Anti-c-fms Antibody Prevents Osteoclast Formation and Bone Resorption in Co-Culture of Osteoblasts and Osteoclast Precursors In Vitro and in Ovariectomized Mice
Abstract
:1. Introduction
2. Results
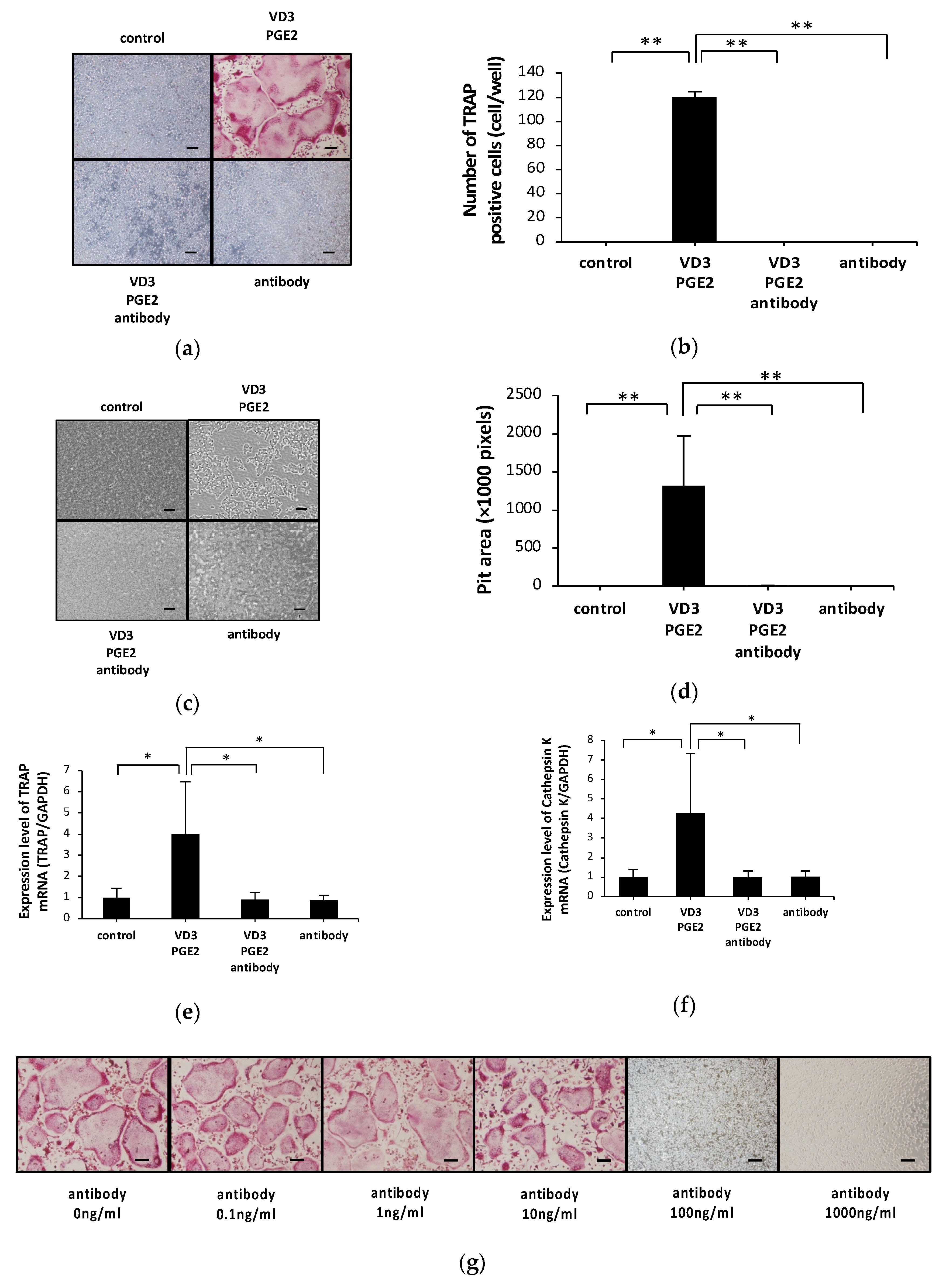
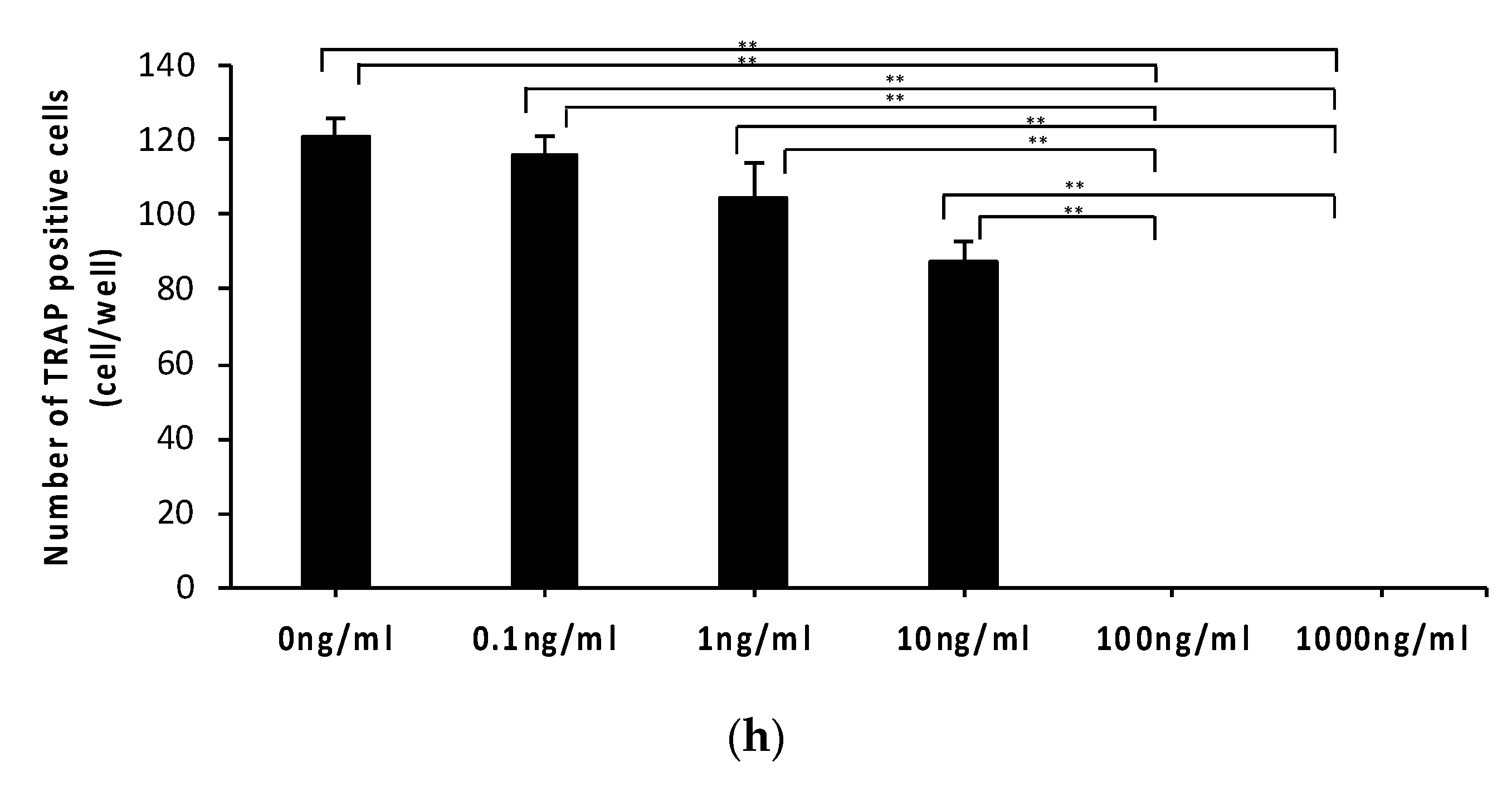
2.1. Anti-c-fms Antibodies Inhibit Osteoclast Formation and Bone Resorption in an Osteoblast–Osteoclast Co-Culture System
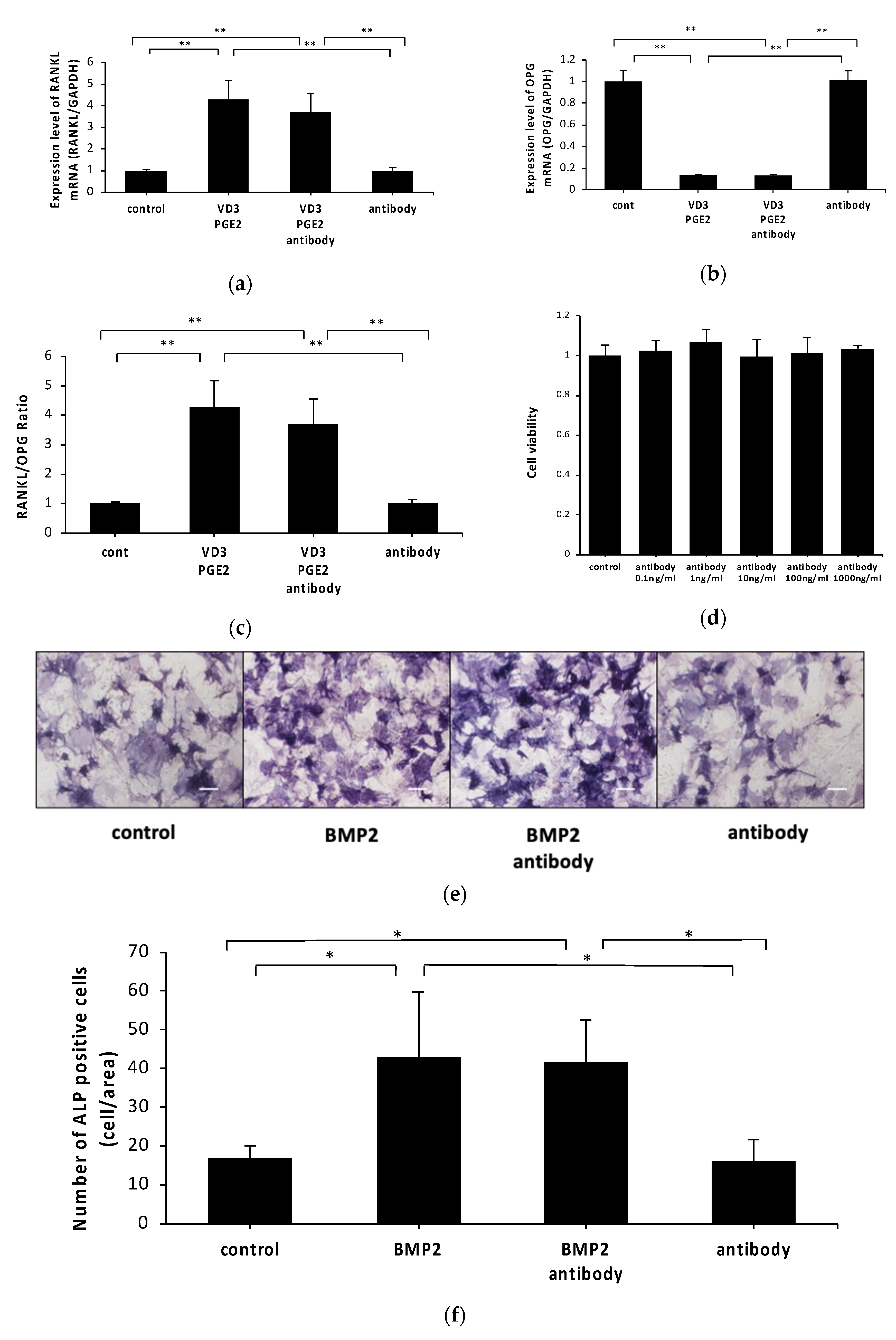
2.2. Anti-c-fms Antibodies Do Not Affect RANKL and OPG mRNA Levels or Osteoblast Viability or Differentiation
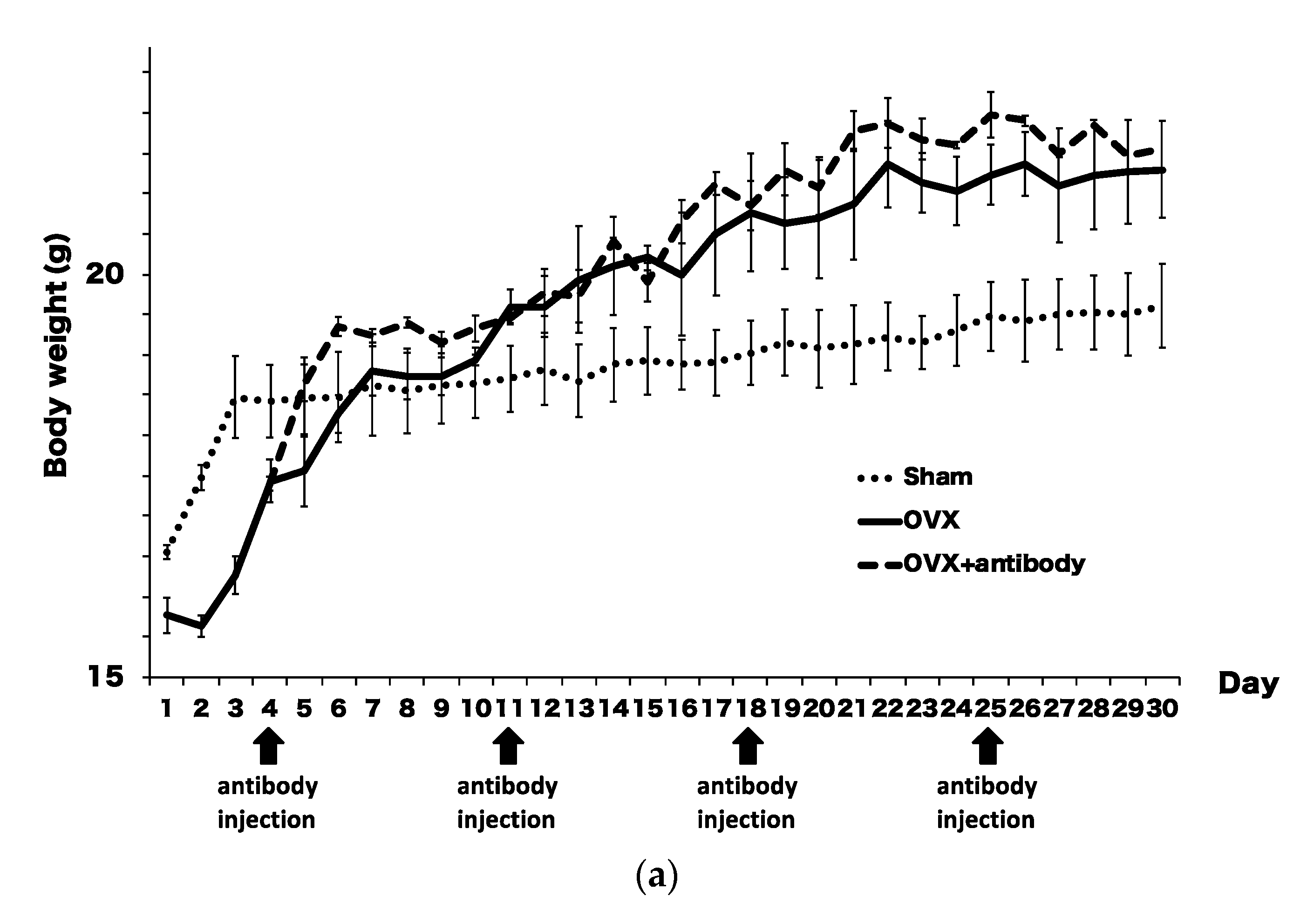
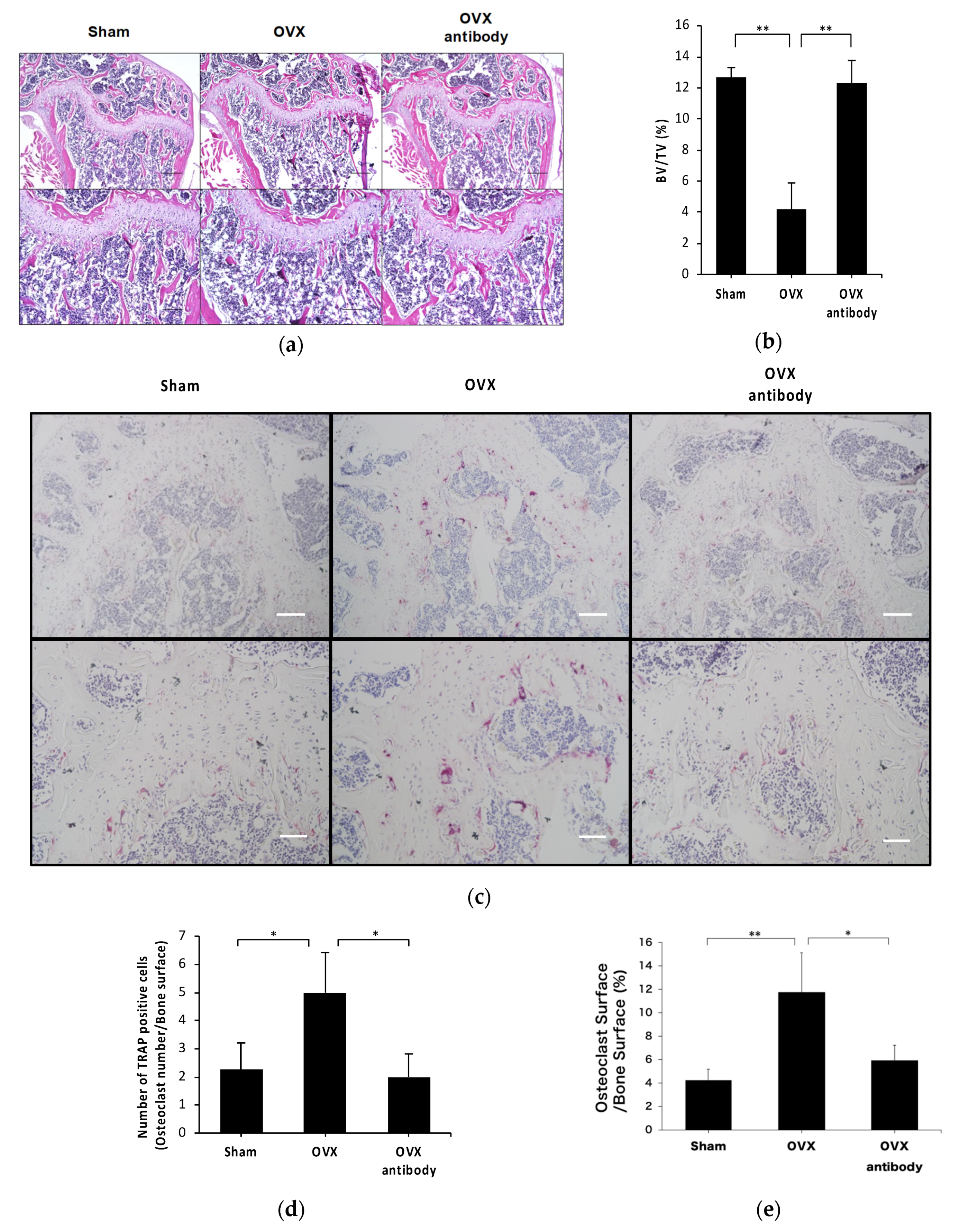
2.3. Anti-c-fms Antibodies Inhibit Bone Resorption and Bone Loss in OVX Mice
2.4. Anti-c-fms Antibodies Inhibit Osteoclast Formation in OVX Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Mouse Ovariectomy
4.3. Preparation for Histological Evaluation
4.4. Preparation of Osteoclast Precursors and Osteoblasts
4.5. Co-Culture of Osteoclast Precursors and Osteoblasts
4.6. Preparation of RNA and Real-Time PCR Analysis
4.7. Evaluation of Bone Loss
4.8. Evaluation of Serum CTX Levels
4.9. Evaluation of Cell Viability
4.10. Evaluation of Osteoblast Differentiation
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| M-CSF | macrophage colony-stimulating factor |
| OVX | ovariectomy |
| RANKL | receptor activator of nuclear factor kb ligand |
