier, T.; Böck, A. Generation of active [NiFe] hydrogenase in vitro from a nickel-free precursor form. Biochemistry 1996, 35, 10089–10093. [Google Scholar] [CrossRef] [PubMed]
- Raleiras, P.; Khanna, N.; Miranda, H.; Mészáros, L.S.; Krassen, H.; Ho, F.; Battchikova, N.; Aro, E.-M.; Magnuson, A.; Lindblad, P.; et al. Turning around the electron flow in an uptake hydrogenase. EPR spectroscopy and in vivo activity of a designed mutant in HupSL from Nostoc punctiforme. Energy Environ. Sci. 2016, 9, 581–594. [Google Scholar] [CrossRef]
- Sun, J.; Hopkins, R.C.; Jenney, F.E.; McTernan, P.M.; Adams, M.W.W. Heterologous Expression and Maturation of an NADP-Dependent [NiFe]-Hydrogenase: A Key Enzyme in Biofuel Production. PLoS ONE 2010, 5, e10526. [Google Scholar] [CrossRef] [PubMed]
- Schink, B.; Schlegel, H.G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. BBA—Enzymol. 1979, 567, 315–324. [Google Scholar] [CrossRef]
- Hartmann, S.; Frielingsdorf, S.; Ciaccafava, A.; Lorent, C.; Fritsch, J.; Seibert, E.; Priebe, J.; Haumann, M.; Zebger, I.; Lenz, O. O2 -tolerant H2 activation by an isolated large subunit of a [NiFe] hydrogenase. Biochemistry 2018, 57, 5339–5349. [Google Scholar]
- Lenz, O.; Gleiche, A.; Strack, A.; Friedrich, B. Requirements for heterologous production of a complex metalloenzyme: The membrane-bound [NiFe] hydrogenase. J. Bacteriol. 2005, 187, 6590–6595. [Google Scholar] [CrossRef]
- Buhrke, T.; Lenz, O.; Krauss, N.; Friedrich, B. Oxygen tolerance of the H2-sensing [NiFe] hydrogenase from Ralstonia eutropha H16 is based on limited access of oxygen to the active site. J. Biol. Chem. 2005, 280, 23791–23796. [Google Scholar] [CrossRef]
- Schiffels, J.; Pinkenburg, O.; Schelden, M.; Aboulnaga, E.H.A.A.; Baumann, M.E.M.; Selmer, T. A innovative cloning platform enables large-scale production and maturation of an oxygen-tolerant [NiFe]-hydrogenase from Cupriavidus necator in Escherichia coli. PLoS ONE 2013, 8, e68812. [Google Scholar] [CrossRef]
- Massanz, C.; Schmidt, S.; Friedrich, B. Subforms and in vitro reconstitution of the NAD-reducing hydrogenase of Alcaligenes eutrophus. J. Bacteriol. 1998, 180, 1023–1029. [Google Scholar] [CrossRef]
- Porthun, A.; Bernhard, M.; Friedrich, B. Expression of a functional NAD-reducing [NiFe] hydrogenase from the gram-positive Rhodococcus opacus in the gram-negative Ralstonia eutropha. Arch. Microbiol. 2002, 177, 159–166. [Google Scholar] [CrossRef]
- Wells, M.A.; Mercer, J.; Mott, R.A.; Pereira-Medrano, A.G.; Burja, A.M.; Radianingtyas, H.; Wright, P.C. Engineering a non-native hydrogen production pathway into Escherichia coli via a cyanobacterial [NiFe] hydrogenase. Metab. Eng. 2011, 13, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Grzeszik, C.; Lübbers, M.; Reh, M.; Schlegel, H.G. Genes encoding the NAD-reducing hydrogenase of Rhodococcus opacus MR11. Microbiology 1997, 143, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence Analysis of the Genome of the Unicellular Cyanobacterium Synechocystis sp. Strain PCC6803. II. Sequence Determination of the Entire Genome and Assignment of Potential Protein-coding Regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Rousset, M.; Montet, Y.; Guigliarelli, B.; Forget, N.; Asso, M.; Bertrand, P.; Fontecilla-Camps, J.C.; Hatchikian, E.C. [3Fe-4S] to [4Fe-4S] cluster conversion in Desulfovibrio fructosovorans [NiFe] hydrogenase by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 11625–11630. [Google Scholar] [CrossRef]
