Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis
Abstract
1. Introduction
2. Results
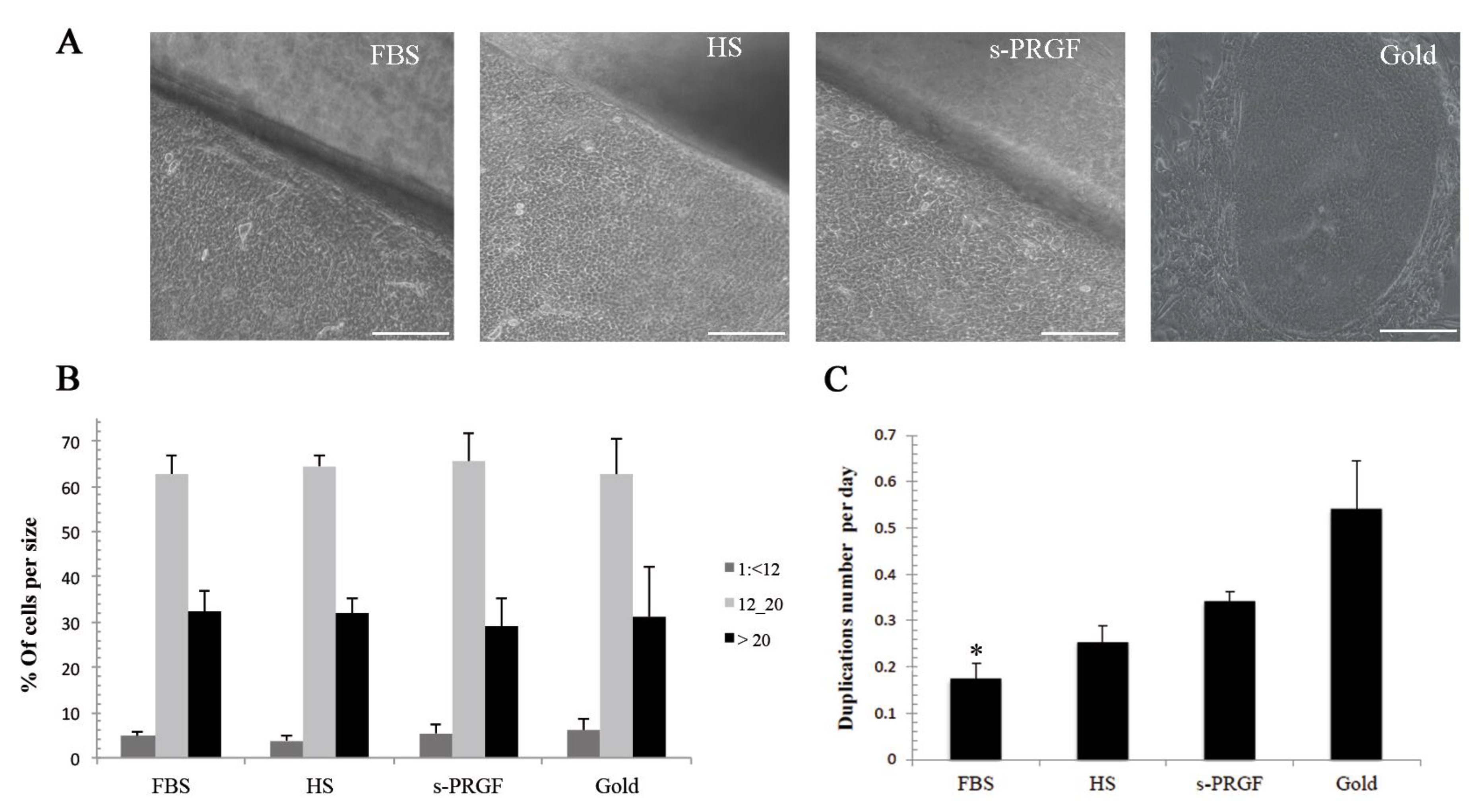
2.1. Cell Size
2.2. Cell Growth Rate
2.3. LESC Protein Marker Analysis
2.4. Gene Expression Analysis
2.5. Quantification of Growth Factor and Cytokines in Human Sera
2.6. Correlations
2.7. Multivariate Analysis of Data
3. Discussion
4. Materials and Methods
4.1. Human Sclerocorneal Tissue
4.2. Preparation of Blood-Derived Products
- Human serum (HS): Spontaneous coagulation for 2 h at room temperature followed by centrifugation at 1000 g for 15 min. The complete supernatant fraction was collected.
