Hexobarbital Sleep Test for Predicting the Susceptibility or Resistance to Experimental Posttraumatic Stress Disorder
Abstract
:1. Introduction
2. Results
2.1. Effect of Microsomal Oxidation Phenotype and Predator Stress on Rats Behavior
2.2. Effect of Microsomal Oxidation Phenotype and Predator Stress on Plasma Corticosterone Concentration
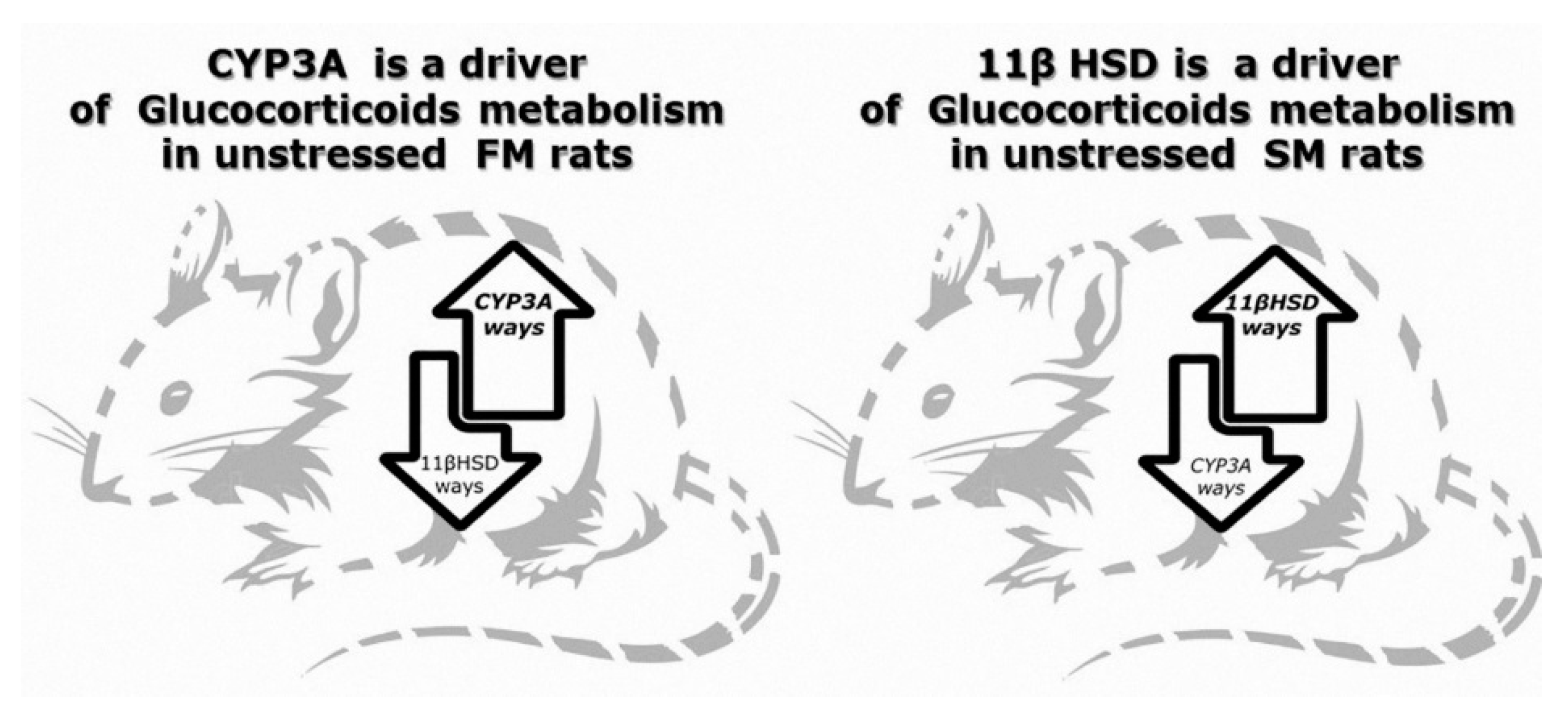
2.3. Effect of Microsomal Oxidation Phenotype and Predator Stress on Corticosterone Metabolites Concentrations
2.4. Effect of Microsomal Oxidation Phenotype and Predator Stress on Kidneys 11βHSD-2 Activity
3. Discussion
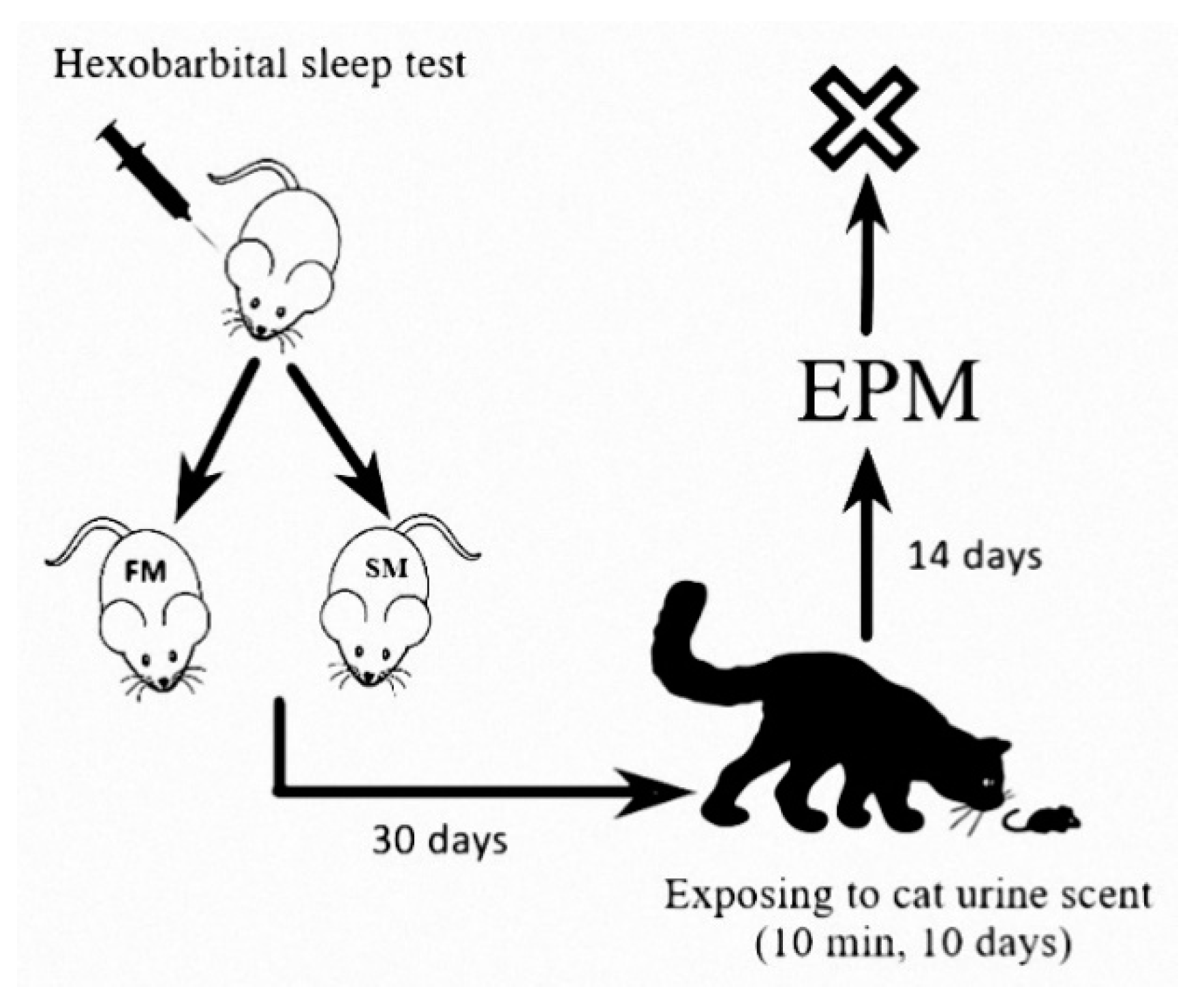
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 11Dehydro Cor | 11-dehydrocorticosterone (endogenous corticosteroid) |
| 11βHSD-2 | 11β-hydroxysteroid dehydrogenase type 2 (enzyme) |
| 6βCort | 6 beta-hydroxycorticosterone (endogenous corticosteroid) |
| AI | anxiety index |
| ANOVA | analysis of variance (collection of statistical models) |
| CYP3A | cytochrome P450 (enzyme) |
| EDTA EPM | ethylenediaminetetraacetic acid (chemical) elevated plus maze test |
| FM | fast metabolizers |
| HPLC | high performance liquid chromatography (method). |
| HST | hexobarbital sleep test |
| PSS | predator scent stress |
| PTSD | posttraumatic stress disorder |
| SM | slow metabolizers |
References
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.C.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef]
- Yehuda, R.; Seckl, J. Minireview: Stress-related psychiatric disorders with low cortisol levels: A metabolic hypothesis. Endocrinology 2011, 152, 4496–4503. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef]
- Tseilikman, O.B.; Kozochkin, D.A.; Manukhina, E.B.; Downey, H.F.; Misharina, M.E.; Komelkova, M.V.; Nikitina, A.A.; Golodnii, S.V.; Dodohova, M.A.; Tseilikman, V.E. Predicting anxiety responses to halogenated glucocorticoid drugs using the hexobarbital sleep time test. Stress 2016, 19, 390–394. [Google Scholar] [CrossRef]
