Intramolecular Hydrogen Bond Driven Conformational Selectivity of Coumarin Derivatives of Resorcin[4]arene
Abstract
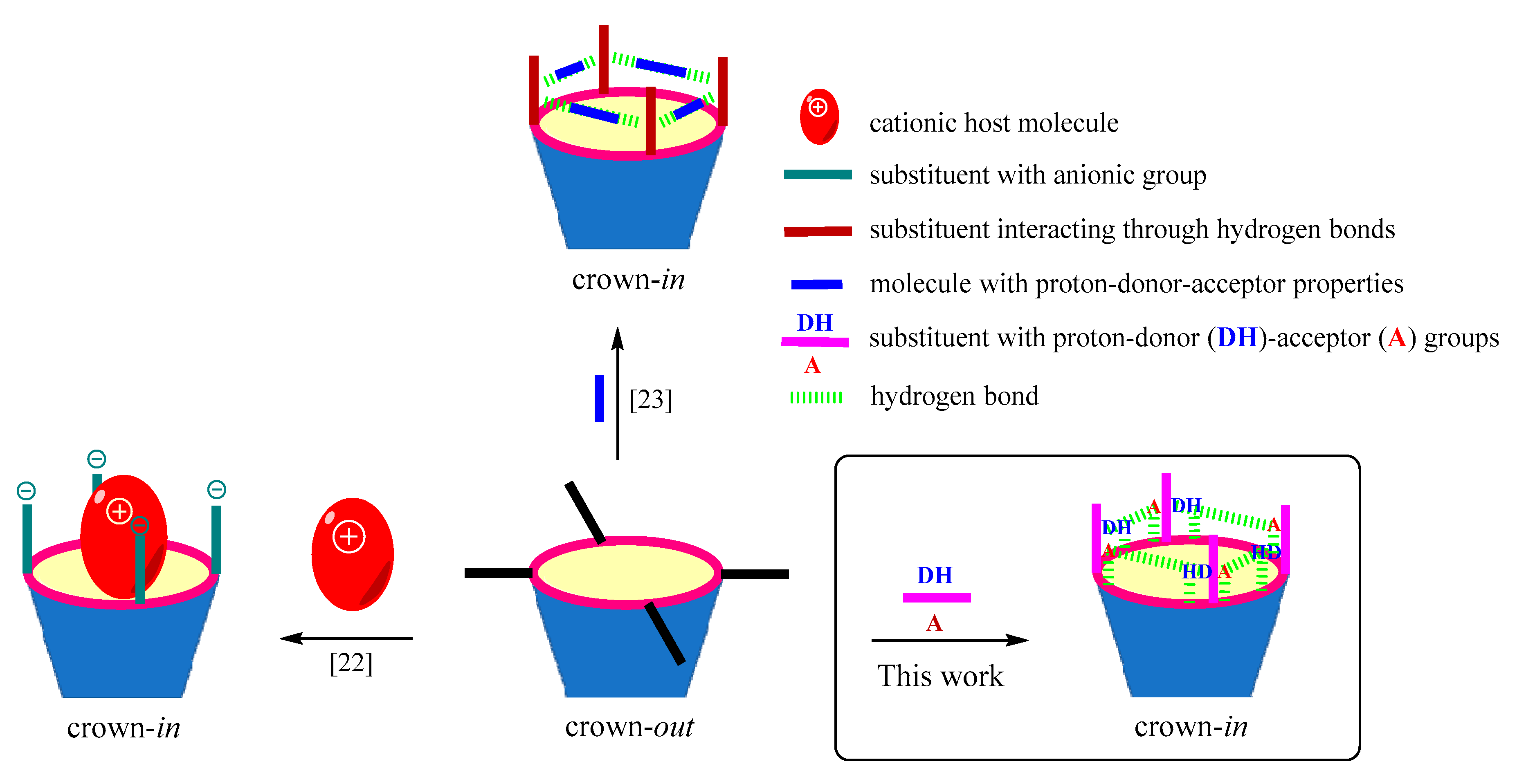