| OPG | osteoprotegrin |
| TNF-α | Tumor necrosis factor-α |
| LPS | lipopolysaccharide |
| 1,25(OH)2D3 | 1,25-dihydroxyvitamin D3 |
| PGE2 | prostaglandin E2 |
| BMP | bone morphogenetic protein |
| ALP | alkaline phosphatase |
| BV/TV | bone volume per tissue volume |
| Tb.Th | trabecular thickness |
| Tb.N | trabecular number |
| BS/BV | bone surface area per bone volume |
| CTX | C-terminal cross-linked telopeptide of type I collagen |
| N.Oc/BS | number of osteoclast per bone surface |
| Oc.S/BS | osteoclast surface per bone surface |
| EDTA | ethylenediaminetetraacetic acid |
References
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R.; National Osteoporosis, F. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Khan, M.; Cheung, A.M.; Khan, A.A. Drug-Related Adverse Events of Osteoporosis Therapy. Endocrinol. Metab. Clin. N. Am. 2017, 46, 181–192. [Google Scholar] [CrossRef]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef] [Green Version]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.; Murphy, C.; Kirstein, B.; Fox, S.W.; Chambers, T.J. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 2002, 143, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Sands, M.S.; Aya, K.; Zhou, P.; Hirayama, T.; Uthgenannt, B.; Wei, S.; Takeshita, S.; Novack, D.V.; Silva, M.J.; et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J. Immunol. 