- Wilson, A.M.; McFarlane, L.C.; Lipworth, B.J. Effects of low and high doses of inhaled flunisolide and triamcinolone acetonide on basal and dynamic measures of adrenocortical activity in healthy volunteers. J. Clin. Endocrinol. Metab. 1998, 83, 922–925. [Google Scholar] [CrossRef]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manukhina, E.B.; Tseilikman, V.E.; Tseilikman, O.B.; Komelkova, M.V.; Kondashevskaya, M.V.; Goryacheva, A.V.; Lapshin, M.S.; Platkovskii, P.O.; Alliluev, A.V.; Downey, H.F. Intermittent hypoxia improves behavioral and adrenal gland dysfunction induced by posttraumatic stress disorder in rats. J. Appl. Physiol. 2018, 125, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.E.; Kozochkin, D.A.; Manukhina, E.B.; Downey, H.F.; Tseilikman, O.B.; Misharina, M.E.; Nikitina, A.A.; Komelkova, M.V.; Lapshin, M.S.; Kondashevskaya, M.V.; et al. Duration of hexobarbital-induced sleep and monoamine oxidase activities in rat brain: Focus on the behavioral activity and on the free-radical oxidation. Gen. Physiol. Biophys. 2016, 35, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Dremencov, E.; Lapshin, M.; Komelkova, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Tseilikman, V. Chronic predator scent stress alters serotonin and dopamine levels in the rat thalamus and hypothalamus, respectively. Gen. Physiol. Biophys. 2019, 38, 187–190. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Conrad, C.D.; Fleshner, M.; Diamond, D.M. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress 2008, 11, 259–281. [Google Scholar] [CrossRef] [Green Version]
- Danan, D.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. Blunted basal corticosterone pulsatility predicts post-exposure susceptibility to PTSD phenotype in rats. Psychoneuroendocrinology 2018, 87, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; McLaughlin, L.D.; Nair, A.; Ebenezer, P.J.; Dange, R.; Francis, J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE 2013, 8, e76146. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Zohar, J.; Gidron, Y.; Matar, M.A.; Belkind, D.; Loewenthal, U.; Kozlovsky, N.; Kaplan, Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol. Psychiatry 2006, 59, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R. Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 1997, 821, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Draper, N.