- Báscones, E.; Imperial, J.; Ruiz-Argüeso, T.; Palacios, J.M. Generation of new hydrogen-recycling Rhizobiaceae strains by introduction of a novel hup minitransposon. Appl. Environ. Microbiol. 2000, 66, 4292–4299. [Google Scholar] [CrossRef]
- Raleiras, P.; Kellers, P.; Lindblad, P.; Styring, S.; Magnuson, A. Isolation and characterization of the small subunit of the uptake hydrogenase from the cyanobacterium Nostoc punctiforme. J. Biol. Chem. 2013, 288, 18345–18352. [Google Scholar] [CrossRef]
- Casalot, L.; Rousset, M. Maturation of the [NiFe] hydrogenases. Trends Microbiol. 2001, 9, 228–237. [Google Scholar] [CrossRef]
- Kleihues, L.; Lenz, O.; Bernhard, M.; Buhrke, T.; Friedrich, B. The H2 sensor of Ralstonia eutropha is a member of the subclass of regulatory [NiFe] hydrogenases. J. Bacteriol. 2000, 182, 2716–2724. [Google Scholar] [CrossRef]
- Burgdorf, T.; Lenz, O.; Buhrke, T.; van der Linden, E.; Jones, A.K.; Albracht, S.P.; Friedrich, B. [NiFe]-hydrogenases of Ralstonia eutropha H16: Modular enzymes for oxygen-tolerant biological hydrogen oxidation. J. Mol. Microbiol. Biotechnol. 2005, 10, 181–196. [Google Scholar] [CrossRef]
- Lenz, O.; Zebger, I.; Hamann, J.; Hildebrandt, P.; Friedrich, B. Carbamoylphosphate serves as the source of CN-, but not of the intrinsic CO in the active site of the regulatory [NiFe]-hydrogenase from Ralstonia eutropha. FEBS Lett. 2007, 581, 3322–3326. [Google Scholar] [CrossRef]
- Van der Linden, E.; Burgdorf, T.; de Lacey, A.L.; Buhrke, T.; Scholte, M.; Fernandez, V.M.; Friedrich, B.; Albracht, S.P. An improved purification procedure for the soluble [NiFe]-hydrogenase of Ralstonia eutropha: New insights into its (in) stability and spectroscopic properties. J. Biol. Inorg. Chem. 2006, 11, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, M.J.; Sebastiampillai, S.; Côté, J.-P.; Hodkinson, N.; Brown, E.D.; Zamble, D.B. A whole-cell, high-throughput hydrogenase assay to identify factors that modulate [NiFe]-hydrogenase activity. J. Biol. Chem. 2019, 294, 15373–15385. [Google Scholar] [CrossRef] [PubMed]
- Soboh, B.; Krüger, S.; Kuhns, M.; Pinske, C.; Lehmann, A.; Sawers, R.G. Development of a cell-free system reveals an oxygen-labile step in the maturation of [NiFe]-hydrogenase 2 of Escherichia coli. FEBS Lett. 2010, 584, 4109–4114. [Google Scholar] [CrossRef] [PubMed]
- Vignais, P.M. H/D exchange reactions and mechanistic aspects of the hydrogenases. Coord. Chem. Rev. 2005, 249, 1677–1690. [Google Scholar] [CrossRef]
- Fontecilla-Camps, J.C.; Frey, M.; Garcin, E.; Hatchikian, C.; Montet, Y.; Piras, C.; Vernede, X.; Volbeda, A. Hydrogenase: A hydrogen-metabolizing enzyme. What do the crystal structures tell us about its mode of action? Biochimie 1997, 79, 661–666. [Google Scholar] [CrossRef]
- Albracht, S.P.J. Nickel hydrogenases: In search of the active site. BBA—Bioenerg. 1994, 1188, 167–204. [Google Scholar] [CrossRef]