- Serum derived from plasma rich in growth factors (s-PRGF): Centrifugation at 460 g for 8 min, followed by a collection of the complete supernatant fraction and induction of clot formation by adding calcium chloride (Braun, Barcelona, Spain) at a final concentration of 22.8 mM in the absence of red and white blood cells. After 2 h at 36 °C, the clot was retracted, and the supernatant was collected.
4.3. Amniotic Membrane Preparation
4.4. Tissue Explants and Limbal Epithelial Cell Isolation
4.5. Explants and Single Cell Cultures
4.6. Analysis of Cell Growth and Cell Size
4.7. Real-Time RT-PCR
4.8. Immunocytochemistry
4.9. Quantification of Growth Factors and Bioactive Molecules in Human Sera
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davanger, M.; Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef]
- Tseng, S.C.G. Concept and application of limbal stem cells. Eye 1989, 3, 141–157. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Seyed-Safi, A.G.; Daniels, J.T. The limbus: Structure and function. Exp. Eye Res. 2020, 197, 108074. [Google Scholar] [CrossRef]
- Dua, H.S.; Shanmuganathan, V.A.; Powell-Richards, A.O.; Tighe, P.J.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar] [CrossRef]
- Shortt, A.J.; Secker, G.A.; Notara, M.D.; Limb, G.A.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Transplantation of ex vivo cultured limbal epithelial stem cells: A review of techniques and clinical results. Surv. Ophthalmol. 2007, 52, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.G. Regulation and clinical implications of corneal epithelial stem cells. Mol. Biol. Rep. 1996, 23, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Notara, M.; Alatza, A.; Gilfillan, J.; Harris, A.R.; Levis, H.J.; Schrader, S.; Vernon, A.; Daniels, J.T. In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Exp. Eye Res. 2010, 90, 188–195. [Google Scholar] [CrossRef]
- Shahdadfar, A.; Haug, K.; Pathak, M.; Drolsum, L.; Olstad, O.K.; Johnsen, E.O.; Petrovski, G.; Moe, M.C.; Nicolaissen, B. Ex vivo expanded autologous limbal epithelial cells on amniotic membrane using a culture medium with human serum as single supplement. Exp. Eye Res. 2012, 97, 1–9. [Google Scholar] [CrossRef]
- Menzel-Severing, J.; Kruse, F.E.; Schlotzer-Schrehardt, U. Stem cell-based therapy for corn ealepithelial reconstruction: Present and future. Proc. Can. J. Ophthalmol. 2013, 48, 13–21. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; de Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Stasi, K.; Goings, D.; Huang, J.; Herman, L.; Pinto, F.; Addis, R.C.; Klein, D.; Massaro-Giordano, G.; Gearhart, J.D. Optimal isolation and xeno-free culture conditions for limbal stem cell function. Investig. Ophthalmol. Vis. Sci. 2014, 55, 375–386. [Google Scholar] [CrossRef]
- Ang, L.P.K.; Jain, P.; Phan, T.T.; Reza, H.M. Human umbilical cord lining cells as novel feeder layer for ex vivo cultivation of limbal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Mei, H.; Nakatsu, M.N.; Baclagon, E.R.; Deng, S.X. A 3D culture system enhances the ability of human bone marrow stromal cells to support the growth of limbal stem/progenitor cells. Stem Cell Res. 2016, 16, 358–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mei, H.; González, S.; Nakatsu, M.N.; Baclagon, E.R.; Chen, F.V.; Deng, S.X. Human adipose-derived stem cells support the growth of limbal stem/progenitor cells. PLoS ONE 2017, 12, e0186238. [Google Scholar] [CrossRef]