2004, 173, 4838–4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaura, H.; Zhou, P.; Kim, H.J.; Novack, D.V.; Ross, F.P.; Teitelbaum, S.L. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Investig. 2005, 115, 3418–3427. [Google Scholar] [CrossRef] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kitaura, H.; Fujii, T.; Hakami, Z.W.; Takano-Yamamoto, T. Anti-c-Fms antibody inhibits lipopolysaccharide-induced osteoclastogenesis in vivo. FEMS Immunol. Med. Microbiol. 2012, 64, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, K.; Kitaura, H.; Fujii, T.; Ishida, M.; Hakami, Z.W.; Takano-Yamamoto, T. An anti-c-Fms antibody inhibits osteoclastogenesis in a mouse periodontitis model. Oral. Dis. 2014, 20, 319–324. [Google Scholar] [CrossRef]
- Kitaura, H.; Yoshimatsu, M.; Fujimura, Y.; Eguchi, T.; Kohara, H.; Yamaguchi, A.; Yoshida, N. An anti-c-Fms antibody inhibits orthodontic tooth movement. J. Dent. Res. 2008, 87, 396–400. [Google Scholar] [CrossRef]
- Kitaura, H.; Fujimura, Y.; Yoshimatsu, M.; Eguchi, T.; Kohara, H.; Jang, I.; Morita, Y.; Yoshida, N. An M-CSF receptor c-Fms antibody inhibits mechanical stress-induced root resorption during orthodontic tooth movement in mice. Angle Orthod. 2009, 79, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Kitaura, H.; Shen, W.R.; Kishikawa, A.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Mizoguchi, I. Establishment of an orthodontic retention mouse model and the effect of anti-c-Fms antibody on orthodontic relapse. PLoS ONE 2019, 14, e0214260. [Google Scholar] [CrossRef] [Green Version]
