Sperm Proteome after Interaction with Reproductive Fluids in Porcine: From the Ejaculation to the Fertilization Site
Abstract
:1. Introduction
2. Results
2.1. Proteins Identified in Different Biological Fluids
2.2. Identification of Sperm-Interacting Proteins According to Incubation with Different Reproductive Biological Fluids
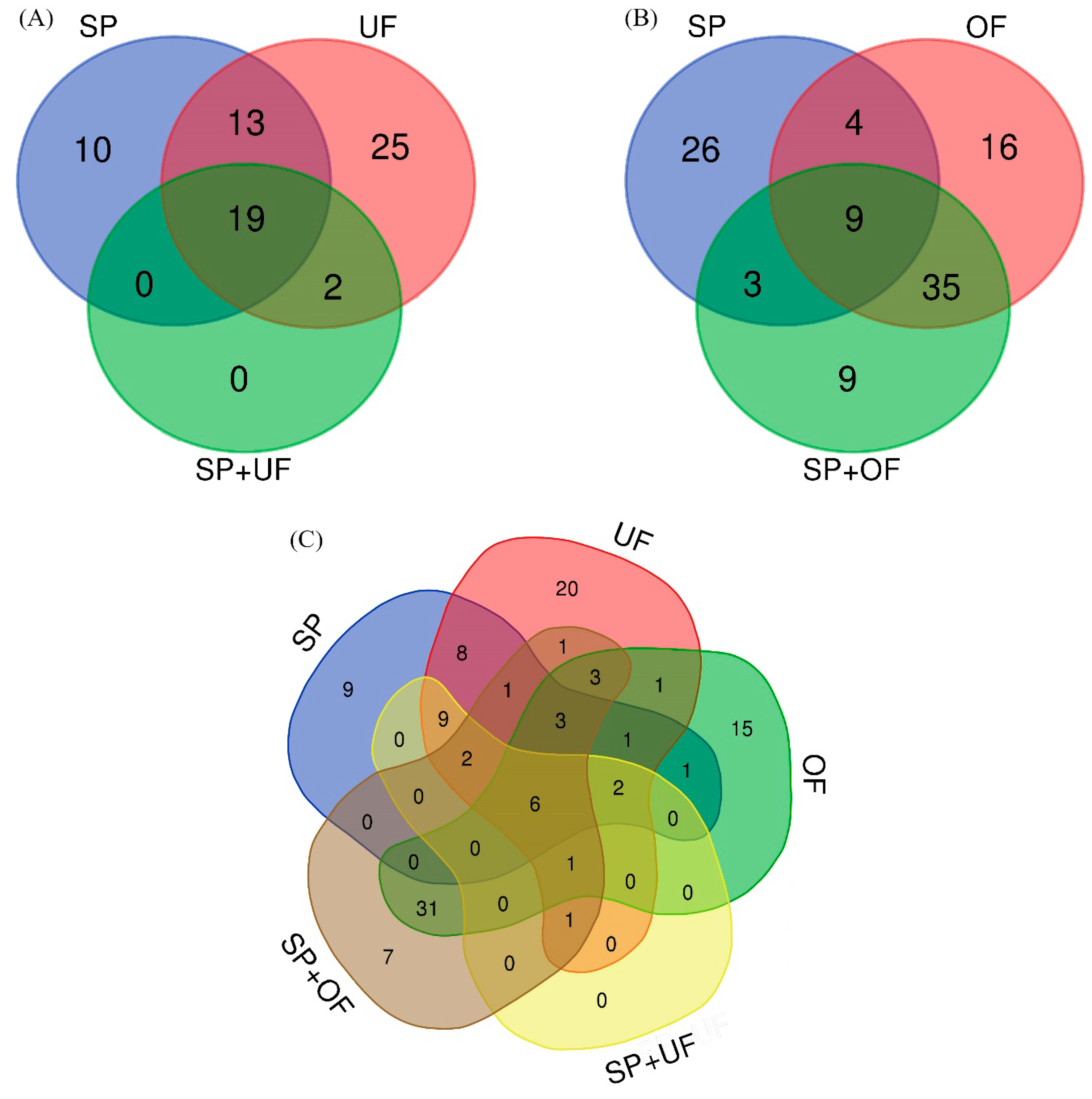
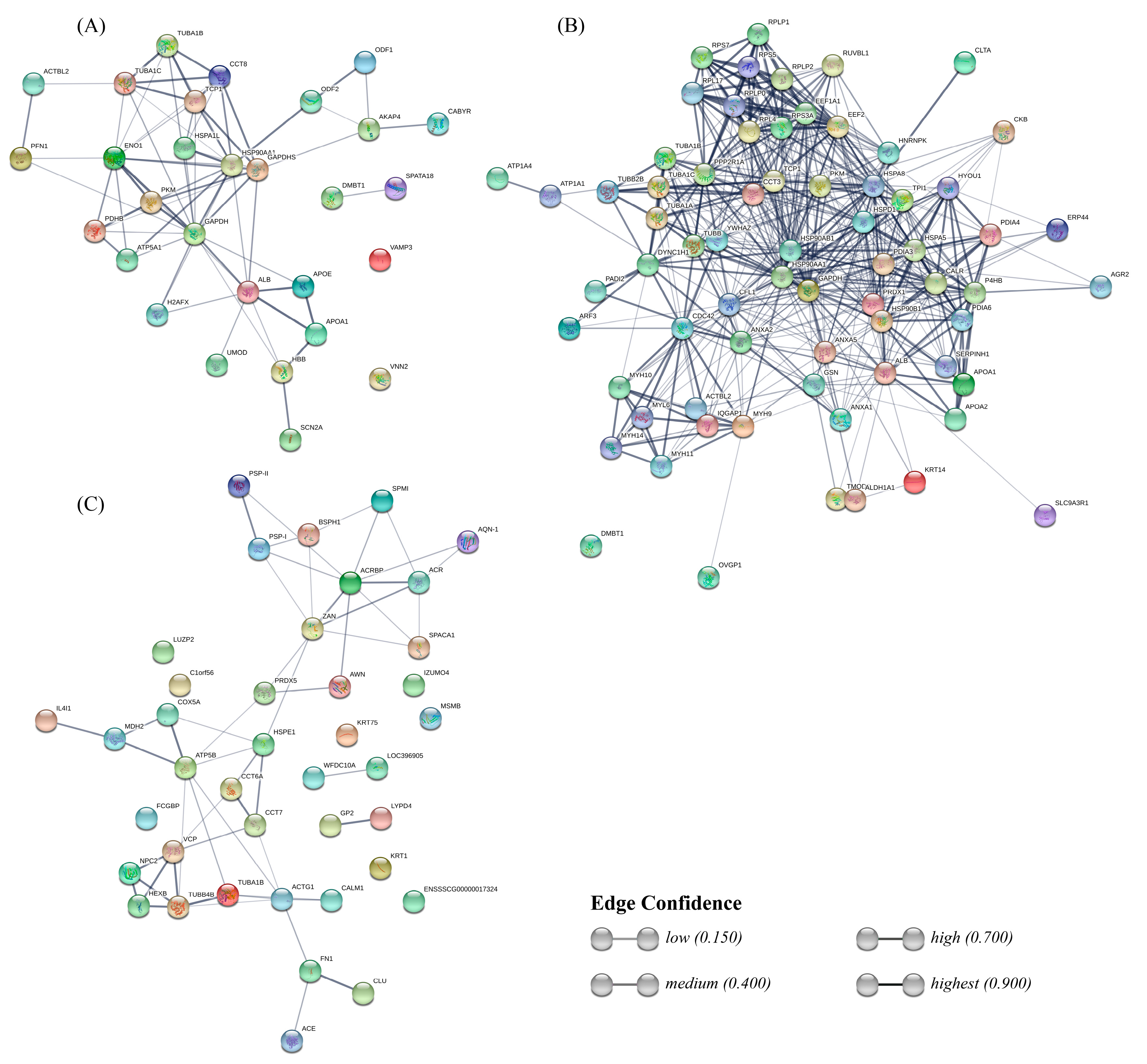
2.2.1. Sperm-Interacting Proteins after Incubation with UF and/or SP
2.2.2. Sperm-Interacting Proteins after Incubation with OF and/or SP
2.2.3. Sperm-Interacting Proteins after Incubation with Different Reproductive Fluids and Their Relationship
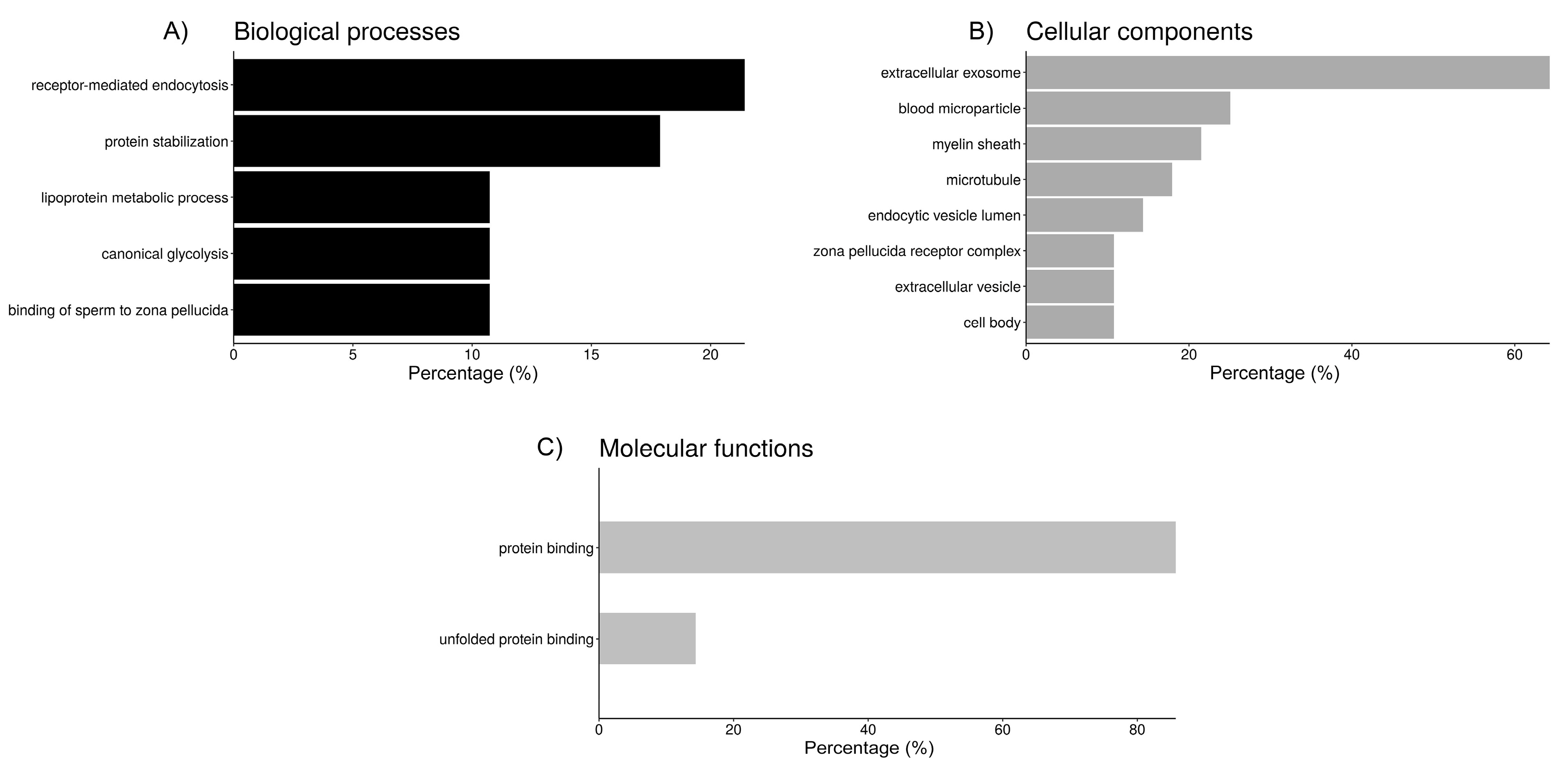
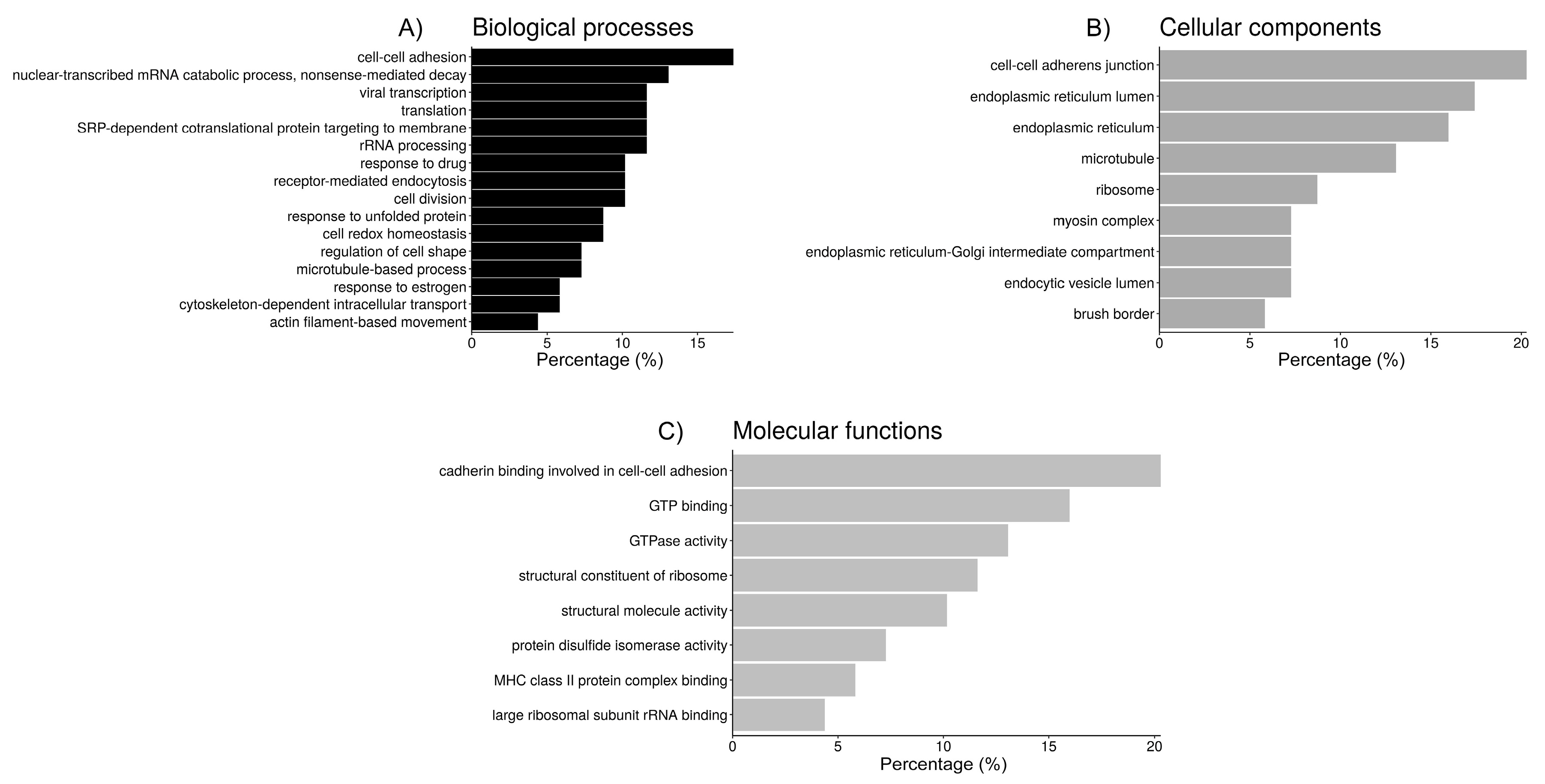
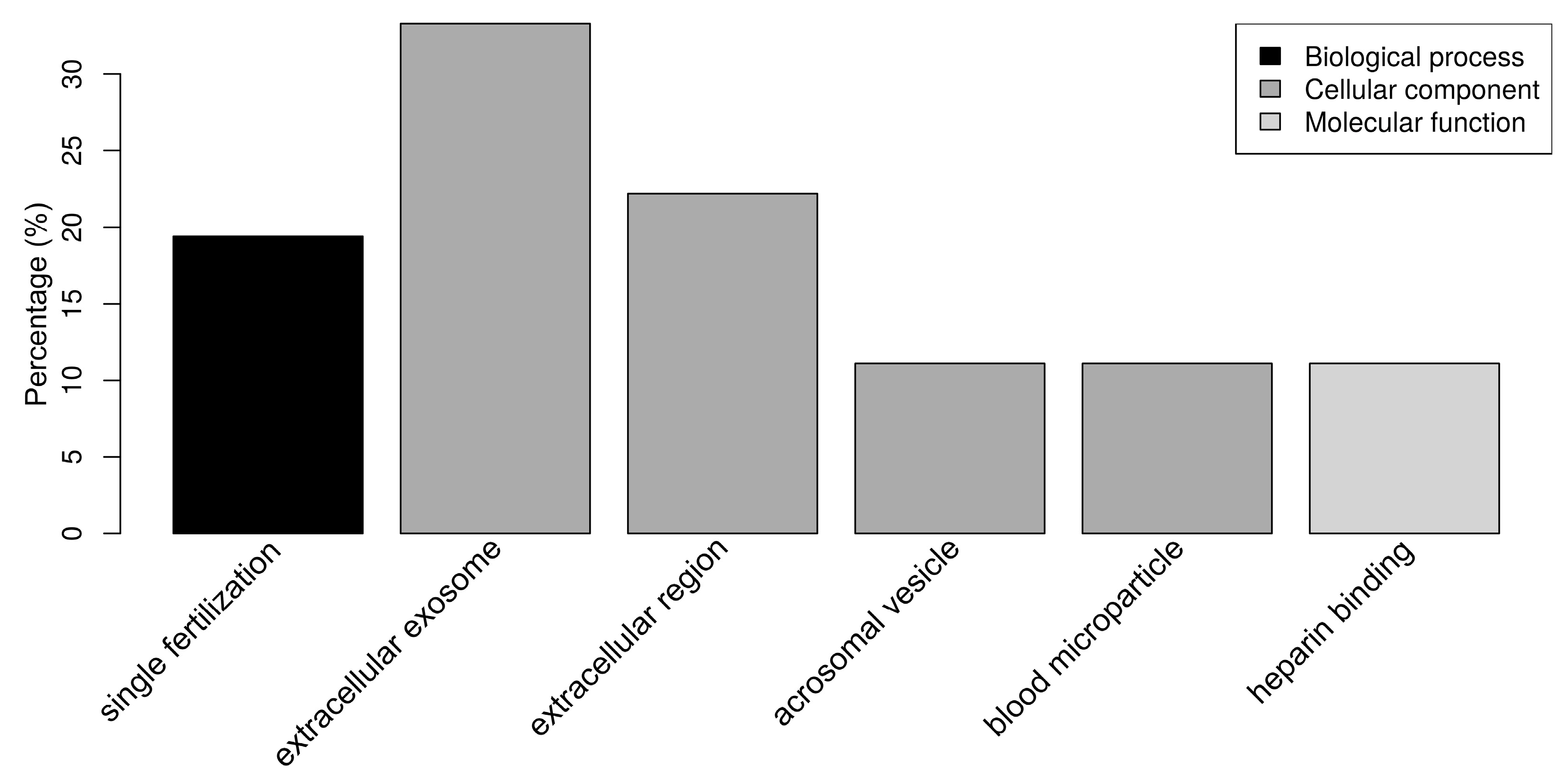
2.3. Functional Analysis of Sperm-Interacting Proteins
3. Discussion
3.1. Uterine Fluid (UF)-Sperm Protein Interaction
3.2. Oviductal Fluid (OF)–Sperm Protein Interaction
3.3. Seminal Plasma (SP)-Sperm Protein Interaction
3.4. Common Sperm Proteins after Interaction with Different Reproductive Fluids
4. Material and Methods
4.1. Ethics
4.2. Ejaculated Spermatozoa Collection
4.3. Collection and Preparation of Biological Fluids (SP, UF, and OF)
4.4. Spermatozoa Incubation with Biological Fluids
4.5. Protein Extraction
4.6. Trypsin Digestion
4.7. High-Performance Liquid Chromatography-Mass Spectrometry Analysis (HPLC-MS/MS Analysis)
4.8. Bioinformatic Analysis
4.8.1. Venn Diagram
4.8.2. Annotation of Human Homologs and Gene Ontology Analysis
4.8.3. Protein-Protein Interaction Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunter, R.H.F. Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J. Reprod. Fertil. 1981, 63, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Holt, W.V.; Van Look, K.J.W. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 2004, 127, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Kawano, N.; Araki, N.; Yoshida, K.; Hibino, T.; Ohnami, N.; Makino, M.; Kanai, S.; Hasuwa, H.; Yoshida, M.; Miyado, K.; et al. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc. Natl. Acad. Sci. USA 2014, 111, 4145–4150. [Google Scholar] [CrossRef] [Green Version]
- Viring, S.; Einarsson, S. Sperm distribution within the genital tract of naturally inseminated gilts. Nord. Vet. Med. 1981, 33, 145–149. [Google Scholar] [PubMed]
- Taylor, U.; Schuberth, H.J.; Rath, D.; Michelmann, H.W.; Sauter-Louis, C.; Zerbe, H. Influence of inseminate components on porcine leucocyte migration in vitro and in vivo after pre- and post-ovulatory insemination. Reprod. Domest. Anim. 2009, 44, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, A.; Engel, B.; Woelders, H. Neutrophil recruitment and phagocytosis of boar spermatozoa after artificial insemination of sows, and the effects of inseminate volume, sperm dose and specific additives in the extender. Reproduction 2003, 125, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Sumransap, P.; Tummaruk, P.; Kunavongkrit, A. Sperm distribution in the reproductive tract of sows after intrauterine insemination. Reprod. Domest. Anim. 2007, 42, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Tummaruk, P.; Tienthai, P. Number of Spermatozoa in the Crypts of the Sperm Reservoir at About 24 h After a Low-Dose Intrauterine and Deep Intrauterine Insemination in Sows. Reprod. Domest. Anim. 2010, 45, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cerezales, S.; Ramos-Ibeas, P.; Acuña, O.S.; Avilés, M.; Coy, P.; Rizos, D.; Gutiérrez-Adán, A. The oviduct: From sperm selection to the epigenetic landscape of the embryo. Biol. Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef] [Green Version]
- Gadella, B.M. Reproductive tract modifications of the boar sperm surface. Mol. Reprod. Dev. 2017, 84, 822–831. [Google Scholar] [CrossRef] [Green Version]
- Perez-Patiño, C.; Parrilla, I.; Li, J.; Barranco, I.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. The proteome of pig spermatozoa is remodeled during ejaculation. Mol. Cell. Proteom. 2018, 18, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadella, B.M.; Luna, C. Cell biology and functional dynamics of the mammalian sperm surface. Theriogenology 2014, 81, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Al-Dossary, A.A.; Martin-Deleon, P.A. Role of exosomes in the reproductive tract Oviductosomes mediate interactions of oviductal secretion with gametes/early embryo. Front. Biosci. 2016, 21, 1278–1285. [Google Scholar]
- Garner, D.L.; Hafez, E.S.E. Spermatozoa and seminal plasma. In Reproduction in Farm Animals; Hafez, B., Havez, E.S.E., Eds.; Wiley and Sons: Hoboken, NJ, USA, 2000; pp. 96–109. ISBN 9781119265306. [Google Scholar]
- Rozeboom, K.J.; Troedsson, M.H.T.; Hodson, H.H.; Shurson, G.C.; Crabo, B.G. The importance of seminal plasma on the fertility of subsequent artificial inseminations in swine. J. Anim. Sci. 2000, 78, 443–448. [Google Scholar] [CrossRef]
- Dostál, J.; Veselský, L.; Marounek, M.; Zelezná, B.; Jonáková, V. Inhibition of bacterial and boar epididymal sperm immunogenicity by boar seminal immunosuppressive component in mice. J. Reprod. Fertil. 1997, 111, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Rozeboom, K.J.; Troedsson, M.H.; Crabo, B.G. Characterization of uterine leukocyte infiltration in gilts after artificial insemination. J. Reprod. Fertil. 1998, 114, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Rozeboom, K.J.; Troedsson, M.H.; Molitor, T.W.; Crabo, B.G. The effect of spermatozoa and seminal plasma on leukocyte migration into the uterus of gilts. J. Anim. Sci. 1999, 77, 2201–2206. [Google Scholar] [CrossRef]