:1. Introduction
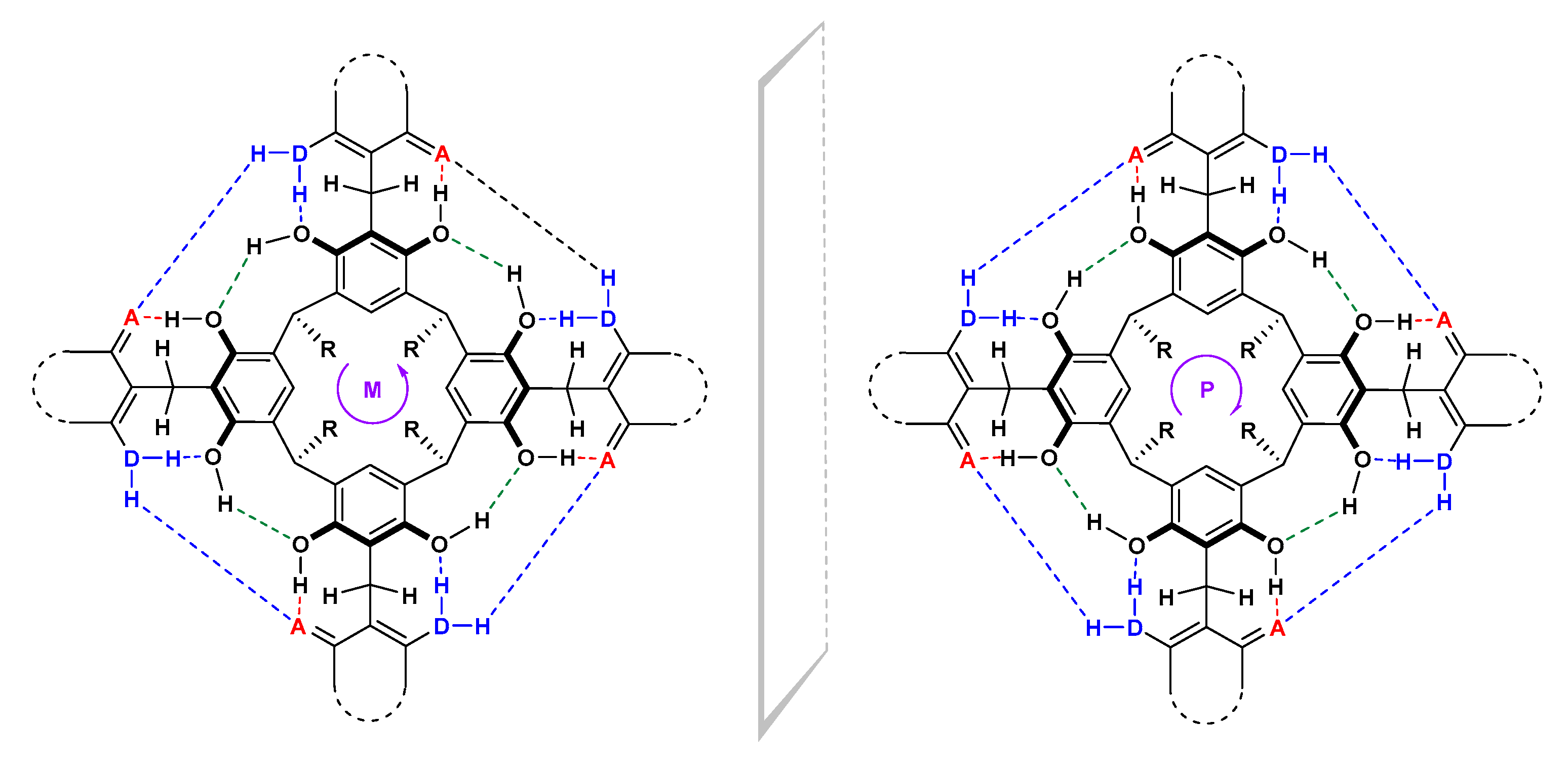