- Bettina, A.; Zhang, Z.; Michels, K.; Cagnina, R.E.; Vincent, I.S.; Burdick, M.D.; Kadl, A.; Mehrad, B. M-CSF Mediates Host Defense during Bacterial Pneumonia by Promoting the Survival of Lung and Liver Mononuclear Phagocytes. J. Immunol. 2016, 196, 5047–5055. [Google Scholar] [CrossRef] [Green Version]
- Van Overmeire, E.; Stijlemans, B.; Heymann, F.; Keirsse, J.; Morias, Y.; Elkrim, Y.; Brys, L.; Abels, C.; Lahmar, Q.; Ergen, C.; et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res. 2016, 76, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lum, L.; Wong, B.R.; Josien, R.; Becherer, J.D.; Erdjument-Bromage, H.; Schlondorff, J.; Tempst, P.; Choi, Y.; Blobel, C.P. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J. Biol. Chem. 1999, 274, 13613–13618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, T.; Kobayashi, Y.; Yamasaki, S.; Kawakami, A.; Eguchi, K.; Sasaki, H.; Sakai, H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: Modulation of the expression by osteotropic factors and cytokines. Biochem. Biophys. Res. Commun. 2000, 275, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Hikita, A.; Yana, I.; Wakeyama, H.; Nakamura, M.; Kadono, Y.; Oshima, Y.; Nakamura, K.; Seiki, M.; Tanaka, S. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. J. Biol. Chem. 2006, 281, 36846–36855. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O′Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Marahleh, A.; Kitaura, H.; Ishida, M.; Shima, K.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Ohori, F.; Noguchi, T.; et al. Effect of Anti-c-fms Antibody on Osteoclast Formation and Proliferation of Osteoclast Precursor In Vitro. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 2096–2097. [Google Scholar] [CrossRef]
- Eastell, R.; O′Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [Green Version]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M.T. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology 1998, 139, 4743–4746. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell. Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jilka, R.L.; Hangoc, G.; Girasole, G.; Passeri, G.; Williams, D.C.; Abrams, J.S.; Boyce, B.; Broxmeyer, H.; Manolagas, S.C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 1992, 257, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.; Islander, U.; Egecioglu, E.; Lof, E.; Swanson, C.; Moverare-Skrtic, S.; Sjogren, K.; Lindberg, M.K.; Carlsten, H.; Ohlsson, C. Investigation of central versus peripheral effects of estradiol in ovariectomized mice. J. Endocrinol. 2005, 187, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.; Weitzmann, M.N.; Gentile, M.A.; Aisa, M.C.; Pacifici, R. M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss. J. Clin. Investig. 2000, 105, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Martinez, A.; Munoz-Islas, E.; Ramirez-Rosas, M.B.; Acosta-Gonzalez, R.I.; Torres-Rodriguez, H.F.; Jimenez-Andrade, J.M. Blockade of the colony-stimulating factor-1 receptor reverses bone loss in osteoporosis mouse models. Pharmacol. Rep. 2020. [Google Scholar] [CrossRef]
- Sudo, T.; Nishikawa, S.; Ogawa, M.; Kataoka, H.; Ohno, N.; Izawa, A.; Hayashi, S.; Nishikawa, S. Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene 1995, 11, 2469–2476. [Google Scholar]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 2000, 15, 1477–1488. [Google Scholar] [CrossRef]
- Bakker, A.D.; Klein-Nulend, J. Osteoblast isolation from murine calvaria and long bones. Methods Mol. Biol. 2012, 816, 19–29. [Google Scholar] [CrossRef]
- Ohori, F.; Kitaura, H.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. IL-33 Inhibits TNF-alpha-Induced Osteoclastogenesis and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 1130. [Google Scholar] [CrossRef] [Green Version]