- Caserta, G.; Lorent, C.; Ciaccafava, A.; Keck, M.; Breglia, R.; Greco, C.; Limberg, C.; Hildebrandt, P.; Cramer, S.P.; Zebger, I.; et al. The large subunit of the regulatory [NiFe]-hydrogenase from Ralstonia eutropha—A minimal hydrogenase? Chem. Sci. 2020, 11, 5453–5465. [Google Scholar] [CrossRef]
- Gallucci, F.; Comite, A.; Capannelli, G.; Basile, A. Steam reforming of methane in a membrane reactor: An industrial case study. Ind. Eng. Chem. Res. 2006, 45, 2994–3000. [Google Scholar] [CrossRef]
- de Jong, W. Sustainable Hydrogen Production by Thermochemical Biomass Processing. In Hydrogen Fuel; CRC Press: Boca Raton, FL, USA, 2008; pp. 197–238. [Google Scholar]
- Chen, W.-H.; Syu, Y.-J. Hydrogen production from water gas shift reaction in a high gravity (Higee) environment using a rotating packed bed. Int. J. Hydrogen Energy 2010, 35, 10179–10189. [Google Scholar] [CrossRef]
- Mohan, S.V.; Pandey, A. Biohydrogen Production: an introduction. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–24. [Google Scholar]
- Barahona, E.; Jiménez-Vicente, E.; Rubio, L.M. Hydrogen overproducing nitrogenases obtained by random mutagenesis and high-throughput screening. Sci. Rep. 2016, 6, 38291. [Google Scholar] [CrossRef]
- Krishnan, A.; Qian, X.; Ananyev, G.; Lun, D.S.; Dismukes, G.C. Rewiring of Cyanobacterial Metabolism for Hydrogen Production: Synthetic Biology Approaches and Challenges. In Synthetic Biology of Cyanobacteria; Springer: Singapore, 2018; pp. 171–213. [Google Scholar]
- Milton, R.D.; Minteer, S.D. Nitrogenase Bioelectrochemistry for Synthesis Applications. Acc. Chem. Res. 2019, 52, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Kawelke, S.; Happe, T. Light driven hydrogen production in protein based semi-artificial systems. Bioresour. Technol. 2011, 102, 8493–8500. [Google Scholar] [CrossRef]
- Utschig, L.M.; Soltau, S.R.; Tiede, D.M. Light-driven hydrogen production from Photosystem I-catalyst hybrids. Curr. Opin. Chem. Biol. 2015, 25, 1–8. [Google Scholar] [CrossRef]
- Martin, B.A.; Frymier, P.D. A Review of Hydrogen Production by Photosynthetic Organisms Using Whole-Cell and Cell-Free Systems. Appl. Biochem. Biotechnol. 2017, 183, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.R.; Berggren, G.; Bacchi, M.; Fontecave, M.; Artero, V. Mimicking hydrogenases: From biomimetics to artificial enzymes. Coord. Chem. Rev. 2014, 270, 127–150. [Google Scholar] [CrossRef]
- Caserta, G.; Roy, S.; Atta, M.; Artero, V.; Fontecave, M. Artificial hydrogenases: Biohybrid and supramolecular systems for catalytic hydrogen production or uptake. Curr. Opin. Chem. Biol. 2015, 25, 36–47. [Google Scholar] [CrossRef]
- Bren, K.L. Multidisciplinary approaches to solar hydrogen. Interface Focus 2015, 5, 20140091. [Google Scholar] [CrossRef][Green Version]
- Benemann, J. Hydrogen Production: Progress and prospects. Nat. Biotechnol. 1996, 14, 1101–1103. [Google Scholar] [CrossRef]
- Hallenbeck, P. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Vignais, P.M. Hydrogenases and H+-reduction in primary energy conservation. In Results and Problems in Cell Differentiation; Springer: Berlin, Germany, 2008; pp. 223–252. [Google Scholar]