- Nguyen, K.N.; Bobba, S.; Richardson, A.; Park, M.; Watson, S.L.; Wakefield, D.; di Girolamo, N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2018, 65, 21–35. [Google Scholar] [CrossRef]
- Figueiredo, G.S.; Mudhar, H.S.; Lako, M.; Figueiredo, F.C. Corneal regeneration. Essentials in ophthalmology. In Corneal Epithelial Stem Cells: Methods for ex Vivo Expansion; Alió, J., Alió del Barrio, J., Arnalich-Montiel, F., Eds.; Springer: Cham, Switzerland, 2019; pp. 77–97. [Google Scholar]
- Shahdadfar, A.; Frønsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 2005, 23, 1357–1366. [Google Scholar] [CrossRef]
- Kolli, S.A.I.; Ahmad, S.; Lako, M.; Figueiredo, F. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells 2010, 28, 597–610. [Google Scholar] [CrossRef]
- Tarle, S.A.; Shi, S.; Kaigler, D. Development of a serum-free system to expand dental-derived stem cells: PDLSCs and SHEDs. J. Cell. Physiol. 2011, 226, 66–73. [Google Scholar] [CrossRef]
- Shafaei, H.; Esmaeili, A.; Mardani, M.; Razavi, S.; Hashemibeni, B.; Nasr-Esfahani, M.H.; Shiran, M.B.; Esfandiari, E. Effects of human placental serum on proliferation and morphology of human adipose tissue-derived stem cells. Bone Marrow Transplant. 2011, 46, 1464–1471. [Google Scholar] [CrossRef]
- Shih, D.T.B.; Burnouf, T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N. Biotechnol. 2015, 32, 199–211. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Chen, L.; Deng, S.X. comparative study of xenobiotic-free media for the cultivation of human limbal epithelial stem/progenitor cells. Tissue Eng. Part C Methods 2017, 23, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Hecker, A.; Kocaömer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klüter, H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 2009, 27, 2331–2341. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Ang, L.P.K.; Koizumi, N.; Yokoi, N.; Kinoshita, S. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology 2006, 113, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Basu, S.; Vemuganti, G.K.; Sejpal, K.; Subramaniam, S.V.; Bandyopadhyay, S.; Krishnaiah, S.; Gaddipati, S.; Tiwari, S.; Balasubramanian, D.; et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br. J. Ophthalmol. 2011, 95, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Higa, K.; Morito, F.; Dogru, M.; Kawakita, T.; Satake, Y.; Shimmura, S.; Tsubota, K. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am. J. Ophthalmol. 2007, 143, 945–953. [Google Scholar] [CrossRef]
- Kolli, S.; Lako, M.; Figueiredo, F.; Mudhar, H.; Ahmad, S. Loss of corneal epithelial stem cell properties in outgrowths from human limbal explants cultured on intact amniotic membrane. Regen. Med. 2008, 3, 329–342. [Google Scholar] [CrossRef]
- Suri, K.; Gong, H.K.; Yuan, C.; Kaufman, S.C. Human platelet lysate as a replacement for fetal bovine serum in limbal stem cell therapy. Curr. Eye Res. 2016, 41, 1266–1273. [Google Scholar] [CrossRef]
- Chen, D.; Qu, Y.; Hua, X.; Zhang, L.; Liu, Z.; Pflugfelder, S.C.; Li, D.Q. A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells. Eye 2017, 31, 962–971. [Google Scholar] [CrossRef]
- Lužnik, Z.; Breda, C.; Barbaro, V.; Ferrari, S.; Migliorati, A.; di Iorio, E.; Ferrari, B.; Griffoni, C.; Grassetto, A.; Elbadawy, H.M.; et al. Towards xeno-free cultures of human limbal stem cells for ocular surface reconstruction. Cell Tissue Bank. 2017, 18, 461–474. [Google Scholar] [CrossRef]
