Photodynamic Activity of Tribenzoporphyrazines with Bulky Periphery against Wound Bacteria
Abstract
:1. Introduction
2. Results and Discussion
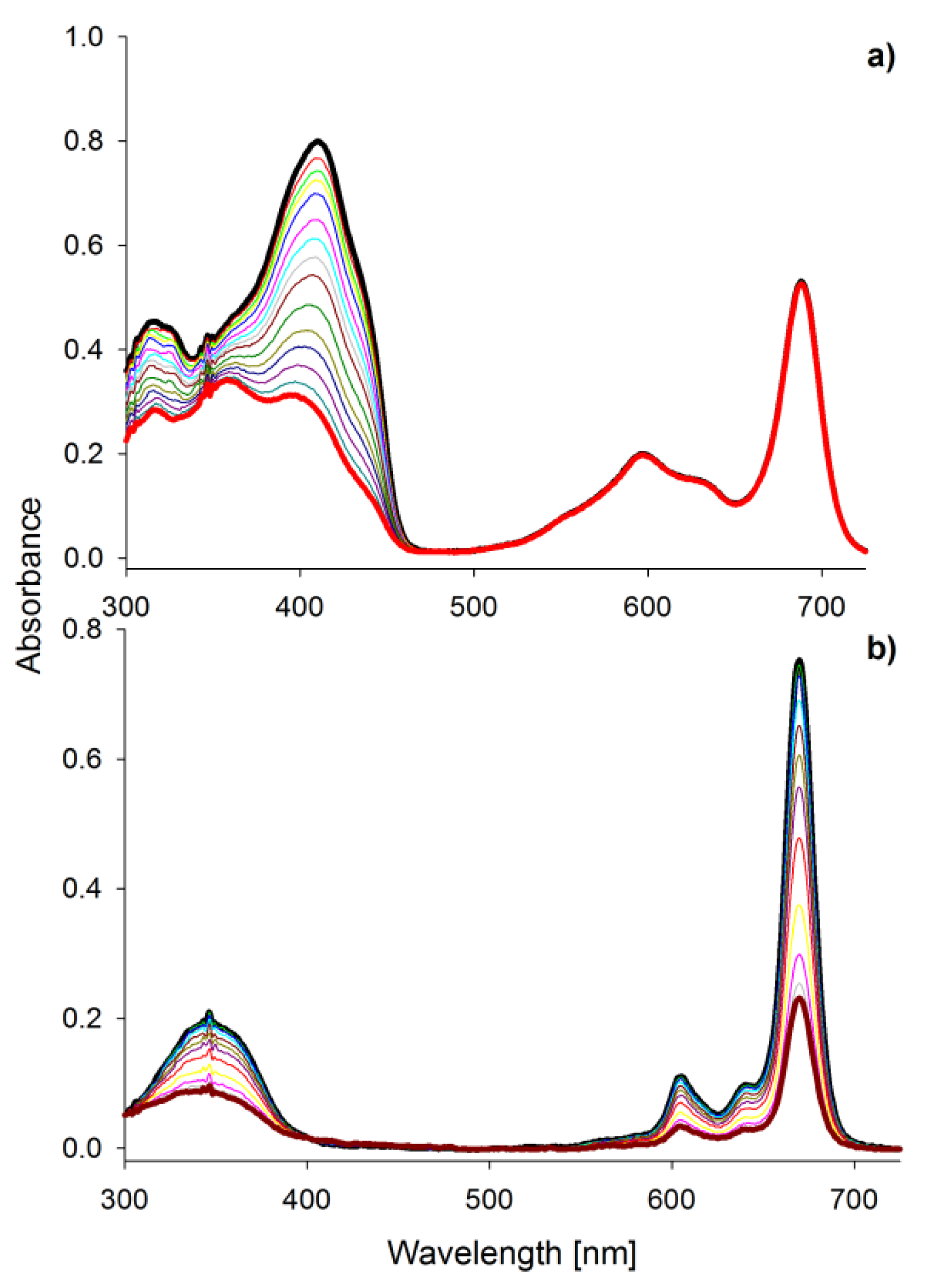
2.1. Spectral Properties
2.2. Singlet Oxygen Formation
2.3. Photostability
2.4. Photodynamic Activity against Bacteria
3. Materials and Methods
3.1. Materials
3.2. Spectral Properties
3.3. Singlet Oxygen Generation
3.4. Photostability Determination
3.5. Photodynamic Activity against Bacteria
3.5.1. Liposomes Preparation and Determination
3.5.2. Bacterial Strains and Culture Conditions
3.5.3. Photodynamic Activity
3.5.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ana Cristina de Oliveira, G.; Costa, T.F.; De Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [Green Version]
- Drago, F.; Gariazzo, L.; Cioni, M.; Trave, I.; Parodi, A. The microbiome and its relevance in complex wounds. Eur. J. Dermatol. 2019, 29, 6–13. [Google Scholar] [CrossRef]
- Cardona, A.F.; Wilson, S.E. Skin and Soft-Tissue Infections: A Critical Review and the Role of Telavancin in Their Treatment. Clin. Infect. Dis. 2015, 61, S69–S78. [Google Scholar] [CrossRef] [Green Version]
- Collier, M. Recognition and management of wound infections. World Wide Wounds 2004, 7, 8–14. [Google Scholar]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Momeni badeleh, S.; Maleki, Y. Current status and future outlook of nano--based systems for burn wound management. J. Biomed. Mater. Res. 2020, 108, 1934–1952. [Google Scholar] [CrossRef]
- Shariati, A.; Moradabadi, A.; Azimi, T.; Ghaznavi-Rad, E. Wound healing properties and antimicrobial activity of platelet-derived biomaterials. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonamuthu, J.; Cai, Y.; Liu, H.; Kasim, M.S.M.; Vasanthakumar, V.R.; Pandi, B.; Wang, H.; Yao, J. MMP-9 responsive dipeptide-tempted natural protein hydrogel-based wound dressings for accelerated healing action of infected diabetic wound. Int. J. Biol. Macromol. 2020, 153, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; He, X.; Li, L.; Li, T.; Liu, Q.; Zhou, X.; Ji, X.; Li, W.; Qian, Z. An injectable photopolymerized hydrogel with antimicrobial and biocompatible properties for infected skin regeneration. NPG Asia Mater. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Yang, W.-T.; Ke, C.-Y.; Wu, W.-T.; Tseng, Y.-H.; Lee, R.-P. Antimicrobial and anti-inflammatory potential of Angelica dahurica and Rheum officinale extract accelerates wound healing in Staphylococcus aureus-infected wounds. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [Green Version]