- Kishikawa, A.; Kitaura, H.; Kimura, K.; Ogawa, S.; Qi, J.; Shen, W.R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; et al. Docosahexaenoic Acid Inhibits Inflammation-Induced Osteoclast Formation and Bone Resorption in vivo Through GPR120 by Inhibiting TNF-alpha Production in Macrophages and Directly Inhibiting Osteoclast Formation. Front. Endocrinol. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.R.; Kimura, K.; Ishida, M.; Sugisawa, H.; Kishikawa, A.; Shima, K.; Ogawa, S.; Qi, J.; Kitaura, H. The Glucagon-Like Peptide-1 Receptor Agonist Exendin-4 Inhibits Lipopolysaccharide-Induced Osteoclast Formation and Bone Resorption via Inhibition of TNF-alpha Expression in Macrophages. J. Immunol. Res. 2018, 2018, 5783639. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Sequence | |
|---|---|---|
| GAPDH | Forward | 5′-GGTGGAGCCAAAAGGGTCA-3′ |
| Reverse | 5′-GGGGGCTAAGCAGTTGGT-3′ | |
| TRAP | Forward | 5′-AACTTGCGACCATTGTTA-3′ |
| Reverse | 5′-GGGGACCTTTCGTTGATGT-3′ | |
| RANKL | Forward | 5′-CCTGAGGCCAGCCATTT-3′ |
| Reverse | 5′-CTTGGCCCAGCCTCGAT-3′ | |
| OPG | Forward | 5′-ATCAGAGCCTCATCACCTT-3′ |
| Reverse | 5′-TTAGGTCCAACTACAGAGGAAC-3′ | |
| Cathepsin K | Forward | 5′-GCAGAGGTTGTACTATGA-3′ |
| Reverse | 5′-GCAGGCGTTGTTCTTATT-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nara, Y.; Kitaura, H.; Ogawa, S.; Shen, W.-R.; Qi, J.; Ohori, F.; Noguchi, T.; Marahleh, A.; Pramusita, A.; Kinjo, R.; et al. Anti-c-fms Antibody Prevents Osteoclast Formation and Bone Resorption in Co-Culture of Osteoblasts and Osteoclast Precursors In Vitro and in Ovariectomized Mice. Int. J. Mol. Sci. 2020, 21, 6120. https://doi.org/10.3390/ijms21176120