- Barranco, I.; Rubér, M.; Perez-Patiño, C.; Atikuzzaman, M.; Martinez, E.A.; Roca, J.; Rodriguez-Martinez, H. The Seminal Plasma of the Boar is Rich in Cytokines, with Significant Individual and Intra-Ejaculate Variation. Am. J. Reprod. Immunol. 2015, 74, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Barranco, I.; Padilla, L.; Pérez-Patiño, C.; Vazquez, J.M.; Martínez, E.A.; Rodríguez-Martínez, H.; Roca, J.; Parrilla, I. Seminal Plasma Cytokines Are Predictive of the Outcome of Boar Sperm Preservation. Front. Vet. Sci. 2019, 6, 436. [Google Scholar] [CrossRef]
- Luongo, C.; Abril-Sánchez, S.; Hernández, J.G.; García-Vázquez, F.A. Seminal plasma mitigates the adverse effect of uterine fluid on boar spermatozoa. Theriogenology 2019, 136, 28–35. [Google Scholar] [CrossRef]
- First, N.L.; Short, R.E.; Peters, J.B.; Stratman, F.W. Transport and Loss of Boar Spermatozoa in the Reproductive Tract of the Sow. J. Anim. Sci. 1968, 27, 1037–1040. [Google Scholar] [CrossRef]
- Parada-Bustamante, A.; Oróstica, M.L.; Reuquen, P.; Zuñiga, L.M.; Cardenas, H.; Orihuela, P.A. The role of mating in oviduct biology. Mol. Reprod. Dev. 2016, 83, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Jelinkova, P.; Manaskova, P.; Ticha, M.; Jonakova, V. Proteinase inhibitors in aggregated forms of boar seminal plasma proteins. Int. J. Biol. Macromol. 2003, 32, 99–107. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal plasma proteins as markers of sperm fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Saravia, F.; Wallgren, M.; Martinez, E.A.; Sanz, L.; Roca, J.; Vazquez, J.M.; Calvete, J.J. Spermadhesin PSP-I/PSP-II heterodimer induces migration of polymorphonuclear neutrophils into the uterine cavity of the sow. J. Reprod. Immunol. 2010, 84, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leahy, T.; Gadella, B.M. Capacitation and capacitation-like sperm surface changes induced by handling boar semen. Reprod. Domest. Anim. 2011, 46 (Suppl. 2), 7–13. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, W.M.; Johnson, L.A. Physiology of spermatozoa at high dilution rates: The influence of seminal plasma. Theriogenology 1999, 52, 1353–1362. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Evans, J.; Nguyen, H.P.T.; Edgell, T.A. The Microenvironment of Human Implantation: Determinant of Reproductive Success. Am. J. Reprod. Immunol. 2016, 75, 218–225. [Google Scholar] [CrossRef]
- Rath, D.; Knorr, C.; Taylor, U. Communication requested: Boar semen transport through the uterus and possible consequences for insemination. Theriogenology 2016, 85, 94–104. [Google Scholar] [CrossRef]
- Yu, H.; Hackenbroch, L.; Meyer, F.R.L.; Reiser, J.; Razzazi-Fazeli, E.; Nöbauer, K.; Besenfelder, U.; Vogl, C.; Brem, G.; Mayrhofer, C. Identification of Rabbit Oviductal Fluid Proteins Involved in Pre-Fertilization Processes by Quantitative Proteomics. Proteomics 2019, 19, 1800319. [Google Scholar] [CrossRef]
- Soleilhavoup, C.; Riou, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Kohnke, P.; Reynaud, K.; de Graaf, S.P.; Gerard, N.; Druart, X. Proteomes of the Female Genital Tract During the Oestrous Cycle. Mol. Cell. Proteom. 2016, 15, 93–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloney, S.E.; Khan, F.A.; Chenier, T.S.; de Amorim, M.D.; Hayes, M.A.; Scholtz, E.L. A comparison of the uterine proteome of mares in oestrus and dioestrus. Reprod. Domest. Anim. 2019, 54, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Azkargorta, M.; Escobes, I.; Iloro, I.; Osinalde, N.; Corral, B.; Ibañez-Perez, J.; Exposito, A.; Prieto, B.; Elortza, F.; Matorras, R. Differential proteomic analysis of endometrial fluid suggests increased inflammation and impaired glucose metabolism in non-implantative IVF cycles and pinpoints PYGB as a putative implantation marker. Hum. Reprod. 2018, 33, 1898–1906. [Google Scholar] [CrossRef] [Green Version]
- Iritani, A.; Sato, E.; Nishikawa, Y. Secretion rates and chemical composition of oviduct and uterine fluids in sows. J. Anim. Sci. 1974, 39, 582–588. [Google Scholar] [CrossRef]
- Li, R.; Whitworth, K.; Lai, L.; Wax, D.; Spate, L.; Murphy, C.N.; Rieke, A.; Isom, C.; Hao, Y.; Zhong, Z.; et al. Concentration and composition of free amino acids and osmolalities of porcine oviductal and uterine fluid and their effects on development of porcine IVF embryos. Mol. Reprod. Dev. 2007, 74, 1228–1235. [Google Scholar] [CrossRef] [Green Version]
- Coy, P.; Cánovas, S.; Mondéjar, I.; Saavedra, M.D.; Romar, R.; Grullón, L.; Matás, C.; Avilés, M. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl. Acad. Sci. USA 2008, 105, 15809–15814. [Google Scholar] [CrossRef] [Green Version]
- Ghersevich, S.; Massa, E.; Zumoffen, C. Oviductal secretion and gamete interaction. Reproduction 2015, 149, R1–R14. [Google Scholar] [CrossRef]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef] [Green Version]