- Oyama, J.; Fernandes Herculano Ramos-Milaré, Á.C.; Lopes Lera-Nonose, D.S.S.; Nesi-Reis, V.; Galhardo Demarchi, I.; Alessi Aristides, S.M.; Juarez Vieira Teixeira, J.; Gomes Verzignassi Silveira, T.; Campana Lonardoni, M.V. Photodynamic therapy in wound healing in vivo: A systematic review. Photodiagn. Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagn. Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Dong, W.; Zhong, Y.; Zhang, X.; Gong, Y.; Zhan, R.; Xing, M.; Zhang, J.; Luo, G.; et al. A dual-targeted platform based on graphene for synergistic chemo-photothermal therapy against multidrug-resistant Gram-negative bacteria and their biofilms. Chem. Eng. J. 2020, 393, 124595. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, B.L.; Situ, X.; Scholle, F.; Bartelmess, J.; Weare, W.W.; Ghiladi, R.A. Antiviral, Antifungal and Antibacterial Activities of a BODIPY-Based Photosensitizer. Molecules 2015, 20, 10604–10621. [Google Scholar] [CrossRef] [Green Version]
- Piskorz, J.; Dlugaszewska, J.; Porolnik, W.; Teubert, A.; Mielcarek, J. Boron-dipyrromethene derivatives bearing N-alkyl phthalimide and amine substituents of potential application in the photoinactivation of bacteria. Dyes Pigments 2020, 178, 108322. [Google Scholar] [CrossRef]
- Kumar, P.S.M.; Francis, A.P.; Devasena, T. Biosynthesized and Chemically Synthesized Titania Nanoparticles: Comparative Analysis of Antibacterial Activity. J. Environ. Nanotechnol. 2014, 3, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, A.; Hassani, L.; Mohammadi, F.; Jahangoshayi, P.; Mohammadi, K. The biological effects of vanadyl curcumin and vanadyl diacetylcurcumin complexes: The effect on structure, function and oxidative stability of the peroxidase enzyme, antibacterial activity and cytotoxic effect. J. Enzyme Inhib. Med. Chem. 2016, 31, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Elashnikov, R.; Radocha, M.; Panov, I.; Rimpelova, S.; Ulbrich, P.; Michalcova, A.; Svorcik, V.; Lyutakov, O. Porphyrin-silver nanoparticles hybrids: Synthesis, characterization and antibacterial activity. Mater. Sci. Eng. C 2019, 102, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, D.; Lijewski, S.; Falkowski, M.; Piskorz, J.; Szczolko, W.; Sobotta, L.; Stolarska, M.; Popenda, L.; Jurga, S.; Konopka, K.; et al. Dendrimeric sulfanyl porphyrazines: Synthesis, physico-chemical characterization and biological activity for potential applications in anticancer photodynamic therapy. ChemPlusChem 2016, 81, 460. [Google Scholar] [CrossRef] [PubMed]
- Günsel, A.; Güzel, E.; Bilgiçli, A.T.; Atmaca, G.Y.; Erdoğmuş, A.; Yarasir, M.N. Synthesis and investigation of photophysicochemical properties of novel ketone-substituted gallium (III) and indium (III) phthalocyanines with high singlet oxygen yield for photodynamic therapy. J. Lumin. 2017, 192, 888–892. [Google Scholar] [CrossRef]
- Wierzchowski, M.; Łażewski, D.; Tardowski, T.; Grochocka, M.; Czajkowski, R.; Sobiak, S.; Sobotta, L. Nanomolar photodynamic activity of porphyrins bearing 1,4,7-trioxanonyl and 2-methyl-5-nitroimidazole moieties against cancer cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111703. [Google Scholar] [CrossRef]
- Sobotta, L.; Ziental, D.; Sniechowska, J.; Dlugaszewska, J.; Potrzebowski, M.J. Lipid vesicle-loaded meso-substituted chlorins of high in vitro antimicrobial photodynamic activity. Photochem. Photobiol. Sci. 2019, 18, 213–223. [Google Scholar] [CrossRef]
- Sobotta, L.; Dlugaszewska, J.; Ziental, D.; Szczolko, W.; Koczorowski, T.; Goslinski, T.; Mielcarek, J. Optical properties of a series of pyrrolyl-substituted porphyrazines and their photoinactivation potential against Enterococcus faecalis after incorporation into liposomes. J. Photochem. Photobiol. A Chem. 2019, 368, 104–109. [Google Scholar] [CrossRef]
- Kandaz, M.; Özkaya, A.R.; Koca, A.; Salih, B. Water and alcohol-soluble octakis-metalloporphyrazines bearing sulfanyl polyetherol substituents: Synthesis, spectroscopy and electrochemistry. Dyes Pigments 2007, 74, 483–489. [Google Scholar] [CrossRef]
- Michel, S.L.J.; Hoffman, B.M.; Baum, S.M.; Barrett, A.G.M. Peripherally Functionalized Porphyrazines: Novel Metallomacrocycles with Broad, Untapped Potential. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 473–590. ISBN 978-0-471-22711-3. [Google Scholar]
- Kobayashi, N. Spectroscopically and/or Structurally Intriguing Phthalocyanines and Related Compounds. Part 1. Monomeric systems. IVUZKKT 2019, 62, 4–46. [Google Scholar] [CrossRef]