- Vardar-Schara, G.; Maeda, T.; Wood, T.K. Metabolically engineered bacteria for producing hydrogen via fermentation. Microb. Biotechnol. 2008, 1, 107–125. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Xiu, Z.; Li, X.; Zhang, D. Stoichiometric analysis of biological hydrogen production by fermentative bacteria. Int. J. Hydrogen Energy 2006, 31, 539–549. [Google Scholar] [CrossRef]
- Levin, D.; Islam, R.; Cicek, N.; Sparling, R. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Int. J. Hydrogen Energy 2006, 31, 1496–1503. [Google Scholar] [CrossRef]
- Ust’ak, S.; Havrland, B.; Muñoz, J.O.J.; Fernández, E.C.; Lachman, J. Experimental verification of various methods for biological hydrogen production. Int. J. Hydrogen Energy 2007, 32, 1736–1741. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Zhang, Z.; Lee, D.J.; Zhou, X.; Jing, Y.; Ge, X.; Jiang, D.; Hu, J.; He, C. Photo-fermentative hydrogen production from crop residue: A mini review. Bioresour. Technol. 2017, 229, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 2007, 226, 1075–1086. [Google Scholar] [CrossRef]
- Volgusheva, A.A.; Jokel, M.; Allahverdiyeva, Y.; Kukarshikh, G.P.; Lukashev, E.P.; Lambreva, M.D.; Krendeleva, T.E.; Antal, T.K. Comparative analyses of H2 photoproduction in magnesium- and sulfur-starved Chlamydomonas reinhardtii cultures. Physiol. Plant. 2017, 161, 124–137. [Google Scholar] [CrossRef]
- Avilan, L.; Roumezi, B.; Risoul, V.; Bernard, C.S.; Kpebe, A.; Belhadjhassine, M.; Rousset, M.; Brugna, M.; Latifi, A. Phototrophic hydrogen production from a clostridial [FeFe] hydrogenase expressed in the heterocysts of the cyanobacterium Nostoc PCC 7120. Appl. Microbiol. Biotechnol. 2018, 102, 5775–5783. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Kanakagiri, S.; Jin, E.S.; Masuda, T.; Melis, A. Truncated chlorophyll antenna size of the photosystems—A practical method to improve microalgal productivity and hydrogen production in mass culture. Int. J. Hydrogen Energy 2002, 27, 1257–1264. [Google Scholar] [CrossRef]
- Singh, H.; Das, D. Biofuels from Microalgae: Biohydrogen. In Green Energy and Technology; Springer: Berlin, Germany, 2018; pp. 201–228. [Google Scholar]
- Kim, Y.M.; Cho, H.-S.; Jung, G.Y.; Park, J.M. Engineering the pentose phosphate pathway to improve hydrogen yield in recombinant Escherichia coli. Biotechnol. Bioeng. 2011, 108, 2941–2946. [Google Scholar] [CrossRef]
- Wong, Y.M.; Wu, T.Y.; Ling, T.C.; Show, P.L.; Lee, S.Y.; Chang, J.S.; Ibrahim, S.; Juan, J.C. Evaluating new bio-hydrogen producers: Clostridium perfringens strain JJC, Clostridium bifermentans strain WYM and Clostridium sp. strain Ade.TY. J. Biosci. Bioeng. 2018, 125, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Hemschemeier, A.; Melis, A.; Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res. 2009, 102, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, K.; Chen, X.; Schreiber, K.; Kaspar, U.; Makam, S.; Appel, J. The Bidirectional NiFe-hydrogenase in Synechocystis sp. PCC 6803 Is Reduced by Flavodoxin and Ferredoxin and Is Essential under Mixotrophic, Nitrate-limiting Conditions. J. Biol. Chem. 2014, 289, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef]
- Karube, I.; Urano, N.; Yamada, T.; Hirochika, H.; Sakaguchi, K. Cloning and expression of the hydrogenase gene from Clostridium butyricum in Escherichia coli. FEBS Lett. 1983, 158, 119–122. [Google Scholar] [CrossRef][Green Version]