- Coy, P.; Lloyd, R.; Romar, R.; Satake, N.; Matas, C.; Gadea, J.; Holt, W.V. Effects of porcine pre-ovulatory oviductal fluid on boar sperm function. Theriogenology 2010, 74, 632–642. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Yang, X.; Kan, F.W.K. Recombinant hamster oviductin is biologically active and exerts positive effects on sperm functions and sperm-oocyte binding. PLoS ONE 2015, 10, e0123003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Rodriguez, M.; Vicente-Carrillo, A.; Rodriguez-Martinez, H. Hyaluronan improves neither the long-term storage nor the cryosurvival of liquid-stored CD44-bearing ai boar spermatozoa. J. Reprod. Dev. 2018, 64, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pini, T.; Leahy, T.; Soleilhavoup, C.; Tsikis, G.; Labas, V.; Combes-Soia, L.; Harichaux, G.; Rickard, J.P.; Druart, X.; De Graaf, S.P. Proteomic Investigation of Ram Spermatozoa and the Proteins Conferred by Seminal Plasma. J. Proteome Res. 2016, 15, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.P.; Pool, K.R.; Druart, X.; de Graaf, S.P. The fate of spermatozoa in the female reproductive tract: A comparative review. Theriogenology 2019, 137, 104–112. [Google Scholar] [CrossRef]
- Casado-Vela, J.; Rodriguez-Suarez, E.; Iloro, I.; Ametzazurra, A.; Alkorta, N.; Garcı, J.A.; Matorras, R.; Nagore, D.; Simo, L.; Elortza, F. Comprehensive Proteomic Analysis of Human Endometrial Fluid Aspirate research articles. J. Proteome Res. 2009, 8, 4622–4632. [Google Scholar] [CrossRef] [Green Version]
- Kasvandik, S.; Saarma, M.; Kaart, T.; Rooda, I.; Velthut-Meikas, A.; Ehrenberg, A.; Gemzell, K.; Lalitkumar, P.G.; Salumets, A.; Peters, M. Uterine Fluid Proteins for Minimally Invasive Assessment of Endometrial Receptivity. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Zeng, S.; Ulbrich, S.E.; Bauersachs, S. Spatial organization of endometrial gene expression at the onset of embryo attachment in pigs. BMC Genom. 2019, 20, 895. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Park, J.E.; Yoo, I.; Han, J.; Kim, N.; Lim, W.J.; Cho, E.S.; Choi, B.; Choi, S.; Kim, T.H.; et al. Integrated transcriptomes throughout swine oestrous cycle reveal dynamic changes in reproductive tissues interacting networks. Sci. Rep. 2018, 8, 5436. [Google Scholar] [CrossRef]
- Germeyer, A.; Capp, E.; Schlicksupp, F.; Jauckus, J.; von Rango, U.; von Wolff, M.; Strowitzki, T. Cell-type specific expression and regulation of apolipoprotein D and E in human endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 487–491. [Google Scholar] [CrossRef]
- Mariani, F.; Roncucci, L. Role of the Vanins-Myeloperoxidase Axis in Colorectal Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 918. [Google Scholar] [CrossRef] [Green Version]
- Sayasith, K.; Sirois, J.; Lussier, J.G. Expression, Regulation, and Promoter Activation of Vanin-2 (VNN2) in Bovine Follicles Prior to Ovulation1. Biol. Reprod. 2013, 89, 98. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Watanabe, T.; Sakurai, S.; Ohtake, K.; Kinoshita, T.; Araki, A.; Fujita, T.; Takei, H.; Takeda, Y.; Sato, Y.; et al. A novel glycosylphosphatidyl inositol-anchored protein on human leukocytes: A possible role for regulation of neutrophil adherence and migration. J. Immunol. 1999, 162, 4277–4284. [Google Scholar] [PubMed]
- Aurrand-Lions, M.; Galland, F.; Bazin, H.; Zakharyev, V.M.; Imhof, B.A.; Naquet, P. Vanin-1, a novel GPI-linked perivascular molecule involved in thymus homing. Immunity 1996, 5, 391–405. [Google Scholar] [CrossRef] [Green Version]
- Smits, K.; Willems, S.; Van Steendam, K.; Van De Velde, M.; De Lange, V.; Ververs, C.; Roels, K.; Govaere, J.; Van Nieuwerburgh, F.; Peelman, L.; et al. Proteins involved in embryo-maternal interaction around the signalling of maternal recognition of pregnancy in the horse. Sci. Rep. 2018, 8, 5249. [Google Scholar] [CrossRef]
- Chen, D.; Xu, X.; Zhu, L.J.; Angervo, M.; Li, Q.; Bagchi, M.K.; Bagchi, I.C. Cloning and uterus/oviduct-specific expression of a novel estrogen- regulated gene (ERG1). J. Biol. Chem. 1999, 274, 32215–32224. [Google Scholar] [CrossRef] [Green Version]
- Holt, W.V.; Fazeli, A. Do sperm possess a molecular passport? Mechanistic insights into sperm selection in the female reproductive tract. Mol. Hum. Reprod. 2015, 21, 491–501. [Google Scholar] [CrossRef]
- Nip, M.M.C.; Miller, D.; Taylor, P.V.; Gannon, M.J.; Hancock, K.W. Infertility: Expression of heat shock protein 70 kDa in human endometrium of normal and infertile women. Hum. Reprod. 1994, 9, 1253–1256. [Google Scholar] [CrossRef]
- Liman, N. Heat shock proteins (HSP)-60, -70, -90, and 105 display variable spatial and temporal immunolocalization patterns in the involuting rat uterus. Anim. Reprod. 2017, 14, 1072–1086. [Google Scholar] [CrossRef]
- Lloyd, R.E.; Elliott, R.M.A.; Fazeli, A.; Watson, P.F.; Holt, W. V Effects of oviductal proteins, including heat shock 70 kDa protein 8, on survival of ram spermatozoa over 48 h in vitro. Reprod. Fertil. Dev. 2009, 21, 408–418. [Google Scholar] [CrossRef]
- Coy, P.; Yanamachi, R. The Common and Species-Specific Roles of Oviductal Proteins in Mammalian Fertilization and Embryo Development. BioScience 2015, 65. [Google Scholar] [CrossRef] [Green Version]