- Brejchova, K.; Trosan, P.; Studeny, P.; Skalicka, P.; Utheim, T.P.; Bednar, J.; Jirsova, K. Characterization and comparison of human limbal explant cultures grown under defined and xeno-free conditions. Exp. Eye Res. 2018, 176, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Nam, S.M.; Choi, S.K.; Seo, K.Y.; Kim, H.O.; Chung, S.H. Comparative study of substrate free and amniotic membrane scaffolds for cultivation of limbal epithelial sheet. Sci. Rep. 2018, 8, 14628. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Velasco-García, A.M.; Aloy-Reverté, C.; Vilarrodona, A.; Casaroli-Marano, R.P. Xenofree generation of limbal stem cells for ocular surface advanced cell therapy. Stem Cell Res. Ther. 2019, 10, 374. [Google Scholar] [CrossRef]
- Freire, V.; Andollo, N.; Etxebarria, J.; Durán, J.A.; Morales, M.C. In vitro effects of three blood derivatives on human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5571–5578. [Google Scholar] [CrossRef]
- Freire, V.; Andollo, N.; Etxebarria, J.; Hernáez-Moya, R.; Durán, J.A.; Morales, M.C. Corneal wound healing promoted by 3 blood derivatives: An in vitro and in vivo comparative study. Cornea 2014, 33, 614–620. [Google Scholar] [CrossRef]
- Etxebarria, J.; Sanz-Lázaro, S.; Hernáez-Moya, R.; Freire, V.; Durán, J.A.; Morales, M.C.; Andollo, N. Serum from plasma rich in growth factors regenerates rabbit corneas by promoting cell proliferation, migration, differentiation, adhesion and limbal stemness. Acta Ophthalmol. 2017, 95. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Barrio, C.; Etxebarria, J.; Hernáez-Moya, R.; del Val-Alonso, M.; Rodriguez-Astigarraga, M.; Urkaregi, A.; Freire, V.; Morales, M.C.; Durán, J.; Vicario, M.; et al. Hyaluronic acid combined with serum rich in growth factors in corneal epithelial defects. Int. J. Mol. Sci. 2019, 20, 1655. [Google Scholar] [CrossRef]
- Grueterich, M.; Espana, E.M.; Tseng, S.C.G. Ex vivo expansion of limbal epithelial stem cells: Amniotic membrane serving as a stem cell niche. Surv. Ophthalmol. 2003, 48, 631–646. [Google Scholar] [CrossRef]
- Koizumi, N.; Rigby, H.; Fullwood, N.J.; Kawasaki, S.; Tanioka, H.; Koizumi, K.; Kociok, N.; Joussen, A.M.; Kinoshita, S. Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells. Graefe Arch. Clin. Exp. Ophthalmol. 2007, 245, 123–134. [Google Scholar] [CrossRef]
- Shortt, A.J.; Secker, G.A.; Lomas, R.J.; Wilshaw, S.P.; Kearney, J.N.; Tuft, S.J.; Daniels, J.T. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials 2009, 30, 1056–1065. [Google Scholar] [CrossRef]
- Meller, D.; Dabul, V.; Tseng, S.C.G. Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Exp. Eye Res. 2002, 74, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.G.; He, H.; Zhang, S.; Chen, S.Y. niche regulation of limbal epithelial stem cells: Relationship between inflammation and regeneration. Ocul. Surf. 2016, 14, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Ferrari, S.; Fasolo, A.; Böhm, E.; Ponzin, D.; Barbaro, V. Techniques for culture and assessment of limbal stem cell grafts. Ocul. Surf. 2010, 8, 146–153. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; de Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Notara, M.; Haddow, D.B.; MacNeil, S.; Daniels, J.T. A xenobiotic-free culture system for human limbal epithelial stem cells. Regen. Med. 2007, 2, 919–927. [Google Scholar] [CrossRef]