- Lee, S.Y.; Lee, H.J.; Park, J.M.; Lee, J.H.; Park, J.S.; Shin, H.S.; Kim, Y.H.; Min, J. Bacterial hydrogen production in recombinant Escherichia coli harboring a HupSL hydrogenase isolated from Rhodobacter sphaeroides under anaerobic dark culture. Int. J. Hydrogen Energy 2010, 35, 1112–1116. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Jones, P.R. Engineering of a synthetic hydF–hydE–hydG–hydA operon for biohydrogen production. Anal. Biochem. 2008, 373, 170–172. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Jones, P.R. Construction of a synthetic YdbK-dependent pyruvate: H2 pathway in Escherichia coli BL21 (DE3). Metab. Eng. 2009, 11, 139–147. [Google Scholar] [CrossRef]
- Mishra, J.; Khurana, S.; Kumar, N.; Ghosh, A.K.; Das, D. Molecular cloning, characterization, and overexpression of a novel [Fe]-hydrogenase isolated from a high rate of hydrogen producing Enterobacter cloacae IIT-BT 08. Biochem. Biophys. Res. Commun. 2004, 324, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Chittibabu, G.; Nath, K.; Das, D. Feasibility studies on the fermentative hydrogen production by recombinant Escherichia coli BL21. Process Biochem. 2006, 41, 682–688. [Google Scholar] [CrossRef]
- Maeda, T.; Vardar, G.; Self, W.T.; Wood, T.K. Inhibition of hydrogen uptake in Escherichia coli by expressing the hydrogenase from the cyanobacterium Synechocystis sp. PCC 6803. BMC Biotechnol. 2007, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Heberle, J.; Riesle, J.; Thiedemann, G.; Oesterhelt, D.; Dencher, N.A. Proton migration along the membrane surface and retarded surface to bulk transfer. Nature 1994, 370, 379–382. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, B.H.; Jo, Y.; Cha, H.J. Improved production of biohydrogen in light-powered Escherichia coli by co-expression of proteorhodopsin and heterologous hydrogenase. Microb. Cell Fact. 2012, 11, 2. [Google Scholar] [CrossRef]
- Honda, Y.; Hagiwara, H.; Ida, S.; Ishihara, T. Application to Photocatalytic H2 Production of a Whole-Cell Reaction by Recombinant Escherichia coli Cells Expressing [FeFe]-Hydrogenase and Maturases Genes. Angew. Chem. 2016, 128, 8177–8180. [Google Scholar] [CrossRef]
- Masukawa, H.; Sakurai, H.; Hausinger, R.P.; Inoue, K. Sustained photobiological hydrogen production in the presence of N2 by nitrogenase mutants of the heterocyst-forming cyanobacterium Anabaena. Int. J. Hydrogen Energy 2014, 39, 19444–19451. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Y.; Gao, R.; Tong, J.; Yang, Z. Transferring [NiFe] hydrogenase gene from Rhodopeseudomonas palustris into E. coli BL21(DE3) for improving hydrogen production. Int. J. Hydrogen Energy 2015, 40, 4329–4336. [Google Scholar] [CrossRef]
- Lenz, O.; Lauterbach, L.; Frielingsdorf, S.; Friedrich, B. Oxygen-tolerant hydrogenases and their biotechnological potential. In Biohydrogen; De Gruyter: Berlin, Germany, 2015; pp. 61–96. [Google Scholar]
- Plumeré, N.; Ruediger, O.; Oughli, A.A.; Williams, R.; Vivekananthan, J.; Poeller, S.; Schuhmann, W.; Lubitz, W. A redox hydrogel protects hydrogenase from high-potential deactivation and oxygen damage. Nat. Chem. 2014, 6, 822–827. [Google Scholar] [CrossRef]