- Elliott, R.M.A.; Lloyd, R.E.; Fazeli, A.; Sostaric, E.; Georgiou, A.S.; Satake, N.; Watson, P.F.; Holt, W.V. Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 2009, 137, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-J.; Hong, S.-H.; Yoon, M.-J.; Lee, K.-A.; Ko, J.-J.; Koo, H.S.; Kim, J.H.; Choi, D.H.; Kwon, H.; Kang, Y.-J. Endometrial profilin 1: A key player in embryo-endometrial crosstalk. Clin. Exp. Reprod. Med. 2020, 47, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, B.; Marot, G.; Dacheux, J.L.; Mercat, M.J.; Schwob, S.; Jaffrézic, F.; Gatti, J.L. The adult boar testicular and epididymal transcriptomes. BMC Genom. 2009, 10, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saewu, A.; Kadunganattil, S.; Raghupathy, R.; Kongmanas, K.; Diaz-Astudillo, P.; Hermo, L.; Tanphaichitr, N. Clusterin in the mouse epididymis: Possible roles in sperm maturation and capacitation. Reproduction 2017, 154, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Wang, Z.; Cheng, G.; Liu, B.; Li, P.; Li, J.; Wang, W.; Yin, C.; Zhang, W. Presence, localization, and origin of clusterin in normal human spermatozoa. J. Assist. Reprod. Genet. 2012, 29, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, M.; Akbari, H.; Heidari, M.H.; Molouki, A.; Murulitharan, K.; Moeini, H.; Novin, M.G.; Aabed, F.; Taheri, H.; Fadaei, F.; et al. Correlation between human clusterin in seminal plasma with sperm protamine deficiency and DNA fragmentation. Mol. Reprod. Dev. 2013, 80, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Wolkowicz, M.J.; Digilio, L.; Klotz, K.; Shetty, J.; Flickinger, C.J.; Herr, J.C. Equatorial segment protein (ESP) is a human alloantigen involved in sperm-egg binding and fusion. J. Androl. 2008, 29, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, Y.; Murakami, M.; Inoue, N.; Satouh, Y.; Kaseda, K.; Ikawa, M.; Okabe, M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J. Cell Sci. 2010, 123, 1531–1536. [Google Scholar] [CrossRef] [Green Version]
- Teijeiro, J.M.; Ignotz, G.G.; Marini, P.E. Annexin A2 is involved in pig (Sus scrofa) sperm-oviduct interaction. Mol. Reprod. Dev. 2009, 76, 334–341. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.; Fernandez-Rufete, M.; Corbin, E.; Tsikis, G.; Uzbekov, R.; Garanina, A.S.; Coy, P.; Almiñana, C.; Mermillod, P. Oviduct fluid extracellular vesicles regulate polyspermy during porcine in vitro fertilisation. Reprod. Fertil. Dev. 2020, 32, 409–418. [Google Scholar] [CrossRef]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef] [Green Version]
- Talevi, R.; Gualtieri, R. Molecules involved in sperm-oviduct adhesion and release. Theriogenology 2010, 73, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Ignotz, G.G.; Cho, M.Y.; Suarez, S.S. Annexins Are Candidate Oviductal Receptors for Bovine Sperm Surface Proteins and Thus May Serve to Hold Bovine Sperm in the Oviductal Reservoir1. Biol. Reprod. 2007, 77, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondéjar, I.; Acuña, O.S.; Izquierdo-Rico, M.J.; Coy, P.; Avilés, M. The Oviduct: Functional Genomic and Proteomic Approach. Reprod. Domest. Anim. 2012, 47, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the many talents of an old protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef] [Green Version]
- Marey, M.A.; Liu, J.; Kowsar, R.; Haneda, S.; Matsui, M.; Sasaki, M.; Shimizu, T.; Hayakawa, H.; Wijayagunawardane, M.P.B.; Hussein, F.M.; et al. Bovine oviduct epithelial cells downregulate phagocytosis of sperm by neutrophils: Prostaglandin E2 as a major physiological regulator. Reproduction 2014, 147, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Almiñana, C.; Bauersachs, S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 2020, 150. [Google Scholar] [CrossRef]
- Cabello-Agüeros, J.F.; Hernández-González, E.O.; Mújica, A. The role of F-actin cytoskeleton-associated gelsolin in the guinea pig capacitation and acrosome reaction. Cell Motil. Cytoskelet. 2003, 56, 94–108. [Google Scholar] [CrossRef]
- Gandolfi, F.; Passoni, L.; Modina, S.; Brevini, T.A.; Varga, Z.; Lauria, A. Similarity of an oviduct-specific glycoprotein between different species. Reprod. Fertil. Dev. 1993, 5, 433–443. [Google Scholar] [CrossRef]
- Kan, F.W.K.; Esperanzate, P.W.B. Surface mapping of binding of oviductin to the plasma membrane of golden hamster spermatozoa during in vitro capacitation and acrosome reaction. Mol. Reprod. Dev. 2006, 73, 756–766. [Google Scholar] [CrossRef]
- Zhao, Y.; Kan, F.W.K. Human OVGP1 enhances tyrosine phosphorylation of proteins in the fibrous sheath involving AKAP3 and increases sperm-zona binding. J. Assist. Reprod. Genet. 2019, 36, 1363–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saccary, L.; She, Y.-M.; Oko, R.; Kan, F.W.K. Hamster Oviductin Regulates Tyrosine Phosphorylation of Sperm Proteins During In Vitro Capacitation1. Biol. Reprod. 2013, 89, 38. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar, I.; Martínez-Martínez, I.; Avilés, M.; Coy, P. Identification of Potential Oviductal Factors Responsible for Zona Pellucida Hardening and Monospermy During Fertilization in Mammals1. Biol. Reprod. 2013, 89. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Tam, M.F.; Hsu, Y.T.; Lin, J.H.; Chen, H.H.; Chuang, C.K.; Chen, M.Y.; King, Y.T.; Lee, W.C. Developmental changes of heat-shock proteins in porcine testis by a proteomic analysis. Theriogenology 2005, 64, 1940–1955. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Kuo, Y.H.; Tsou, H.L.; Lee, Y.P.; King, Y.T.; Huang, H.C.; Yang, P.C.; Lee, W.C. The decline of porcine sperm motility by geldanamycin, a specific inhibitor of heat-shock protein 90 (hsp90). Theriogenology 2000, 53, 1177–1184. [Google Scholar] [CrossRef]
- Saribek, B.; Jin, Y.; Saigo, M.; Eto, K.; Abe, S. HSP90β is involved in signaling prolactin-induced apoptosis in newt testis. Biochem. Biophys. Res. Commun. 2006, 349, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-W.; Lam, K.K.W.; Lee, C.-L.; Yeung, W.S.B.; Zhao, W.E.; Ho, P.-C.; Ou, J.-P.; Chiu, P.C.N. The Roles of Protein Disulphide Isomerase Family A, Member 3 (ERp57) and Surface Thiol/Disulphide Exchange in Human Spermatozoa-Zona Pellucida Binding. Hum. Reprod. 2017, 32, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Ellerman, D.A.; Myles, D.G.; Primakoff, P. A Role for Sperm Surface Protein Disulfide Isomerase Activity in Gamete Fusion: Evidence for the Participation of ERp57. Dev. Cell 2006, 10, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asquith, K.L.; Baleato, R.M.; McLaughlin, E.A.; Nixon, B.; Aitken, R.J. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J. Cell Sci. 2004, 117, 3645–3657. [Google Scholar] [CrossRef] [Green Version]