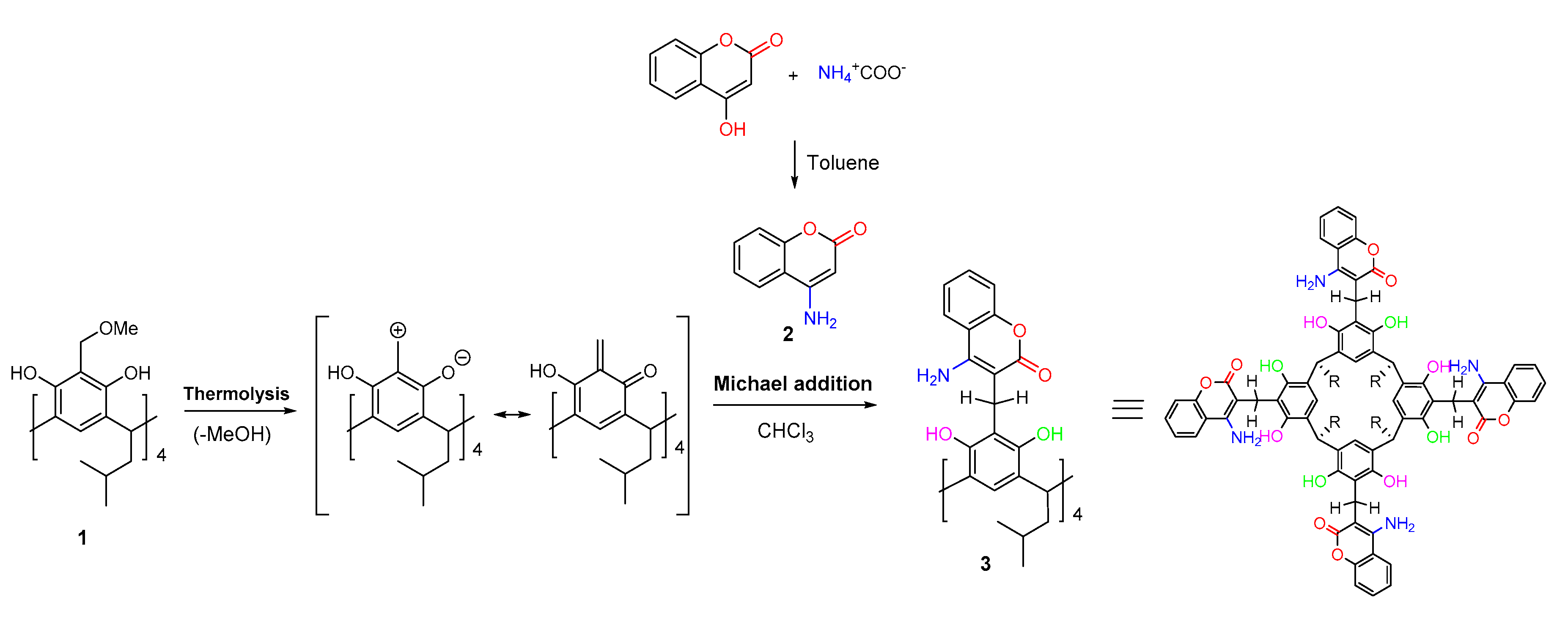
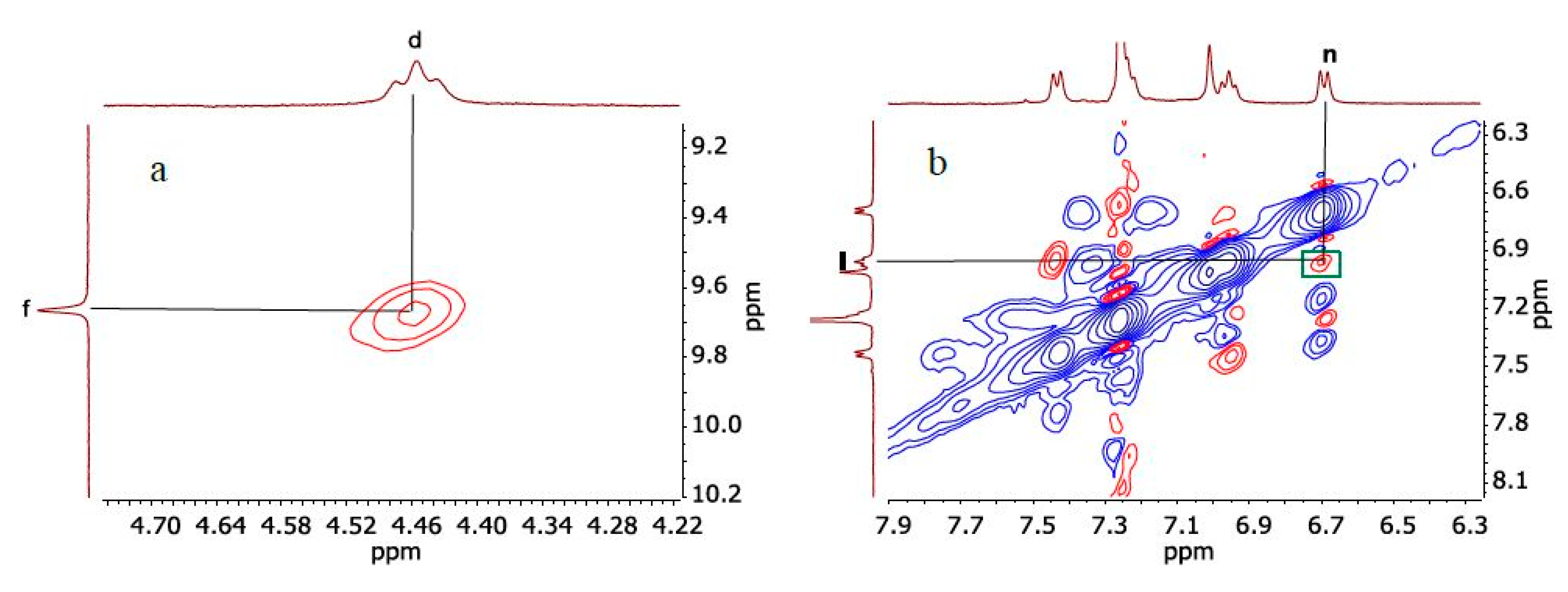