- Fourmond, V.; Stapf, S.; Li, H.; Buesen, D.; Birrell, J.A.; Ruediger, O.; Lubitz, W.; Schuhmann, W.; Plumere, N.; Leger, C. Mechanism of protection of catalysts supported in redox hydrogel films. J. Am. Chem. Soc. 2015, 137, 5494–5505. [Google Scholar] [CrossRef]
- Oughli, A.A.; Ruff, A.; Boralugodage, N.P.; Rodriguez-Macia, P.; Plumere, N.; Lubitz, W.; Shaw, W.J.; Schuhmann, W.; Ruediger, O. Dual properties of a hydrogen oxidation Ni-catalyst entrapped within a polymer promote self-defense against oxygen. Nat. Commun. 2018, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Buesen, D.; Dementin, S.; Léger, C.; Fourmond, V.; Plumeré, N. Complete Protection of O2-Sensitive Catalysts in Thin Films. J. Am. Chem. Soc. 2019, 141, 16734–16742. [Google Scholar] [CrossRef] [PubMed]
- Rumpel, S.; Siebel, J.F.; Diallo, M.; Farès, C.; Reijerse, E.J.; Lubitz, W. Structural Insight into the Complex of Ferredoxin and [FeFe] Hydrogenase from Chlamydomonas reinhardtii. ChemBioChem 2015, 16, 1663–1669. [Google Scholar] [CrossRef] [PubMed]

| Hydrogenase | Expression Host Cofactor | Hyd Yield [mg/g] | Specific Activity [U mg−1] | Ref. |
|---|---|---|---|---|
| DthHmdII | E. coli + MmaHmd FeGP | NR | 8 a | [35] |
| MjaHmd | M. jannaschii | NR | 350 § | [31] |
| MjaHmdII | E. coli + MmaHmd FeGP | NR | 5 a | [35] |
| MjaHmd | E. coli + DthHmdII FeGP | NR | 42 | [35] |
| MjaHmd | E. coli + MmaHmd FeGP | 2.5 | 370–1100 § | [31,36,37] |
| MkaHmd | M. kandleri | 0.4 | 360 § | [32] |
| MkaHmd | E. coli + MjaHmd FeGP | NR | 1100 *,§ | [31] |
| [Fe]-MjaHmd | E. coli + Fe(II)-complex | NR | 2.5 | [37] |
| [Mn]-MjaHmd | E. coli + Mn(I)-complex | NR | 1.5 | [37] |
| Hydrogenase | Host for Recombinant Production | Origin of the Maturation Proteins | Hyd Yield [mg/L] | Whole-Cell Activity | Specific Activity of Purified Enzyme | Ref. |
|---|---|---|---|---|---|---|
| CacHydA | C. acetobutylicum | Host | NR | NR | 162 b | [63] |
| CacHydA | E. coli | C. acetobutylicum | >1 | 96 a | 75 b | [64] |
| CacHydA | E. coli ΔiscR | C. acetobutylicum | 0.003 | 1.3 a,* | 96 b | [65] |
| CacHydA | S. elongatus | C. reinhardtii | NR | NR | 0.05 b | [66] |
| CacHydB | E. coli | C. acetobutylicum | NR | NR | 8.6 b | [64] |
| CbuHydA | E. coli | Host | NR | 500 a | NR | [67] |
| CpaHydA | C. pasteurianum | Host | NR | 1681 a | 1236 b | [68] |
| CpaHydA | in vitro | S. oneidensis | NR | NA | 242 b | [69] |
| CpaHydI | E. coli | S. oneidensis | 8 | NR | 1087 b | [70] |
| CpaHydI | in vitro | S. oneidensis | NR | NA | 1000 a | [71] |
| CpaHydI | in vitro | S. oneidensis | NR | NR | 2000 b | [72] |
| CpaHydI | in vitro | - | NR | NR | 2000 b | [73] |
| CpaHydI | Synechococcus sp. | Host | NR | NR | 4.6 b | [74] |
| CreHydA1 | C. reinhardtii | Host | 0.07 | 13.8 b,* | 741 b | [75] |
| CreHydA1 | C. acetobutylicum | Host | 0.1–1.0 | NR | 625–760 b | [76], [77] |
| CreHydA1 | E. coli | C. reinhardtii | low | NR | 0.4 b | [78] |
| CreHydA1 | E. coli | C. acetobutylicum | 0.8–1.0 | 61a | 150 b | [64] |
| CreHydA1-Fd | E. coli | C. acetobutylicum | NR | NR | 1000 b | [79] |
| CreHydA1 | E. coli | S. oneidensis | 30 | NR | 641 b | [70] |
| CreHydA1 | in vitro | C. reinhardtii | NR | NR | 600 b | [80] |
| CreHydA1 | in vitro | - | NR | NR | 700 b | [81] |