- Kadam, K.; D’Souza, S.; Bandivdekar, A.; Natraj, U. Identification and characterization of oviductal glycoprotein-binding protein partner on gametes: Epitopic similarity to non-muscle myosin IIA, MYH 9. Mol. Hum. Reprod. 2006, 12, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Lamy, J.; Corbin, E.; Blache, M.-C.; Garanina, A.S.; Uzbekov, R.; Mermillod, P.; Saint-Dizier, M. Steroid hormones regulate sperm–oviduct interactions in the bovine. Reproduction 2017, 154, 497–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megnagi, B.; Finkelstein, M.; Shabtay, O.; Breitbart, H. The role and importance of cofilin in human sperm capacitation and the acrosome reaction. Cell Tissue Res. 2015, 362, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, K.G.; Ek, B.; Morrell, J.; Stavreus-Evers, A.; Holst, B.S.; Humblot, P.; Ronquist, G.; Larsson, A. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP). Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4604–4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternlicht, H.; Farr, G.W.; Sternlicht, M.L.; Driscoll, J.K.; Willison, K.; Yaffe, M.B. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 9422–9426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.R.; Pilder, S.H.; Bailey, J.L.; Olds-Clarke, P. Sperm from Mice Carrying One or Two t Haplotypes Are Deficient in investment and Oocyte Penetration. Dev. Biol. 1995, 168, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.; Olds-Clarke, P. Mice Carrying Two t Haplotypes: Sperm Populations with Reduced Zona Pellucida Binding Are Deficient in Capacitation1. Biol. Reprod. 1999, 61, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Redgrove, K.A.; Anderson, A.L.; Dun, M.D.; McLaughlin, E.A.; O’Bryan, M.K.; Aitken, R.J.; Nixon, B. Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae. Dev. Biol. 2011, 356, 460–474. [Google Scholar] [CrossRef] [Green Version]
- Ferramosca, A.; Zara, V. Bioenergetics of Mammalian Sperm Capacitation. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar]
- Valencia, J.; Gómez, G.; López, W.; Mesa, H.; Henao, F.J. Relationship between HSP90a, NPC2 and L-PGDS proteins to boar semen freezability. J. Anim. Sci. Biotechnol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Ecroyd, H.; Jones, R.C.; Aitken, R.J. Tyrosine Phosphorylation of HSP-90 During Mammalian Sperm Capacitation1. Biol. Reprod. 2003, 69, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- González-Cadavid, V.; Martins, J.A.M.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.L.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Moura, A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenwald, E.; Foote, R.H.; Parks, J.E. Bovine oviductal fluid components and their potential role in sperm cholesterol efflux. Mol. Reprod. Dev. 1990, 25, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Thérien, I.; Soubeyrand, S.; Manjunath, P. Major Proteins of Bovine Seminal Plasma Modulate Sperm Capacitation by High-Density Lipoprotein1. Biol. Reprod. 1997, 57, 1080–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, A.J.; Kopf, G.S. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Investig. 2002, 110, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.A.; García-Vázquez, F.A.; Alvau, A.; Escoffier, J.; Krapf, D.; Sánchez-Cárdenas, C.; Salicioni, A.M.; Darszon, A.; Visconti, P.E. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J. Cell. Physiol. 2015, 230, 1758–1769. [Google Scholar] [CrossRef] [Green Version]
- González-Fernández, L.; Macías-García, B.; Calle-Guisado, V.; García-Marín, L.J.; Bragado, M.J. Calmodulin inhibitors increase the affinity of Merocyanine 540 for boar sperm membrane under non-capacitating conditions. J. Reprod. Dev. 2018, 64, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, L.; Li, Y.; Zhao, N.; Zhen, L.; Fu, J.; Yang, Q. Calcium regulates motility and protein phosphorylation by changing cAMP and ATP concentrations in boar sperm in vitro. Anim. Reprod. Sci. 2016, 172, 39–51. [Google Scholar] [CrossRef]
- Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Valero, M.L.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Characterization of the porcine seminal plasma proteome comparing ejaculate portions. J. Proteom. 2016, 142, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Utleg, A.G.; Yi, E.C.; Xie, T.; Shannon, P.; White, J.T.; Goodlett, D.R.; Hood, L.; Lin, B. Proteomic analysis of human prostasomes. Prostate 2003, 56, 150–161. [Google Scholar] [CrossRef]
- Ace, C.I.; Okulicz, W.C. A Progesterone-Induced Endometrial Homolog of a New Candidate Tumor Suppressor, DMBT1 1. J. Clin. Endocrinol. Metab. 1998, 83, 3569–3573. [Google Scholar] [CrossRef] [PubMed]