- Mack, J.; Kobayashi, N. Low Symmetry Phthalocyanines and Their Analogues. Chem. Rev. 2011, 111, 281–321. [Google Scholar] [CrossRef] [PubMed]
- Kasprzycki, P.; Sobotta, L.; Lijewski, S.; Wierzchowski, M.; Goslinski, T.; Mielcarek, J.; Radzewicz, C.; Fita, P. Unusual cis-diprotonated forms and fluorescent aggregates of non-peripherally alkoxy-substituted metallophthalocyanines. Phys. Chem. Chem. Phys. 2017, 19, 21390–21400. [Google Scholar] [CrossRef] [PubMed]
- Lijewski, S.; Gierszewski, M.; Sobotta, L.; Piskorz, J.; Kordas, P.; Kucinska, M.; Baranowski, D.; Gdaniec, Z.; Murias, M.; Karolczak, J.; et al. Photophysical properties and photochemistry of a sulfanyl porphyrazine bearing isophthaloxybutyl substituents. Dyes Pigments 2015, 113, 702–708. [Google Scholar] [CrossRef]
- Ehrlich, L.A.; Skrdla, P.J.; Jarrell, W.K.; Sibert, J.W.; Armstrong, N.R.; Saavedra, S.S.; Barrett, A.G.M.; Hoffman, B.M. Preparation of Polyetherol-Appended Sulfur Porphyrazines and Investigations of Peripheral Metal Ion Binding in Polar Solvents. Inorg. Chem. 2000, 39, 3963–3969. [Google Scholar] [CrossRef]
- Baygu, Y.; Yıldız, B.; Kabay, N.; Gök, Y. Novel magnesium and zinc porphyrazines containing galactose moieties: Synthesis via click reaction and characterization. Inorg. Chem. Commun. 2016, 71, 35–40. [Google Scholar] [CrossRef]
- Harper, S.R.; Pfrunder, M.C.; Esdaile, L.J.; Jensen, P.; McMurtrie, J.C.; Arnold, D.P. Synthetic, Structural, and Spectroscopic Studies of Bis(porphyrinzinc) Complexes Linked by Two-Atom Conjugating Bridges: Bis(porphyrinzinc) Complexes Linked by Two-Atom Bridges. Eur. J. Org. Chem. 2015, 2015, 2807–2825. [Google Scholar] [CrossRef]
- Gierszewski, M.; Falkowski, M.; Sobotta, L.; Stolarska, M.; Popenda, L.; Lijewski, S.; Wicher, B.; Burdzinski, G.; Karolczak, J.; Jurga, S.; et al. Porphyrazines with peripheral isophthaloxyalkylsulfanyl substituents and their optical properties. J. Photochem. Photobiol. A Chem. 2015, 307, 54–67. [Google Scholar] [CrossRef]
- Freyer, W.; Mueller, S.; Teuchner, K. Photophysical properties of benzoannelated metal-free phthalocyanines. J. Photochem. Photobiol. A Chem. 2004, 163, 231–240. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef] [PubMed]