| CreHydA1 | S. oneidensis | Host | 0.4–0.5 | NA | 740 b | [82] |
| CreHydA1 | Synechocystis sp. | Host | NR | NR | 0.1 b | [83] |
| CreHydA2 | E. coli | C. acetobutylicum | 0.8–1.0 | 108 a | 116 b | [64] |
| CsaHydA | in vitro | C. acetobutylicum | NR | NR | 2.5 b | [84] |
| CsuHydA | E. coli | S. oneidensis | NR | NA | 6.5 b | [85] |
| EhaHyd | E. coli | Host | NR | NR | 70 b | [86] |
| EhiHyd | E. coli | Host | NR | NR | 0.04 b | [87] |
| PgrHyd | E. coli | Host | NR | NR | 2131 b | [88] |
| SobHydA1 | C. acetobutylicum | Host | NR | NR | 633 b | [76] |
| SonHydA | Anabaena sp. | S. oneidensis | NR | NR | 0.06 b | [89] |
| Hydrogenase | Host for Recombinant Production | Origin of the Maturation Proteins | Hyd Yield | Whole-Cell Activity | Specific Activity of Purified Enzyme | Ref. |
|---|---|---|---|---|---|---|
| AmaHynSL | A. macleodi ΔHynSL | Host | NR | 0.03 a,* | 0.1 c | [113] |
| AmaHynSL | E. coli | A. macleodii | NR | 3–70 × 10−3 a,* | NR | [114,115] |
| AmaHyaAB | T. roseopersicina | Host, A. macleodii | NR | 5 × 10−3 a | NR | [116] |
| AflHydSL | E. coli | Host | 2.3 f | NA | 77 a | [117] |
| DgiHynAB | D. gigas ΔHynAB | Host | NR | 1.9 a,* | 91 a | [118] |
| DgiHynAB | D. fructosovorans ΔHynAB | Host | NR | 0.2 b | NR | [119] |
| EcoHyd1 | E. coli ΔHyd1 | Host | NR | 4–7 × 10−2 a,* | 1–3 × 10−2 a | [120,121] |
| Eco Hyd1/2 | in vitro | E. coli | NR | NR | 192 e | [122] |
| EcoHyd3 HycE | in vitro | E. coli | NR | NR | 1.2 a | [123] |
| HmaMBH | E. coli | Host | NR | 0.07 a,* | 0.03 a | [121] |
| NpuHupSL | E. coli | N. punctiforme | NR | 208 a | NR | [124] |
| PfuSH | E. coli | P. furiosus | 0.8 f | 2.9 a | 100 a | [125] |
| ReuMBH | R. eutropha H16 | Host | NR | 1.0 c,* | 170 c | [126] |
| ReuMBH preHoxG | in vitro | E. coli | NR | NR | 2 × 10−3 d | [127] |
| ReuMBH | in vitro | R. eutropha | NR | NR | 0.01 d | [127] |
| ReuMBH | P. stutzeri | R. eutropha | NR | 17–19 c,* | NR | [128] |
| ReuRH | E. coli | R. eutropha | 0.3 g | NR | 0.8 b | [129] |
| ReuSH | E. coli | R. eutropha | 0.4 g | 1.2 b,* | 230 b | [130] |
| ReuSH | in vitro | R. eutropha | NR | NR | 2.7 b | [131] |
| RopSH | R. eutropha ΔSH ΔMBH | Host, R. opacus | NR | 5.9 a,* | NR | [132] |
| SynSH | E. coli | Synechocystis sp. | NR | 0.04 a,* | NR | [133] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Neubauer, P.; Lenz, O.; Gimpel, M. Heterologous Hydrogenase Overproduction Systems for Biotechnology—An Overview. Int. J. Mol. Sci. 2020, 21, 5890. https://doi.org/10.3390/ijms21165890
Fan Q, Neubauer P, Lenz O, Gimpel M. Heterologous Hydrogenase Overproduction Systems for Biotechnology—An Overview. International Journal of Molecular Sciences. 2020; 21(16):5890. https://doi.org/10.3390/ijms21165890
Chicago/Turabian StyleFan, Qin, Peter Neubauer, Oliver Lenz, and Matthias Gimpel. 2020. "Heterologous Hydrogenase Overproduction Systems for Biotechnology—An Overview" International Journal of Molecular Sciences 21, no. 16: 5890. https://doi.org/10.3390/ijms21165890
APA StyleFan, Q., Neubauer, P., Lenz, O., & Gimpel, M. (2020). Heterologous Hydrogenase Overproduction Systems for Biotechnology—An Overview. International Journal of Molecular Sciences, 21(16), 5890. https://doi.org/10.3390/ijms21165890