- Ambruosi, B.; Accogli, G.; Douet, C.; Canepa, S.; Pascal, G.; Monget, P.; Moros, C.; Holmskov, U.; Mollenhauer, J.; Robbe-Masselot, C.; et al. Deleted in malignant brain tumor 1 is secreted in the oviduct and involved in the mechanism of fertilization in equine and porcine species. Reproduction 2013, 146, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.L.; Marini, P.E. First evidence of the interaction between deleted in malignant brain tumor 1 and galectin-3 in the mammalian oviduct. Histochem. Cell Biol. 2014, 141, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldán, M.L.; Teijeiro, J.M.; Álvarez, J.R.; Marini, P.E. Sperm binding to porcine oviductal cells is mediated by SRCR domains contained in DMBT1. J. Cell. Biochem. 2018, 119, 3755–3762. [Google Scholar] [CrossRef]
- Bauersachs, S.; Rehfeld, S.; Ulbrich, S.E.; Mallok, S.; Prelle, K.; Wenigerkind, H.; Einspanier, R.; Blum, H.; Wolf, E. Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J. Mol. Endocrinol. 2004, 32, 449–466. [Google Scholar] [CrossRef]
- Tone, A.A.; Begley, H.; Sharma, M.; Murphy, J.; Rosen, B.; Brown, T.J.; Shaw, P.A. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin. Cancer Res. 2008, 14, 4067–4078. [Google Scholar] [CrossRef] [Green Version]
- Rutherfurd, K.J.; Swiderek, K.M.; Green, C.B.; Chen, S.; Shively, J.E.; Kwok, S.C.M. Purification and characterization of PSP-I and PSP-II, two major proteins from porcine seminal plasma. Arch. Biochem. Biophys. 1992, 295, 352–359. [Google Scholar] [CrossRef]
- Centurion, F.; Vazquez, J.M.; Calvete, J.J.; Roca, J.; Sanz, L.; Parrilla, I.; Garcia, E.M.; Martinez, E.A. Influence of Porcine Spermadhesins on the Susceptibility of Boar Spermatozoa to High Dilution. Biol. Reprod. 2003, 69, 640–646. [Google Scholar] [CrossRef]
- Töpfer-Petersen, E.; Romero, A.; Varela, P.F.; Ekhlasi-Hundrieser, M.; Dostàlovà, Z.; Sanz, L.; Calvete, J.J. Spermadhesins: A new protein family. Facts, hypotheses and perspectives. Andrologia 1998, 30, 217–224. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Wallgren, M. Advances in boar semen cryopreservation. Vet. Med. Int. 2010, 2010, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Caballero, I.; Vazquez, J.M.; Mayor, G.M.; Almiñana, C.; Calvete, J.J.; Sanz, L.; Roca, J.; Martinez, E.A. PSP-I/PSP-II spermadhesin exert a decapacitation effect on highly extended boar spermatozoa. Int. J. Androl. 2009, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Sinowatz, F.; Amselgruber, W.; Töpfer-Petersen, E.; Calvete, J.J.; Sanz, L.; Plendl, J. Immunohistochemical localization of spermadhesin AWN in the porcine male genital tract. Cell Tissue Res. 1995, 282, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.C.; Romar, R.; Avilés, M.; Gadea, J.; Coy, P. Determination of glycosidase activity in porcine oviductal fluid at the different phases of the estrous cycle. Reproduction 2008, 136, 833–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Vázquez, F.A.; Hernández-Caravaca, I.; Matás, C.; Soriano-Úbeda, C.; Abril-Sánchez, S.; Izquierdo-Rico, M.J. Morphological study of boar sperm during their passage through the female genital tract. J. Reprod. Dev. 2015, 61, 407–413. [Google Scholar] [CrossRef]
- Ben, W.X.; Fu, M.T.; Mao, L.K.; Ming, Z.W.; Xiong, W.W. Effects of various concentrations of native seminal plasma in cryoprotectant on viability of human sperm. Syst. Biol. Reprod. Med. 1997, 39, 211–216. [Google Scholar] [CrossRef]
- Moros-Nicolás, C.; Douet, C.; Reigner, F.; Goudet, G. Effect of cumulus cell removal and sperm pre-incubation with progesterone on in vitro fertilization of equine gametes in the presence of oviductal fluid or cells. Reprod. Domest. Anim. 2019, 54, 1095–1103. [Google Scholar] [CrossRef]
- Kumaresan, A.; Johannisson, A.; Humblot, P.; Bergqvist, A.S. Effect of bovine oviductal fluid on motility, tyrosine phosphorylation, and acrosome reaction in cryopreserved bull spermatozoa. Theriogenology 2019, 124, 48–56. [Google Scholar] [CrossRef]
- Dusa, A. venn: Draw Venn diagrams. 2018. [Google Scholar]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Dplyr: A Grammar of Data Manipulation Version 0.8.3 from CRAN. Available online: https://rdrr.io/cran/dplyr/ (accessed on 12 December 2019).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (2nd Edition). J. Stat. Softw. 2016, 77, 260. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002, 30, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
| Experimental Groups | Protein Name | Accession Number |
|---|---|---|
| UF | A-kinase anchoring protein 4 | A0A286ZWH7 |
| Apolipoprotein E | P18650 | |
| ATP synthase subunit alpha | A0A287BBS4 | |
| Chaperonin containing TCP1 subunit 8 | I3LCA2 | |
| Enolase 1 | A0A287B6S5 | |
| Glyceraldehyde-3-phosphate dehydrogenase | F1RM74 | |
| Heat shock 70 kDa protein 1-like | A5A8V7 | |
| Heat shock protein HSP 90-alpha | A0A287AQK7 | |
| Hemoglobin subunit alpha | P01965 | |
| Hemoglobin subunit beta | A0A5G2QRW3 | |
| Histone H2A | A0A5G2QMX0 | |
| IgA heavy chain constant region | A0A287B626 | |
| Ig like protein | A0A286ZTC9 | |
| Mitochondria-eating protein | A0A5G2R8N5 | |
| Multifunctional fusion protein | A0A287B7L9 | |
| Outer dense fiber protein 1 | Q29077 | |
| Outer dense fiber protein 2 | F1RR82 | |
| Profilin | F1RFY1 | |
| Pyruvate dehydrogenase E1 component subunit beta | A0A5G2QSU5 | |
| RIIa domain-containing protein | A0A5G2R5J1 | |
| Serum albumin | A0A287BAY9 | |
| T-complex protein 1 subunit alpha | F1SB63 | |
| Uromodulin | F1RPA9 | |
| Vanin 2 | F1S3Q9 | |
| V-SNARE coiled-coil homology domain-containing protein | A0A287BEC7 | |
| SP | Beta-hexosaminidase | D0G6 × 8 |
| Beta-microseminoprotein | A0A2C9F3B6 | |
| Cytochrome c oxidase subunit 5A | F1SJ34 | |
| Fc fragment of IgG binding protein | A0A287BCE6 | |
| Glycoprotein 2 | F1RPA7 | |
| Heat shock 10kDa protein 1 | F1SMZ6 | |
| Keratin 1 | F1SGG3 | |