- Piskorz, J.; Lijewski, S.; Gierszewski, M.; Gorniak, K.; Sobotta, L.; Wicher, B.; Tykarska, E.; Düzgüneş, N.; Konopka, K.; Sikorski, M.; et al. Sulfanyl porphyrazines: Molecular barrel-like self-assembly in crystals, optical properties and in vitro photodynamic activity towards cancer cells. Dyes Pigments 2016, 136, 898–908. [Google Scholar] [CrossRef]
- Piskorz, J.; Skupin, P.; Lijewski, S.; Korpusinski, M.; Sciepura, M.; Konopka, K.; Sobiak, S.; Goslinski, T.; Mielcarek, J. Synthesis, physical–chemical properties and in vitro photodynamic activity against oral cancer cells of novel porphyrazines possessing fluoroalkylthio and dietherthio substituents. J. Fluor. Chem. 2012, 135, 265–271. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Piskorz, J.; Popenda, L.; Stolarska, M.; Szczolko, W.; Konopka, K.; Jurga, S.; Sobotta, L.; Mielcarek, J.; Düzgüneş, N.; et al. S-seco-porphyrazine as a new member of the seco-porphyrazine family—Synthesis, characterization and photocytotoxicity against cancer cells. Bioorg. Chem. 2020, 96, 103634. [Google Scholar] [CrossRef]
- Taştemel, A.; Karaca, B.Y.; Durmuş, M.; Bulut, M. Photophysical and photochemical properties of novel metallophthalocyanines bearing 7-oxy-3-( m -methoxyphenyl)coumarin groups. J. Lumin. 2015, 168, 163–171. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent effects on the photochemical and fluorescence properties of zinc phthalocyanine derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Sobotta, L.; Lijewski, S.; Dlugaszewska, J.; Nowicka, J.; Mielcarek, J.; Goslinski, T. Photodynamic inactivation of Enterococcus faecalis by conjugates of zinc(II) phthalocyanines with thymol and carvacrol loaded into lipid vesicles. Inorg. Chim. Acta 2019, 489, 180–190. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Durmuş, M.; Atilla, D.; Gürek, A.G.; Ahsen, V.; Nyokong, T. Synthesis, photophysical and photochemical studies on long chain zinc phthalocyanine derivatives. Synth. Met. 2008, 158, 839–847. [Google Scholar] [CrossRef]
- Bonnett, R.; Martınez, G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Kaliya, O.L. Oxidative photobleaching of phthalocyanines in solution. J. Porphyr. Phthalocyanines 2012, 16, 705–712. [Google Scholar] [CrossRef]