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 2005, 186, 251–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal’chikova, N.A.; Kuznetsova, N.V.; Selyatitskaya, V.G.; Cherkasova, O.P.; Kuz’mina, O.I. Effects of Intraperitoneal Administration of Mifepristone on Glucocorticoid Status of Experimental Animals. Bull. Exp. Biol. Med. 2016, 161, 257–260. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its behavioral and metabolic correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Arumugam, T.V.; Mattson, M.P. Lowering corticosterone levels reinstates hippocampal brain-derived neurotropic factor and Trkb expression without influencing deficits in hypothalamic brain-derived neurotropic factor expression in leptin receptor-deficient mice. Neuroendocrinology 2011, 93, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Tseilikman, V.; Komelkova, M.; Lapshin, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Kondashevskaya, M.; et al. High and low anxiety phenotypes in a rat model of complex post-traumatic stress disorder are associated with different alterations in regional brain monoamine neurotransmission. Psychoneuroendocrinology 2020, 117, 104691. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Sarapultsev, P.; Dremencov, E.; Komelkova, M.; Tseilikman, O.; Tseilikman, V. Low glucocorticoids in stress-related disorders: The role of inflammation. Stress 2020, 1–11. [Google Scholar] [CrossRef]
- Tseylikman, O.B.; Lapshin, M.S.; Kozochkin, D.A.; Komel’kova, M.V.; Kuzina, O.V.; Golodniy, S.V.; Lazuko, S.S.; Tseylikman, V.E. Behavioral Activity and Some Markers of Posttraumatic Stress Disorder among Serotoninergic System Indicators and Glucocorticoid Metabolizing Enzymes in Rats with Different Duration of Hexenal Sleep. Bull. Exp. Biol. Med. 2016, 161, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Kozochkin, D.A.; Manukhina, E.B.; Downey, H.F.; Tseilikman, O.B.; Komelkova, M.V.; Vasilyeva, M.V.; Lapshin, M.S.; Sahabutdinov, M.N.; Lazuko, S.S.; Tseilikman, V.E. The role of microsomal oxidation in the regulation of monoamine oxidase activity in the brain and liver of rats. Gen. Physiol. Biophys. 2017, 36, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N. The epidemiology of posttraumatic stress disorder: What is the extent of the problem? J. Clin. Psychiatry 2001, 62 (Suppl. 17), 16–22. [Google Scholar] [PubMed]