| NPC intracellular cholesterol transporter 2 | A0A5G2RMF7 | |
| T-complex protein 1 subunit zeta | I3L9J4 | |
| WAP four-disulfide core domain 10A-like (WFDC10AL) | A0A287AEV7 | |
| UF *, SP * | Acrosin | A0A287AFN9 |
| Angiotensin-converting enzyme | F1RRW5 | |
| Calmodulin | A0A5G2QWK6 | |
| IZUMO family member 4 | A0A5G2QPK2 | |
| Keratin 75 | F1SGI7 | |
| LY6/PLAUR domain containing 4 | D3K5J4 | |
| Malate dehydrogenase | A0A5G2RGL7 | |
| Peroxiredoxin 1 | A0A286ZND5 | |
| Sperm equatorial segment protein 1 precursor | A0A287B423 | |
| T-complex protein 1 subunit eta | A0A5G2RAV7 | |
| Transitional endoplasmic reticulum ATPase | A0A286ZUM8 | |
| Uncharacterized protein (leucine-rich repeat-containing protein 37A-like isoform X2) | A0A287B9V6 | |
| Zonadhesin | A0A5G2QZP6 | |
| UF *, SP + UF * | Deleted in malignant brain tumors 1 protein | Q4A3R3 |
| Apolipoprotein A-I | K7GM40 | |
| UF *, SP *, SP + UF * | Acrosin-binding protein | F1SL45 |
| Actin, cytoplasmic 1/Actin gamma 1 | A0A287AA77 | |
| Amine oxidase | F1RHU4 | |
| ATP synthase subunit beta | K7GLT8 | |
| Carbohydrate-binding protein AQN-1 | Q4R0H3 | |
| Chromosome 1 open reading frame 56 | A0A5G2QRQ5 | |
| Clusterin | A0A5S6I5T1 | |
| Fibronectin 1 | F1SS24 | |
| Jacalin-type lectin domain-containing protein | A0A287AVU8 | |
| Leucine zipper protein 2/LUZP2 | A0A287BT68 | |
| Major seminal plasma glycoprotein PSP-I | P35495 | |
| Major seminal plasma glycoprotein PSP-II | P35496 | |
| Seminal plasma protein pB1 | A0A2C9F357 | |
| Seminal plasma sperm motility inhibitor | I7HJH6 | |
| Sperm acrosome membrane-associated protein 1 | D5K8A9 | |
| Spermadhesin AWN | Q4R0H8 | |
| Sperm-associated acrosin inhibitor isoform X1 | A0A2C9F3F5 | |
| Tubulin alpha chain | F2Z5T5 | |
| Tubulin beta chain | A0A5G2QGK1 |
| Experimental Groups | Protein Name | Accession Number |
|---|---|---|
| OF | 40S ribosomal protein S3a | F2Z5C7 |
| 40S ribosomal protein S5 | F2Z5E6 | |
| 60S acidic ribosomal protein P0 | A0A5S6HGK5 | |
| 60S ribosomal protein L17 isoform a | A0A287B386 | |
| Apolipoprotein A-II preproprotein | A0A481BCM9 | |
| Creatine kinase B-type | A0A5G2R6 × 7 | |
| Dynein cytoplasmic 1 heavy chain 1 | A0A287B9W3 | |
| Elongation factor 1-alpha | A0A287A391 | |
| Glyceraldehyde-3-phosphate dehydrogenase | F1RM74 | |
| Myosin heavy chain 14 | I3LIE3 | |
| Myosin light polypeptide 6 | A0A5G2QAD4 | |
| Oviduct-specific glycoprotein | Q28990 | |
| Peptidyl arginine deiminase 2 | I3LNE4 | |
| Peptidylprolyl isomerase | A0A287B5T6 | |
| RuvB-like helicase | I3L742 | |
| T-complex protein 1 subunit gamma | A0A287AMZ2 | |
| SP | Acrosin | A0A287AFN9 |
| Acrosin-binding protein | F1SL45 | |
| Amine oxidase | F1RHU4 | |
| Angiotensin-converting enzyme | F1RRW5 | |
| Beta-hexosaminidase | D0G6 × 8 | |
| Beta-microseminoprotein | A0A2C9F3B6 | |
| Chromosome 1 open reading frame 56 | A0A5G2QRQ5 | |
| Clusterin | A0A5S6I5T1 | |
| Cytochrome c oxidase subunit 5A | F1SJ34 | |
| Fc fragment of IgG binding protein | A0A287BCE6 | |
| Glycoprotein 2 | F1RPA7 | |
| Heat shock 10kDa protein 1 | F1SMZ6 | |
| IZUMO family member 4 | A0A5G2QPK2 | |
| Jacalin-type lectin domain-containing protein | A0A287AVU8 | |
| Keratin 1 | F1SGG3 | |
| Keratin 75 | F1SGI7 | |
| Leucine zipper protein 2/LUZP2 | A0A287BT68 | |
| LY6/PLAUR domain containing 4 | D3K5J4 | |
| Malate dehydrogenase | A0A5G2RGL7 | |
| NPC intracellular cholesterol transporter 2 | A0A5G2RMF7 | |
| Seminal plasma protein pB1 | A0A2C9F357 | |
| Sperm acrosome membrane-associated protein 1 | D5K8A9 | |
| Sperm equatorial segment protein 1 precursor | A0A287B423 | |
| Sperm-associated acrosin inhibitor isoform X1 | A0A2C9F3F5 | |
| WAP four-disulfide core domain 10A-like (WFDC10AL) | A0A287AEV7 | |
| Zonadhesin | A0A5G2QZP6 | |
| SP + OF | 40S ribosomal protein S7 | A0A287A9Y6 |
| Anterior gradient 2, protein disulphide isomerase family member | A0A287AM82 | |
| Apolipoprotein A-I | K7GM40 | |
| Heterogeneous nuclear ribonucleoprotein k isoform X1 | I3LQS0 | |
| Hypoxia upregulated 1 | A0A286ZZF0 | |
| Keratin 14 | F1S0J8 | |
| Na(+)/H(+) exchange regulatory cofactor NHE-RF | B8XH67 | |
| Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform | P54612 | |
| T-complex protein 1 subunit alpha | F1SB63 | |
| OF *, SP * | Actin, cytoplasmic 1/Actin gamma 1 | A0A287AA77 |
| ATP synthase subunit beta | K7GLT8 | |
| Peroxiredoxin 1 | A0A286ZND5 | |
| T-complex protein 1 subunit zeta | I3L9J4 | |
| OF *, SP+OF * | 60 kDa heat shock protein, mitochondrial | A0A287ATN8 |
| 60S acidic ribosomal protein P1 | F1SIT7 | |
| 60S acidic ribosomal protein P2 | A0A287B7U0 | |
| 60S ribosomal protein L4 | A0A5G2QSX6 | |
| Aldehyde dehydrogenase 1 family member A1 | I3LRS5 | |
| Annexin I | F1SJB5 | |
| Annexin II | A0A286ZJV6 | |
| Annexin V | F2Z5C1 | |
| Calreticulin | P28491 | |
| Clathrin light chain | A0A287BFJ2 | |
| Cofilin-1 | K7GK75 | |
| Deleted in malignant brain tumors 1 protein | Q4A3R3 | |
| Endoplasmic reticulum chaperone BiP/78 kDa glucose-regulated protein | A0A287BIL8 | |
| Endoplasmin | Q29092 | |
| Eukaryotic translation elongation factor 2 | I3LII3 | |
| Gelsolin | A0A287A6P1 | |
| Heat shock cognate 71kDa protein | A0A286ZWK2 | |
| Heat shock protein HSP 90-alpha | A0A287AQK7 | |
| Heat shock protein HSP 90-beta | A0A286ZKC5 | |
| IQ motif containing GTPase activating protein 1 | IQGAP1 | |
| Multifunctional fusion protein | A0A287B7L9 | |
| Myosin 9 | A0A5G2R8R0 | |
| Myosin heavy chain 14 | MYH9 | |