- Dilber, G.; Altunparmak, H.; Nas, A.; Kantekin, H.; Durmuş, M. The peripheral and non-peripheral 2H-benzotriazole substituted phthalocyanines: Synthesis, characterization, photophysical and photochemical studies of zinc derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Łapok, Ł.; Cyza, M.; Gut, A.; Kępczyński, M.; Szewczyk, G.; Sarna, T.; Nowakowska, M. Synthesis, spectroscopic properties and interaction with a liposomal membrane of a novel iodinated magnesium phthalocyanine. J. Photochem. Photobiol. A Chem. 2014, 286, 55–63. [Google Scholar] [CrossRef]
- Sergeeva, N.N.; Senge, M.O. Photochemical Transformations Involving Magnesium Porphyrins and Phthalocyanines. In PATAI’S Chemistry of Functional Groups; Rappoport, Z., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 978-0-470-68253-1. [Google Scholar]
- Piskorz, J.; Mlynarczyk, D.T.; Szczolko, W.; Konopka, K.; Düzgüneş, N.; Mielcarek, J. Liposomal formulations of magnesium sulfanyl tribenzoporphyrazines for the photodynamic therapy of cancer. J. Inorg. Biochem. 2018, 184, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Falkowski, M.; Popenda, L.; Kryjewski, M.; Szczolko, W.; Jurga, S.; Mielcarek, J.; Goslinski, T. Tribenzoporphyrazines with dendrimeric peripheral substituents and their promising photocytotoxic activity against Staphylococcus aureus. J. Photochem. Photobiol. B Biol. 2020, 204, 111803. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masiera, N.; Bojarska, A.; Gawryszewska, I.; Sadowy, E.; Hryniewicz, W.; Waluk, J. Antimicrobial photodynamic therapy by means of porphycene photosensitizers. J. Photochem. Photobiol. B Biol. 2017, 174, 84–89. [Google Scholar] [CrossRef]
- Dlugaszewska, J.; Szczolko, W.; Koczorowski, T.; Skupin-Mrugalska, P.; Teubert, A.; Konopka, K.; Kucinska, M.; Murias, M.; Düzgüneş, N.; Mielcarek, J.; et al. Antimicrobial and anticancer photodynamic activity of a phthalocyanine photosensitizer with N -methyl morpholiniumethoxy substituents in non-peripheral positions. J. Inorg. Biochem. 2017, 172, 67–79. [Google Scholar] [CrossRef]
- Gajdács, M. The Continuing Threat of Methicillin-Resistant Staphylococcus aureus. Antibiotics 2019, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [Green Version]
- Barnett, T.C.; Bowen, A.C.; Carapetis, J.R. The fall and rise of Group A Streptococcus diseases. Epidemiol. Infect. 2019, 147, e4. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Speer, C.P.; Glaser, K. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence 2018, 9, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Mamone, L.; Di Venosa, G.; Gándara, L.; Sáenz, D.; Vallecorsa, P.; Schickinger, S.; Rossetti, M.V.; Batlle, A.; Buzzola, F.; Casas, A. Photodynamic inactivation of Gram-positive bacteria employing natural resources. J. Photochem. Photobiol. B Biol. 2014, 133, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chauke, V.; Durmuş, M.; Nyokong, T. Photochemistry, photophysics and nonlinear optical parameters of phenoxy and tert-butylphenoxy substituted indium(III) phthalocyanines. J. Photochem. Photobiol. A Chem. 2007, 192, 179–187. [Google Scholar] [CrossRef]
- Sobotta, L.; Fita, P.; Szczolko, W.; Wrotynski, M.; Wierzchowski, M.; Goslinski, T.; Mielcarek, J. Functional singlet oxygen generators based on porphyrazines with peripheral 2,5-dimethylpyrrol-1-yl and dimethylamino groups. J. Photochem. Photobiol. A Chem. 2013, 269, 9–16. [Google Scholar] [CrossRef]
- Seotsanyana-Mokhosi, I.; Kuznetsova, N.; Nyokong, T. Photochemical studies of tetra-2, 3-pyridinoporphyrazines. J. Photochem. Photobiol. A Chem. 2001, 140, 215–222. [Google Scholar] [CrossRef]
- Sobotta, L.; Sniechowska, J.; Ziental, D.; Dlugaszewska, J.; Potrzebowski, M.J. Chlorins with (trifluoromethyl)phenyl substituents—Synthesis, lipid formulation and photodynamic activity against bacteria. Dyes Pigments 2019, 160, 292–300. [Google Scholar] [CrossRef]
- Sobotta, L.; Dlugaszewska, J.; Kasprzycki, P.; Lijewski, S.; Teubert, A.; Mielcarek, J.; Gdaniec, M.; Goslinski, T.; Fita, P.; Tykarska, E. In vitro photodynamic activity of lipid vesicles with zinc phthalocyanine derivative against Enterococcus faecalis. J. Photochem. Photobiol. B Biol. 2018, 183, 111–118. [Google Scholar] [CrossRef]
- Sobotta, L.; Dlugaszewska, J.; Gierszewski, M.; Tillo, A.; Sikorski, M.; Tykarska, E.; Mielcarek, J.; Goslinski, T. Photodynamic inactivation of Enterococcus faecalis by non-peripherally substituted magnesium phthalocyanines entrapped in lipid vesicles. J. Photochem. Photobiol. B Biol. 2018, 188, 100–106. [Google Scholar] [CrossRef]
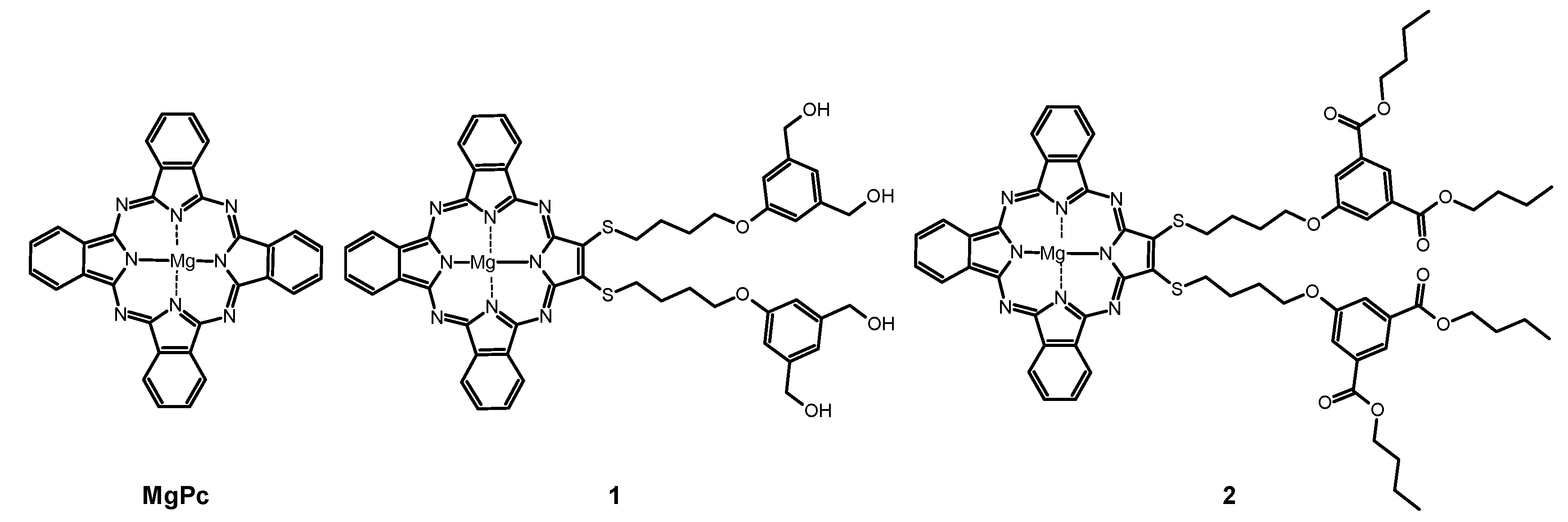
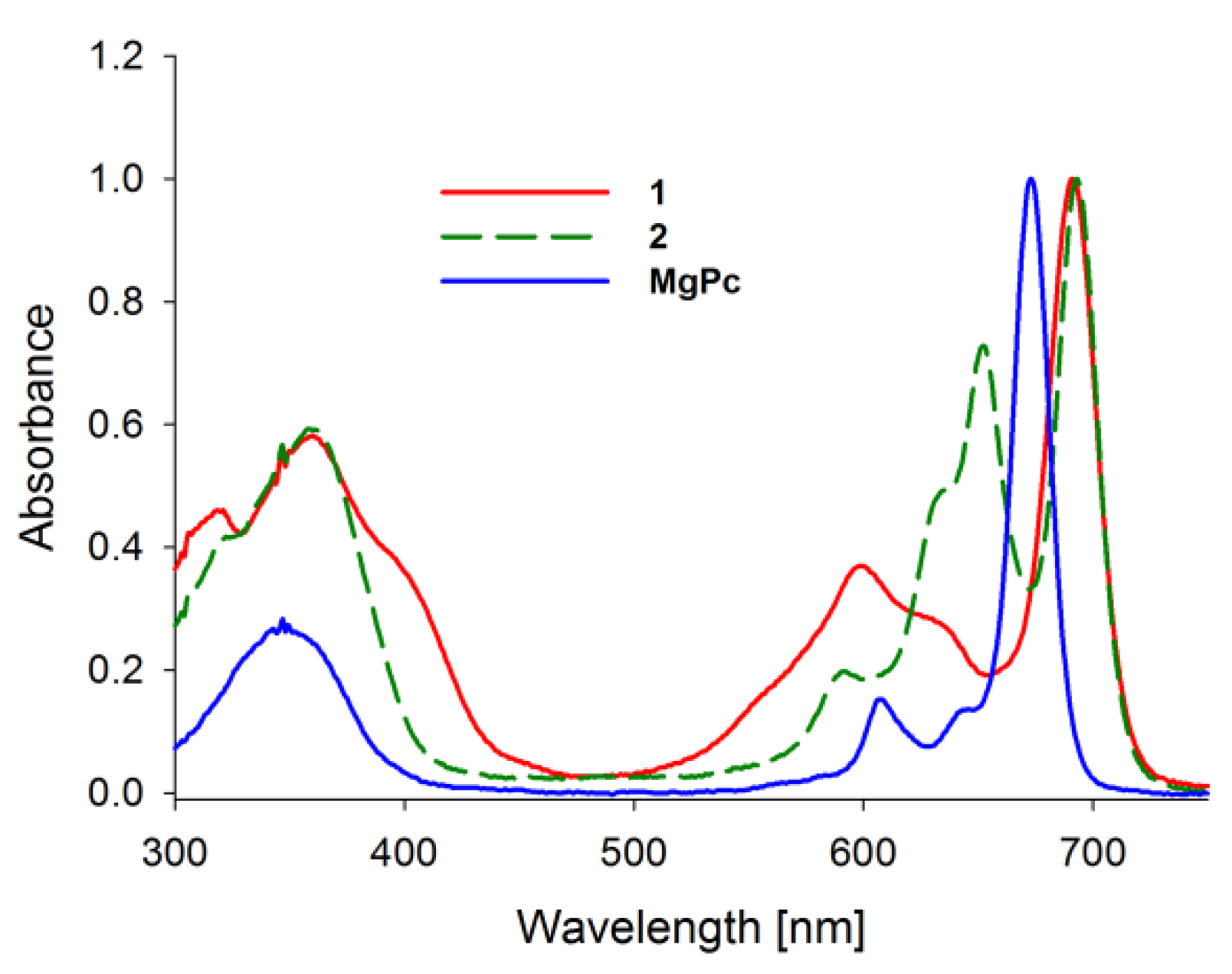
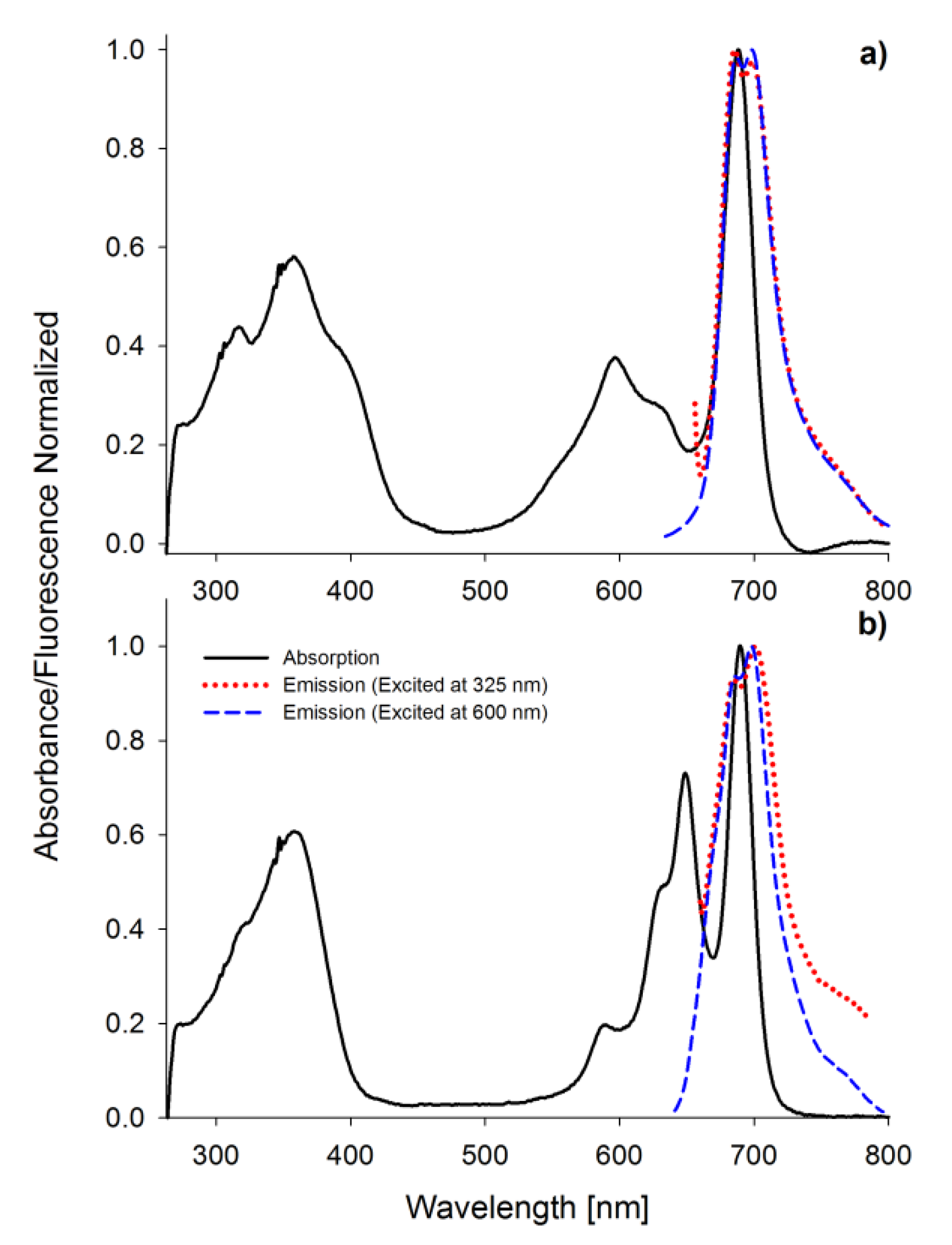
| Compound | Solvent | ΦFL | 106ΦP | ΦΔ | logε[nm] | |
|---|---|---|---|---|---|---|
| 1 | DMF | 0.051 | 86.40 | 0.20 [26] | 4.49[346] | 4.67[688] |
| DMSO | 0.029 | 37.60 | 0.27 | 4.45[346] | 4.67[691] | |
| 2 | DMF | 0.008 | 48.40 | 0.05 [26] | 4.69[346] | 4.83[690] |