- Baylis, O.; Figueiredo, F.; Henein, C.; Lako, M.; Ahmad, S. 13 Years of cultured limbal epithelial cell therapy: A review of the outcomes. J. Cell. Biochem. 2011, 112, 993–1002. [Google Scholar] [CrossRef]
- Utheim, O.; Islam, R.; Lyberg, T.; Roald, B.; Eidet, J.R.; de la Paz, M.F.; Dartt, D.A.; Raeder, S.; Utheim, T.P. Serum-free and xenobiotic-free preservation of cultured human limbal epithelial cells. PLoS ONE 2015, 10, e0118517. [Google Scholar] [CrossRef]
- Behaegel, J.; Zakaria, N.; Tassignon, M.J.; Leysen, I.; Bock, F.; Koppen, C.; Ní Dhubhghaill, S. Short-and long-term results of xenogeneic-free cultivated autologous and allogeneic limbal epithelial stem cell transplantations. Cornea 2019, 38, 1543–1549. [Google Scholar] [CrossRef]
- Zakaria, N.; Possemiers, T.; Dhubhghaill, S.N.; Leysen, I.; Rozema, J.; Koppen, C.; Timmermans, J.P.; Berneman, Z.; Tassignon, M.J. Results of a phase I/II clinical trial: Standardized, non-xenogenic, cultivated limbal stem cell transplantation. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef]
- Trosan, P.; Smeringaiova, I.; Brejchova, K.; Bednar, J.; Benada, O.; Kofronova, O.; Jirsova, K. The enzymatic de-epithelialization technique determines denuded amniotic membrane integrity and viability of harvested epithelial cells. PLoS ONE 2018, 13, e0194820. [Google Scholar] [CrossRef]
- González, S.; Deng, S.X. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Expe. Eye Res. 2013, 116, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Dziasko, M.A.; Daniels, J.T. anatomical features and cell-cell interactions in the human limbal epithelial stem cell niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.C.; Espana, E.M.; Yoo, S.H.; Budak, M.T.; Wolosin, J.M.; Tseng, S.C.G. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5125–5129. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.S.X.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Nowell, C.S.; Radtke, F. Corneal epithelial stem cells and their niche at a glance. J. Cell Sci. 2017, 130, 1021–1025. [Google Scholar] [CrossRef]
- Li, D.Q.; Scheffer, A.; Tsenc, C.G. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J. Cell Physiol. 1995, 163, 61–79. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Vesaluoma, M.; Teppo, A.M.; Grönhagen-Riska, C.; Tervo, T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: A potential modulator of corneal wound healing following photorefractive keratectomy. Curr. Eye Res. 1997, 16, 825–831. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, C.; Fan, T.; Xu, B. Fibronectin regulates the self-renewal of rabbit limbal epithelial stem cells by stimulating the Wnt11/Fzd7/ROCK non-canonical Wnt pathway. Exp. Eye Res. 2019, 185. [Google Scholar] [CrossRef]
- Stepp, M.A.; Spurr-Michaud, S.; Gipson, I.K. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Investig. Ophtalmol. Vis. Sci. 1993, 34, 1829–1844. [Google Scholar]
- Qi, H.; Chuang, E.Y.; Yoon, K.C.; de Paiva, C.S.; Shine, H.D.; Jones, D.B.; Pflugfelder, S.C.; Li, D.Q. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol. Vis. 2007, 13, 1934–1941. [Google Scholar] [PubMed]
- Qi, H.; Li, D.Q.; Bian, F.; Chuang, E.Y.; Jones, D.B.; Pflugfelder, S.C. Expression of glial cell-derived neurotrophic factor and its receptor in the stem-cell-containing human limbal epithelium. Br. J. Ophthalmol. 2008, 92, 1269–1274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueno, H.; Ferrari, G.; Hattori, T.; Saban, D.R.; Katikireddy, K.R.; Chauhan, S.K.; Dana, R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 867–872. [Google Scholar] [CrossRef]