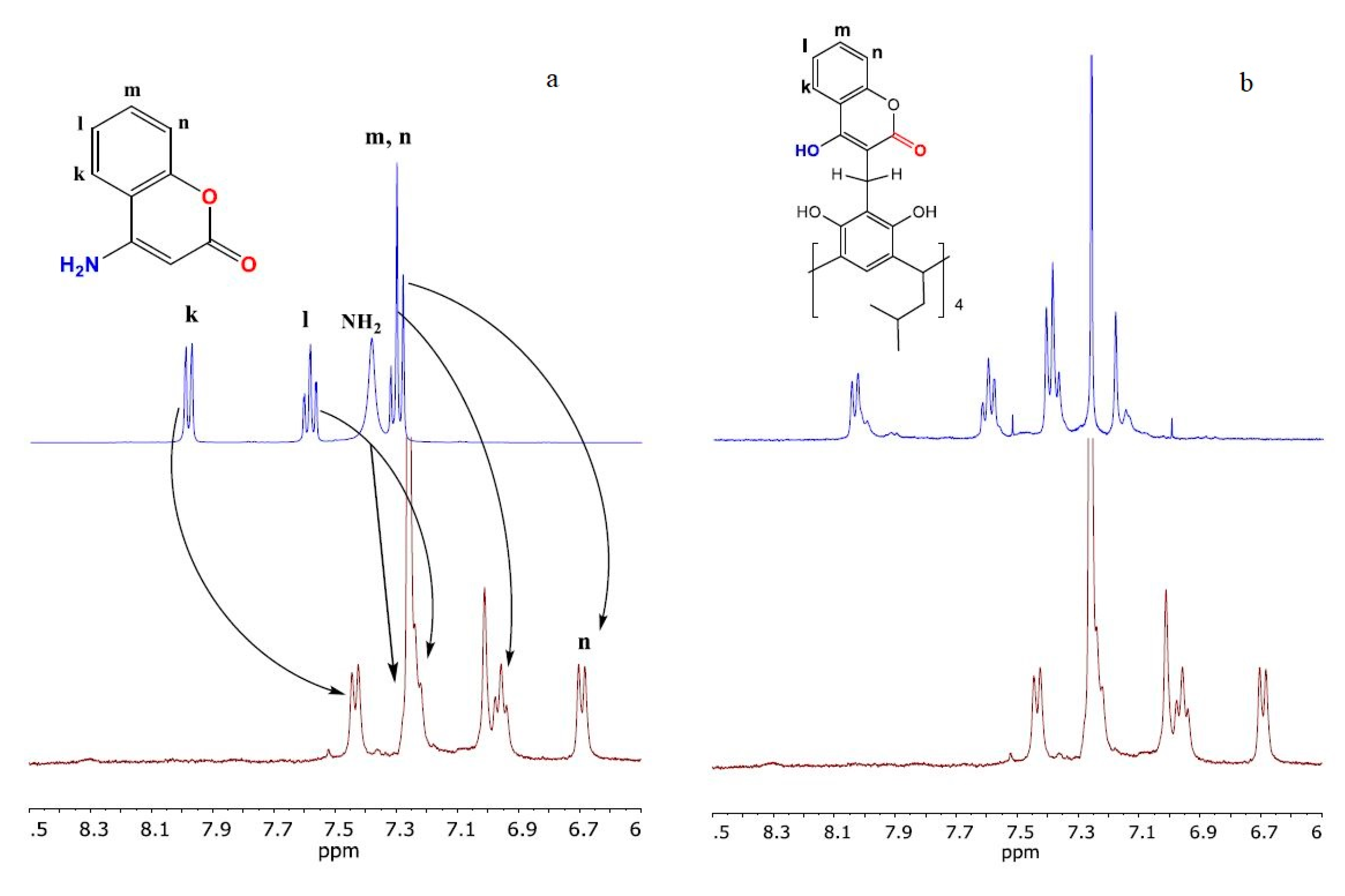