Nara Y, Kitaura H, Ogawa S, Shen W-R, Qi J, Ohori F, Noguchi T, Marahleh A, Pramusita A, Kinjo R, et al. Anti-c-fms Antibody Prevents Osteoclast Formation and Bone Resorption in Co-Culture of Osteoblasts and Osteoclast Precursors In Vitro and in Ovariectomized Mice. International Journal of Molecular Sciences. 2020; 21(17):6120. https://doi.org/10.3390/ijms21176120
Chicago/Turabian StyleNara, Yasuhiko, Hideki Kitaura, Saika Ogawa, Wei-Ren Shen, Jiawei Qi, Fumitoshi Ohori, Takahiro Noguchi, Aseel Marahleh, Adya Pramusita, Ria Kinjo, and et al. 2020. "Anti-c-fms Antibody Prevents Osteoclast Formation and Bone Resorption in Co-Culture of Osteoblasts and Osteoclast Precursors In Vitro and in Ovariectomized Mice" International Journal of Molecular Sciences 21, no. 17: 6120. https://doi.org/10.3390/ijms21176120
APA StyleNara, Y., Kitaura, H., Ogawa, S., Shen, W.-R., Qi, J., Ohori, F., Noguchi, T., Marahleh, A., Pramusita, A., Kinjo, R., & Mizoguchi, I. (2020). Anti-c-fms Antibody Prevents Osteoclast Formation and Bone Resorption in Co-Culture of Osteoblasts and Osteoclast Precursors In Vitro and in Ovariectomized Mice. International Journal of Molecular Sciences, 21(17), 6120. https://doi.org/10.3390/ijms21176120