| DMSO | 0.002 | 4.84 | 0.08 | 4.65[346] | 4.84[693] | |
| MgPc | DMF | 0.230 [46] | 327 [46] | 0.28 [46] | 4.91[346] | 5.36[670] |
| DMSO | 0.181 | 4.77 | 0.14 | 4.80[346] | 5.33[673] | |
| ZnPc | DMF | 0.200 [47] | 10.2 [48] | 0.56 [49] | - | |
| DMSO | 0.170 [47] | 3.5 [48] | 0.67 [49] | |||
| Compound | Mean Diameter [nm] | Dv10 [nm] | Dv50 [nm] | Dv90 [nm] |
|---|---|---|---|---|
| 1 | 301 ± 3 | 126 | 217 | 629 |
| 2 | 188 ± 23 | 71 | 147 | 346 |
| MgPc | 119 ± 4 | 65 | 115 | 171 |
| Compound | 1 | 2 | MgPc |
|---|---|---|---|
| Concentration [M] | log reduction in bacterial growth | ||
| Staphylococcus aureus | |||
| 10−4 | 3.5 ± 0.3 | 3.6 ± 0.2 | 4.9 ± 0.3 |
| 10−5 | >5.9 ± 0.0 | 4.3 ± 0.1 | 4.6 ± 0.2 |
| MRSA | |||
| 10−4 | no activity | 0.7 ± 0.1 | >5.7 ± 0.1 |
| 10−5 | 5.1 ± 0.1 | 3.8 ± 0.0 | >5.7 ± 0.1 |
| Staphylococcus epidermidis | |||
| 10−4 | 3.3 ± 0.2 | 3.6 ± 0.2 | >6.0 ± 0.0 |
| 10−5 | >5.7 ± 0.2 | >5.7 ± 0.3 | >5.7 ± 0.4 |
| Streptococcus pyogenes | |||
| 10−4 | 0.5 ± 0.3 | 1.7 ± 0.2 | >4.7 ± 0.3 |
| 10−5 | >4.7 ± 0.3 | >4.7 ± 0.3 | >4.7 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarska, M.; Glowacka-Sobotta, A.; Mlynarczyk, D.T.; Dlugaszewska, J.; Goslinski, T.; Mielcarek, J.; Sobotta, L. Photodynamic Activity of Tribenzoporphyrazines with Bulky Periphery against Wound Bacteria. Int. J. Mol. Sci. 2020, 21, 6145. https://doi.org/10.3390/ijms21176145
Stolarska M, Glowacka-Sobotta A, Mlynarczyk DT, Dlugaszewska J, Goslinski T, Mielcarek J, Sobotta L. Photodynamic Activity of Tribenzoporphyrazines with Bulky Periphery against Wound Bacteria. International Journal of Molecular Sciences. 2020; 21(17):6145. https://doi.org/10.3390/ijms21176145
Chicago/Turabian StyleStolarska, Magdalena, Arleta Glowacka-Sobotta, Dariusz T. Mlynarczyk, Jolanta Dlugaszewska, Tomasz Goslinski, Jadwiga Mielcarek, and Lukasz Sobotta. 2020. "Photodynamic Activity of Tribenzoporphyrazines with Bulky Periphery against Wound Bacteria" International Journal of Molecular Sciences 21, no. 17: 6145. https://doi.org/10.3390/ijms21176145
APA StyleStolarska, M., Glowacka-Sobotta, A., Mlynarczyk, D. T., Dlugaszewska, J., Goslinski, T., Mielcarek, J., & Sobotta, L. (2020). Photodynamic Activity of Tribenzoporphyrazines with Bulky Periphery against Wound Bacteria. International Journal of Molecular Sciences, 21(17), 6145. https://doi.org/10.3390/ijms21176145