| Myosin-11 | MYH11 | |
| Oviduct-specific glycoprotein | OVGP1 | |
| Protein disulfide-isomerase P4HB | G9F6 × 8 | |
| Protein disulfide-isomerase PDIA3 | F6QA08 | |
| Protein disulfide-isomerase PDIA4 | PDIA4 | |
| SERPIN domain-containing protein/Serpin H1 precursor | A0A286ZRU9 | |
| Serum albumin | A0A287BAY9 | |
| Sodium/potassium-transporting ATPase subunit alpha | F1SAX3 | |
| Thioredoxin domain-containing protein | A0A5G2Q895 | |
| Triosephosphate isomerase | A0A288CFT0 | |
| Tropomodulin 3 | A0A5G2R425 | |
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta | F2Z558 | |
| OF *, SP + OF *, SP * | Calmodulin | A0A5G2QWK6 |
| Carbohydrate-binding protein AQN-1 | Q4R0H3 | |
| Fibronectin 1 | F1SS24 | |
| Seminal plasma sperm motility inhibitor | I7HJH6 | |
| Spermadhesin AWN | Q4R0H8 | |
| T-complex protein 1 subunit eta | A0A5G2RAV7 | |
| Transitional endoplasmic reticulum ATPase | A0A286ZUM8 | |
| Tubulin alpha chain | F2Z5T5 | |
| Tubulin beta chain | A0A5G2QGK1 | |
| SP + OF *, SP * | Leucine-rich repeat-containing protein 37A | A0A287B9V6 |
| Major seminal plasma glycoprotein PSP-I | P35495 | |
| Major seminal plasma glycoprotein PSP-II | P35496 |
| Most Descriptive Categories of DAVID Functional Annotation Clusters by Similar GO Terms | Proteins 1 | Score 2 |
|---|---|---|
| GO Group 1: Endocytosis | 9 | 3.4 |
| blood microparticle (7, 30.0) 3 | ||
| receptor-mediated endocytosis (6, 20.1) | ||
| endocytic vesicle lumen (4, 162.7) | ||
| lipoprotein metabolic process (3, 49.1) | ||
| GO Group 2: Protein folding/binding to zona pellucida | 6 | 2.8 |
| protein stabilization (5, 22.9) | ||
| unfolded protein binding (4, 21.9) | ||
| zona pellucida receptor complex (3, 217.0) | ||
| binding of sperm to zona pellucida (3, 53.3) | ||
| cell body (3, 31.0) |
| Most Descriptive Categories of DAVID Functional Annotation Clusters by Similar GO Terms | Proteins 1 | Score 2 |
|---|---|---|
| GO Group 1: Cell adhesion/junction | 14 | 9.2 |
| cadherin binding involved in cell-cell adhesion (14, 11.8) 3 | ||
| cell-cell adherens junction (14, 11.4) | ||
| cell-cell adhesion (12, 10.9) | ||
| GO Group 2: Protein folding | 14 | 5.2 |
| endoplasmic reticulum lumen (12, 16.5) | ||
| endoplasmic reticulum (11, 3.5) | ||
| cell redox homeostasis (6, 19.2) | ||
| protein disulfide isomerase activity (4, 56.5) | ||
| endoplasmic reticulum-Golgi intermediate compartment (5, 19.4) | ||
| GO Group 3: Ribosome/translation | 9 | 4.2 |
| nuclear-transcribed mRNA catabolic process, nonsense-mediated decay (9, 18.7) | ||
| SRP-dependent cotranslational protein targeting to membrane (8, 21.0) | ||
| viral transcription (8, 17.6) | ||
| translational initiation (8, 14.4) | ||
| rRNA processing (9.2, 8) | ||
| structural constituent of ribosome (8, 8.8) | ||
| translation (8, 7.8) | ||
| ribosome (6, 9.5) | ||
| cytosolic large ribosomal subunit (5, 19.4) | ||
| large ribosomal subunit rRNA binding (3, 104.9) | ||
| GO Group 4: Endocytosis | 7 | 4.1 |
| receptor-mediated endocytosis (7, 9.3) | ||
| endocytic vesicle lumen (5, 82.5) | ||
| GO Group 5: | 7 | 4.0 |
| response to unfolded protein (6, 35.3) | ||
| MHC class II protein complex binding (4, 61.2) | ||
| GO Group 6: Microtubules | 17 | 3.8 |
| GTP binding (11, 7.0) | ||
| GTPase activity (9, 9.4) | ||
| microtubule (9, 7.6) | ||
| structural molecule activity (7, 6.9) | ||
| cell division (7, 4.9) | ||
| structural contstituyent of cytoskeleton (6, 13.3) | ||
| microtubule-based process (5, 34.3) | ||
| cytoskeleton-dependent intracellular transport (4, 54.9) | ||
| GO Group 7: Myosin | 8 | 3.2 |
| actin filament binding (6, 11.0) | ||
| myosin complex (5, 26.4) | ||
| motor activity (5, 18.5) | ||
| regulation of cell shape (5, 8.8) | ||
| actin-dependent ATPase activity (4, 75.3) | ||
| brush border (4, 17.6) | ||
| myosin II filament (3, 264.1) | ||
| myosin II complex (3, 113.2) | ||
| actomyosin (3, 66.0) | ||
| actin filament-based movement (3, 43.6) | ||
| microfilament motor activity (3, 36.7) | ||
| GO Group 8: | 7 | 2.3 |
| response to drug (7, 5.7) | ||
| response to estrogen (4, 15.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luongo, C.; González-Brusi, L.; Cots-Rodríguez, P.; Izquierdo-Rico, M.J.; Avilés, M.; García-Vázquez, F.A. Sperm Proteome after Interaction with Reproductive Fluids in Porcine: From the Ejaculation to the Fertilization Site. Int. J. Mol. Sci. 2020, 21, 6060. https://doi.org/10.3390/ijms21176060
Luongo C, González-Brusi L, Cots-Rodríguez P, Izquierdo-Rico MJ, Avilés M, García-Vázquez FA. Sperm Proteome after Interaction with Reproductive Fluids in Porcine: From the Ejaculation to the Fertilization Site. International Journal of Molecular Sciences. 2020; 21(17):6060. https://doi.org/10.3390/ijms21176060
Chicago/Turabian StyleLuongo, Chiara, Leopoldo González-Brusi, Paula Cots-Rodríguez, Mª José Izquierdo-Rico, Manuel Avilés, and Francisco Alberto García-Vázquez. 2020. "Sperm Proteome after Interaction with Reproductive Fluids in Porcine: From the Ejaculation to the Fertilization Site" International Journal of Molecular Sciences 21, no. 17: 6060. https://doi.org/10.3390/ijms21176060
APA StyleLuongo, C., González-Brusi, L., Cots-Rodríguez, P., Izquierdo-Rico, M. J., Avilés, M., & García-Vázquez, F. A. (2020). Sperm Proteome after Interaction with Reproductive Fluids in Porcine: From the Ejaculation to the Fertilization Site. International Journal of Molecular Sciences, 21(17), 6060. https://doi.org/10.3390/ijms21176060