- You, L.; Ebner, S.; Kruse, F.E. Glial cell-derived neurotrophic factor (GDNF)-induced migration and signal transduction in corneal epithelial cells. Investig. Ophtalmol. Vis. Sci. 2001, 42, 2496–2504. [Google Scholar]
- Tervo, K.; Tervo, T.; Eränkö, L.; Eränkö, O. Substance P immunoreactive nerves in the rodent cornea. Neurosci. Lett. 1981, 25, 95–97. [Google Scholar] [CrossRef]
- Miller, A.; Costa, M.; Furness, J.B.; Chubb, I.W. Substance P immunoreactive sensory nerves supply the rat iris and cornea. Neurosci. Lett. 1981, 23, 243–249. [Google Scholar] [CrossRef]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Wang, P.; Me, R.; Yuan, Y.; Yu, Y.; Li, M.; Ke, B. Substance P inhibits high urea-induced apoptosis through the AKT/GSK-3β pathway in human corneal epithelial cells. J. Cell. Biochem. 2019, 120, 11342–11349. [Google Scholar] [CrossRef]
- Reid, T.W.; Murphy, C.J.; Iwahashi, C.K.; Foster, B.A.; Mannis, M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J. Cell Biochem. 1993, 52, 476–485. [Google Scholar] [CrossRef]
- Garcia-Hirschfeld, J.; Lopez-Briones, L.G.; Belmonte, C. Neurotrophic influences on corneal epithelial cells. Exp. Eye Res. 1994, 59, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Nakamura, M.; Ofuji, K.; Reid, T.W.; Mannis, M.J.; Murphy, C.J. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J. Cell Physiol. 1996, 169, 159–166. [Google Scholar] [CrossRef]
- Ko, J.A.; Yanai, R.; Nishida, T. Up-regulation of ZO-1 expression and barrier function in cultured human corneal epithelial cells by substance P. FEBS Lett. 2009, 583, 2148–2153. [Google Scholar] [CrossRef]
- Nagano, T.; Nakamura, M.; Nakata, K.; Yamaguchi, T.; Takase, K.; Okahara, A.; Ikuse, T.; Nishida, T. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Yanai, R.; Yamada, N.; Inui, M.; Nishida, T. Correlation of proliferative and anti-apoptotic effects of HGF, insulin, IGF-1, IGF-2, and EGF in SV40-transformed human corneal epithelial cells. Exp. Eye Res. 2006, 83, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Son, B.K.; Kim, S.; Son, Y.; Yu, S.Y.; Hong, H.S. Substance P prevents development of proliferative vitreoretinopathy in mice by modulating TNF-α. Mol. Vis. 2017, 23, 933–943. [Google Scholar]
- Lambiase, A.; Rama, P.; Bonini, S.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N. Engl. J. Med. 1998, 338, 1174–1180. [Google Scholar] [CrossRef]
- Bonini, S.; Lambiase, A.; Rama, P.; Sinigaglia, F.; Allegretti, M.; Chao, W.; Mantelli, F.; Bonini, S.; Lambiase, A.; Rama, P.; et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology 2018, 125, 1332–1343. [Google Scholar] [CrossRef]
- Kolli, S.; Bojic, S.; Ghareeb, A.E.; Kurzawa-Akanbi, M.; Figueiredo, F.C.; Lako, M. The role of nerve growth factor in maintaining proliferative capacity, colony-forming efficiency, and the limbal stem cell phenotype. Stem Cells 2019, 37, 139–149. [Google Scholar] [CrossRef]
- Wilson, S.E.; Liang, Q.; Kim, W.J. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2185–2190. [Google Scholar]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.; Pfaffl, M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Barbaro, V.; Ferrari, S.; Ortolani, C.; de Luca, M.; Pellegrini, G. Q-FIHC: Quantification of fluorescence immunohistochemistry to analysep63 isoforms and cell cycle phases in human limbal stem cells. Microsc. Res. Tech. 2006, 69, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Jollife, I.T. Principal Component Analysis; Springer-Verlag: New York, NY, USA, 2002; ISBN 0-387-95442-2. [Google Scholar]