- Lazuko, S.S.; Kuzhel, O.P.; Belyaeva, L.E.; Manukhina, E.B.; Fred Downey, H.; Tseilikman, O.B.; Komelkova, M.V.; Tseilikman, V.E. Posttraumatic Stress Disorder Disturbs Coronary Tone and Its Regulatory Mechanisms. Cell. Mol. Neurobiol. 2018, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lapiz-Bluhm, M.D.S.; Bondi, C.O.; Doyen, J.; Rodriguez, G.A.; Bédard-Arana, T.; Morilak, D.A. Behavioural Assays to Model Cognitive and Affective Dimensions of Depression and Anxiety in Rats. J. Neuroendocrinol. 2008, 20, 1115–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serova, L.I.; Tillinger, A.; Alaluf, L.G.; Laukova, M.; Keegan, K.; Sabban, E.L. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience 2013, 236, 298–312. [Google Scholar] [CrossRef]
- Cohen, H.; Matar, M.A.; Joseph, Z. Animal models of post-traumatic stress disorder. Curr. Protoc. Neurosci. 2013. Chapter 9. [Google Scholar] [CrossRef]
- Selyatitskaya, V.G.; Cherkasova, O.P.; Pankina, T.V.; Palchikova, N.A. Functional state of adrenocortical system in rats with manifest alloxan-induced diabetes mellitus. Bull. Exp. Biol. Med. 2008, 146, 708–710. [Google Scholar] [CrossRef]
- Cherkasova, O.P. Activity of 11β-hydroxysteroid dehydrogenase of rat kidney and liver in inherited stress-induced arterial hypertension. Biochem. Moscow Suppl. Ser. B 2007, 1, 172–175. [Google Scholar] [CrossRef]

| FM (n = 42) | SM (n = 20) | |||
|---|---|---|---|---|
| Unstressed (n = 22) | Stressed (n = 20) | Unstressed (n = 8) | Stressed (n = 12) | |
| Time in open arms, s | 154.2 ± 24.6 | 258.5 ± 19.6 *** | 78.5 ± 16.6 # | 100.2 ± 21.4 ### |
| Time in closed arms, s | 445.8 ± 24.6 | 341.5 ± 19.6 *** | 521.5 ± 16.6 # | 499.8 ± 21.4 ### |
| Entries into open arms | 6.3 ± 0.6 | 8.7 ± 0.4 ** | 4.2 ± 0.5 # | 5.2 ± 0.6 ### |
| Entries into closed arms | 7.8 ± 0.8 | 10.6 ± 0.5 ** | 5.4 ± 0.6 # | 10.0 ± 0.9 ** |