- Pardo, C.; Pardo, C.E.; Campo, P.C. Del combination of factorial methods and cluster analysis in r: The package factoclass. Rev. Colomb. Estadística 2007, 30, 231–245. [Google Scholar]
- Brereton, R.G.; Lloyd, G.R. Partial least squares discriminant analysis: Taking the magic away. J. Chemom. 2014, 28, 213–225. [Google Scholar] [CrossRef]
- Hand, D.J.; Till, R.J. A simple generalisation of the area under the ROC curve for multiple class classification problems. Mach. Learn. 2001, 45, 171–186. [Google Scholar] [CrossRef]





| Blood Derivatives | Pool | EGF (pg/mL) | HGF (pg/mL) | FGFb (pg/mL) | KGF (pg/mL) | PDGF-AB (ng/mL) | TGF-β (ng/mL) | VEGF (pg/mL) |
| HS | 1 | 277.20 ± 0 | 59.06 ± 10.76 | 81.25 ± 0 | 24.38 ± 0.59 | 18.89 ± 1.53 | 5.12 ± 2.34 | 179.06 ± 5.31 |
| 2 | 709.76 ± 0 | 141.11 ± 13.59 | 123.0 ± 0 | 56.39 ± 6.92 | 19.31 ± 2.53 | 6.83 ± 0.75 | 280.66 ± 16.42 | |
| Mean ± SD | 493.48 ± 305.86 | 100.08 ± 48.42 | 109.08 ± 24.1 | 40.39 ± 18.91 | 19.10 ± 1.72 | 5.98 ± 1.73 | 229.86 ± 59.5 | |
| s-PRGF | 1 | 199.24 ± 12.71 | 28.01 ± 6.95 | 39.77 ± 0 | 21.50 ± 0.57 | 22.81 ± 2.83 | 5.66 ± 0.80 | 281.24 ± 233.04 |
| 2 | 544.48 ± 199.94 | 60.80 ± 0.04 | 59.47 ± 0 | 42.56 ± 6.53 | 19.53 ± 5.75 | 9.13 ± 1.08 | 112.43 ± 17.04 | |
| Mean ± SD | 371.85 ± 230.46 | 44.4 ± 19.35 | 49.62 ± 13.93 | 32.03 ± 12.73 | 21.17 ± 4.16 | 7.40 ± 2.15 | 196.83 ± 166.43 | |
| Blood Derivatives | Pool | FIBRONECTIN (µg/mL) | IFN-γ (pg/mL) | TNF-α (pg/mL) | NGF (pg/mL) | IGF-1 (ng/mL) | GDNF (pg/mL) | SP (pg/mL) |
| HS | 1 | 58.82 ± 11.12 | 0.00 ± 0 | 4.11 ± 2.43 | 60.41 ± 26.88 | 3.98 ± 0.49 | 55.21 ± 6.97 | 20.35 ± 0.31 |
| 2 | 112.18 ± 2.73 | 1.06 ± 0.08 | 10.19 ± 0.84 | 30.66 ± 7.95 | 2.47 ± 1.80 | 22.67 ± 9.07 | 11.34 ± 1.92 | |
| Mean ± SD | 85.5 ± 31.51 | 0.53 ± 0.61 | 7.15 ± 3.81 | 45.53 ± 23.6 | 3.23 ± 1.39 | 38.94 ± 19.91 * | 15.84 ± 5.32 * | |
| s-PRGF | 1 | 91.72 ± 11.28 | 0.00 ± 0 | 10.62 ± 3.31 | 42.53 ± 10.89 | 1.38 ± 0.03 | 0.00 ± 0 | 3.40 ± 0.39 |
| 2 | 31.11 ± 2.49 | 0.95 ± 0.22 | 11.54 ± 0.02 | 49.87 ± 31.75 | 5.44 ± 0 | 11.98 ± 0 | 1.25 ± 0.77 | |
| Mean ± SD | 61.41 ± 35.62 | 0.47 ± 0.56 | 11.08 ± 1.99 | 46.2 ± 19.84 | 3.41 ± 2.35 | 5.99 ± 6.91 | 2.32 ± 1.34 |
| <12 μm | 12–20 μm | >20 μm | Duplication Time | ICC K14 | ICC K12 | ICC ΔNp63α | PCR ABCG2 | PCR ΔNp63α | PCR N-cadherin | PCR K14 | PCR K12 | PCR Ki67 | EGF | HGF | FGFb | KGF | PDGF-AB | TGF-β | VEGF | Fibronectin | IFN-γ | TNF-α | NGF | IGF-1 | GDNF | SP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <12 μm | |||||||||||||||||||||||||||
| 12–20 μm | 92% *** | 66% * | |||||||||||||||||||||||||
| >20 μm | 92% *** | ||||||||||||||||||||||||||
| Duplication Time | −80% ** | −75% * | 75% * | ||||||||||||||||||||||||
| ICC K14 | 63% * | 63% * | 63% * | 63% * | −58% * | 58% * | |||||||||||||||||||||
| ICC K12 | −66% * | ||||||||||||||||||||||||||
| ICC ΔNp63α | −74% ** | ||||||||||||||||||||||||||
| PCR ABCG2 | 60% * | ||||||||||||||||||||||||||
| PCR ΔNp63α | 63% * | −66% * | |||||||||||||||||||||||||
| PCR N-cadherin | |||||||||||||||||||||||||||
| PCR K14 | −74% ** | 60% * | 62% * | ||||||||||||||||||||||||
| PCR K12 | −80% ** | 62% * | −65% * | 73% ** | 65% * | −73% ** | −65% * | ||||||||||||||||||||
| PCR Ki67 | 66% * | −67% * |