| Anxiety index | 0.65 ± 0.02 | 0.56 ± 0.02 ** | 0.71 ± 0.02 # | 0.74 ± 0.02 ### |
| Grooming | 2.91 ± 0.55 | 2.63 ± 0.47 | 1.0 ± 0.3 | 2.5 ± 0.42 |
| Cort, nM/l | 197.4 ± 18.6 | 135.0 ± 13.1 * | 270.4 ± 37.1 # | 154.3 ± 25.3 ** |
| 11Dehydro Cort, nM/l | 1.3 ± 0.2 | 3.6 ± 0.8 | 5.5 ± 1.6 ### | 6.5 ± 1.9 |
| 6βCort in liver, nM/g | 0.28 ± 0.01 | 0.25 ± 0.02 | 0.14 ± 0.04 ## | 0.24 ± 0.04 * |
| 6βCort in blood, nM/l | 0.39 ± 0.05 | 0.65 ± 0.1 ** | 0.45 ± 0.04 | 0.52 ± 0.03 |
| 11βHSD-2, nmol min–1 g–1 | 9.73 ± 0.46 | 10.07 ± 0.94 | 13.45 ± 0.67 ## | 11.26 ± 0.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komelkova, M.; Manukhina, E.; Downey, H.F.; Sarapultsev, A.; Cherkasova, O.; Kotomtsev, V.; Platkovskiy, P.; Fedorov, S.; Sarapultsev, P.; Tseilikman, O.; et al. Hexobarbital Sleep Test for Predicting the Susceptibility or Resistance to Experimental Posttraumatic Stress Disorder. Int. J. Mol. Sci. 2020, 21, 5900. https://doi.org/10.3390/ijms21165900
Komelkova M, Manukhina E, Downey HF, Sarapultsev A, Cherkasova O, Kotomtsev V, Platkovskiy P, Fedorov S, Sarapultsev P, Tseilikman O, et al. Hexobarbital Sleep Test for Predicting the Susceptibility or Resistance to Experimental Posttraumatic Stress Disorder. International Journal of Molecular Sciences. 2020; 21(16):5900. https://doi.org/10.3390/ijms21165900
Chicago/Turabian StyleKomelkova, Maria, Eugenia Manukhina, H. Fred Downey, Alexey Sarapultsev, Olga Cherkasova, Viacheslav Kotomtsev, Pavel Platkovskiy, Stanislav Fedorov, Petr Sarapultsev, Olga Tseilikman, and et al. 2020. "Hexobarbital Sleep Test for Predicting the Susceptibility or Resistance to Experimental Posttraumatic Stress Disorder" International Journal of Molecular Sciences 21, no. 16: 5900. https://doi.org/10.3390/ijms21165900
APA StyleKomelkova, M., Manukhina, E., Downey, H. F., Sarapultsev, A., Cherkasova, O., Kotomtsev, V., Platkovskiy, P., Fedorov, S., Sarapultsev, P., Tseilikman, O., Tseilikman, D., & Tseilikman, V. (2020). Hexobarbital Sleep Test for Predicting the Susceptibility or Resistance to Experimental Posttraumatic Stress Disorder. International Journal of Molecular Sciences, 21(16), 5900. https://doi.org/10.3390/ijms21165900