| EGF | HGF | FGFb | KGF | PDGF-AB | TGF-β | VEGF | Fibronectin | IFN-γ | TNF-α | NGF | IGF-1 | GDNF | SP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EGF | 100% *** | 80% ** | 100% *** | 60% * | 95% *** | |||||||||
| HGF | 100% *** | 80% ** | 100% *** | 60% * | 95% *** | |||||||||
| FGFb | 80% ** | 80% ** | 80% ** | −80% ** | −60% * | 80% ** | 60% * | |||||||
| KGF | 100% *** | 100% *** | 80% ** | 60% * | 95% *** | |||||||||
| PDGF-AB | −80% p = 0.002 | 80% ** | −100% *** | −80% ** | ||||||||||
| TGF-β | 60% * | 60% * | 60% * | 0.74% ** | 80% ** | −80% ** | ||||||||
| VEGF | 80% ** | −60% * | −100% *** | |||||||||||
| Fibronectin | 80% ** | −80% ** | −80% ** | |||||||||||
| IFN-γ | 95% *** | 95% *** | 95% *** | 0.74% *** | −63% * | |||||||||
| TNF-α | −60% * | 80% ** | 80% ** | −80% ** | −100% *** | |||||||||
| NGF | −60% * | −80% p = 0.002 | −63% * | 60% * | −63% * | |||||||||
| IGF-1 | −100% * | −80% p = 0.002 | 60% * | |||||||||||
| GDNF | 80% ** | −100% *** | −80% ** | 80% ** | ||||||||||
| SP | 60% * | −80% ** | −80% ** | −100% *** | −63% * | 80% ** |
| s-PRGF1 | s-PRGF2 | HS1 | HS2 | |
|---|---|---|---|---|
| Cornea 1 | Cluster 1 | Cluster 5 | Cluster 8 | Cluster 11 |
| Variables of cultured cells | − ICC ΔNp63α | +++ ICC ΔNp63α | +++ PCR Ki67 | − PCR K14 |
| −−− PCR ABCG2 | ++ Size 3 | + PCR N-Cadherin, ΔNp63α | −− PCR ABCG2, K12 | |
| −− PCR N-Cadherin, Size 2 | −−− PCR K12 | |||
| −−− PCR Ki67 | ||||
| Content of the sera in GFs | +++ PDGF | +++ TGF | +++ GDNF, SP | +++ HGF, FGFb |
| ++ VEGF | ++ IGF-1 | ++ NGF | ++ KGF, EGF | |
| − SP | −− FN, VEGF | −−− TNF | −− NGF | |
| −− EGF, GDNF | ||||
| −−− IGF-1 | ||||
| Cornea 2 | Cluster 2 | Cluster 6 | Cluster 9 | Cluster 12 |
| Variables of cultured cells | + PCR K12 | +++ Size 1 | +++ PCR Ki67 | +++ PCR K14, Size 3 |
| −−− PCR N-Cadherin | ++ PCR K14, ABCG2 | ++ PCR K14, ABCG2 | ++ PCR ABCG2 | |
| + PCR K12 | − Size1 | + PCR K12 | ||
| −− PCR Ki67 | −−−Size 2 | |||
| Content of the sera in GFs | ++ PDGF | ++ TGF-β, IGF-1 | +++ GDNF, SP | +++ HGF, FGFb |
| − EGF, GDNF | −− FN, VEGF | ++ NGF | ++ KGF, EGF | |
| −− IGF-1 | −−− TNF | −− NGF | ||
| Cornea 3 | Cluster 3 | Cluster 7 | Cluster 10 | Cluster 10 |
| Variables of cultured cells | +++ PCR K12 | +++ PCR N-Cadherin | − PCR K12 | − PCR K12 |
| ++ ICC ΔNp63α, Size 2 | −−− PCR ΔNp63α | −−− PCR K14, ΔNp63α | −−− PCR K14, ΔNp63α | |
| − Size 3 | ||||
| Content of the sera in GFs | ++ PDGF | ++ TGF-β, IGF-1 | + FGFb | + FGFb |
| − IGF-1 | −− FN, VEGF |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernáez-Moya, R.; González, S.; Urkaregi, A.; Pijoan, J.I.; Deng, S.X.; Andollo, N. Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis. Int. J. Mol. Sci. 2020, 21, 6132. https://doi.org/10.3390/ijms21176132
Hernáez-Moya R, González S, Urkaregi A, Pijoan JI, Deng SX, Andollo N. Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis. International Journal of Molecular Sciences. 2020; 21(17):6132. https://doi.org/10.3390/ijms21176132
Chicago/Turabian StyleHernáez-Moya, Raquel, Sheyla González, Arantza Urkaregi, Jose Ignacio Pijoan, Sophie X. Deng, and Noelia Andollo. 2020. "Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis" International Journal of Molecular Sciences 21, no. 17: 6132. https://doi.org/10.3390/ijms21176132
APA StyleHernáez-Moya, R., González, S., Urkaregi, A., Pijoan, J. I., Deng, S. X., & Andollo, N. (2020). Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis. International Journal of Molecular Sciences, 21(17), 6132. https://doi.org/10.3390/ijms21176132