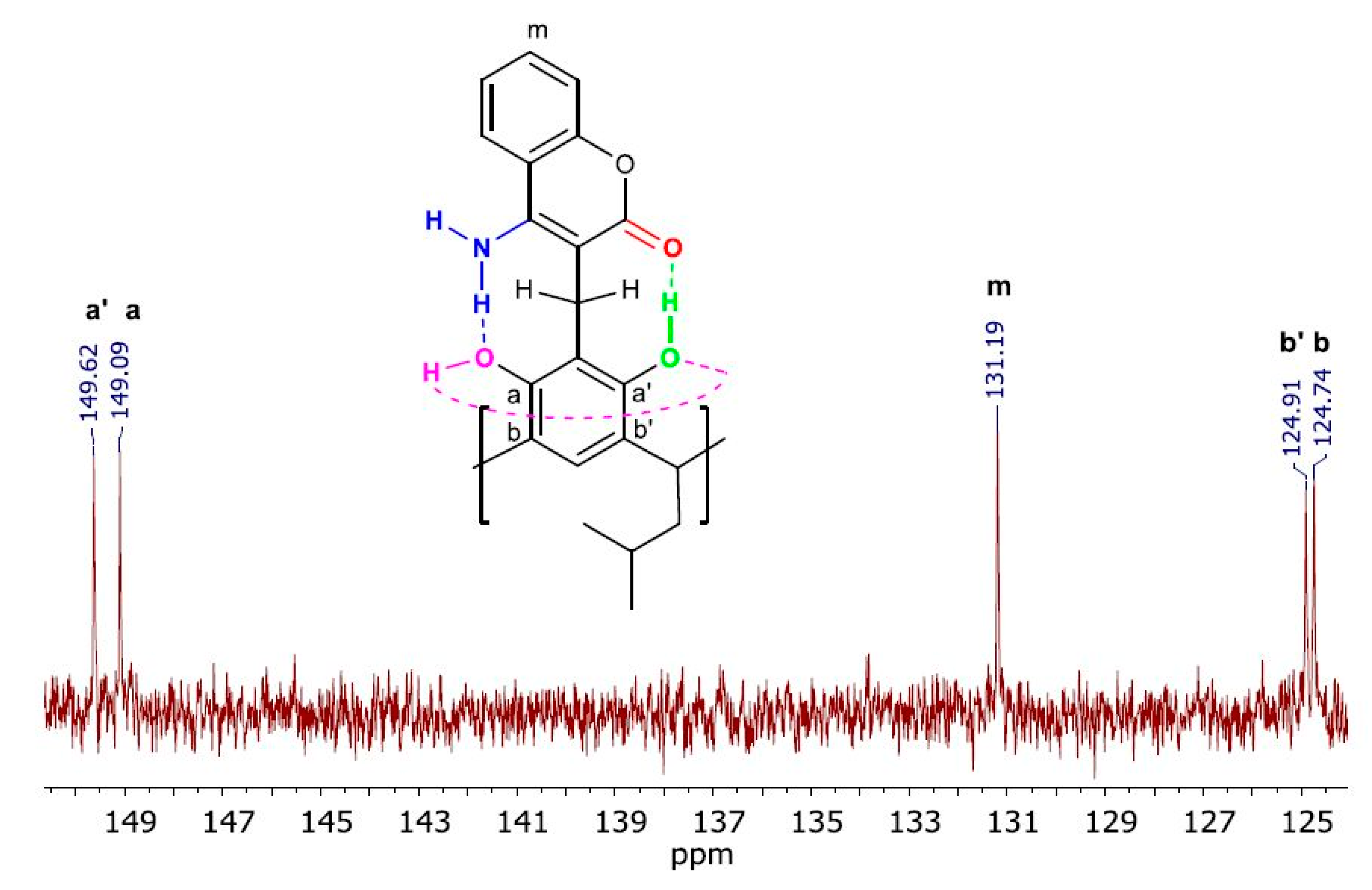
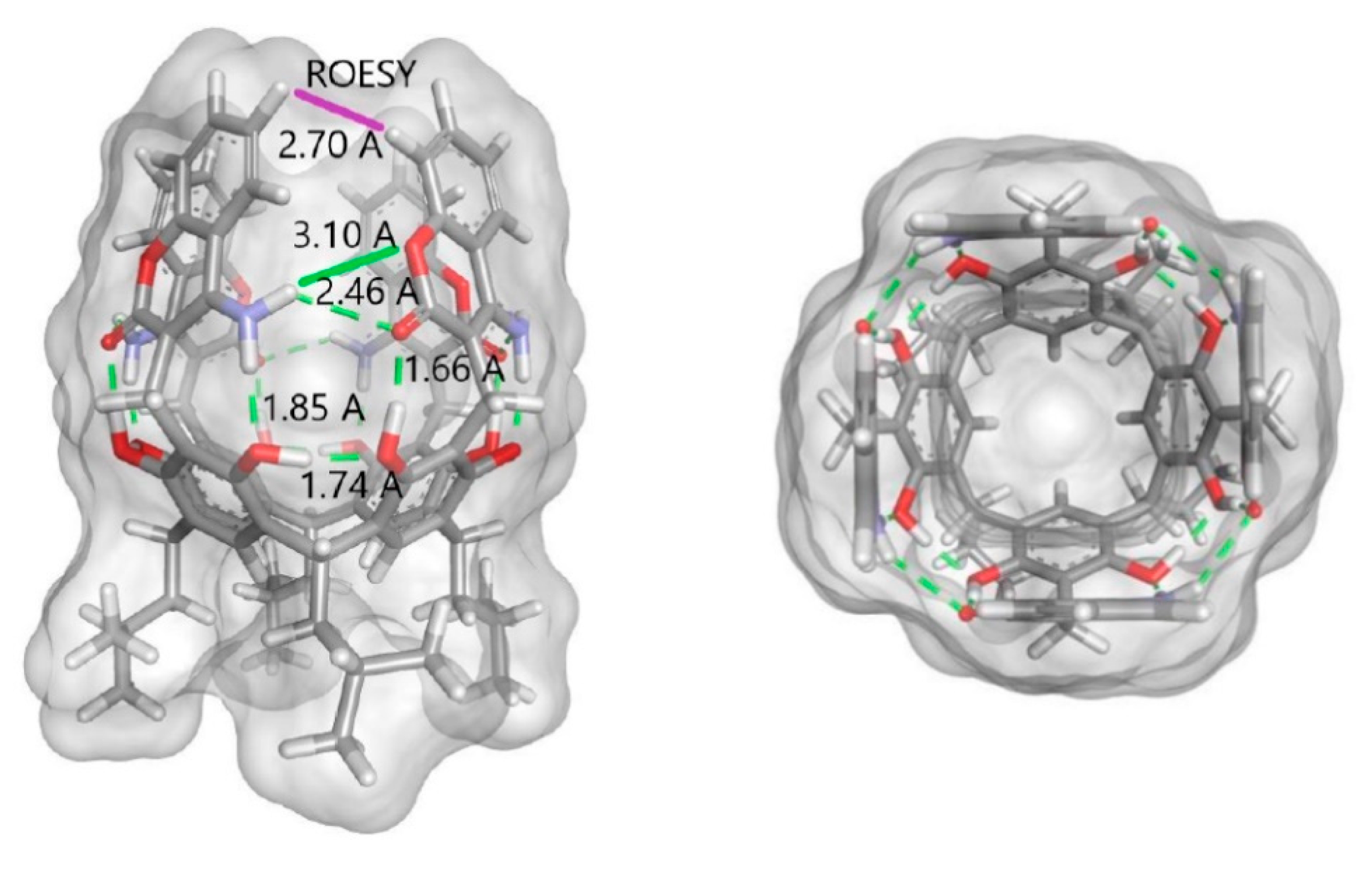
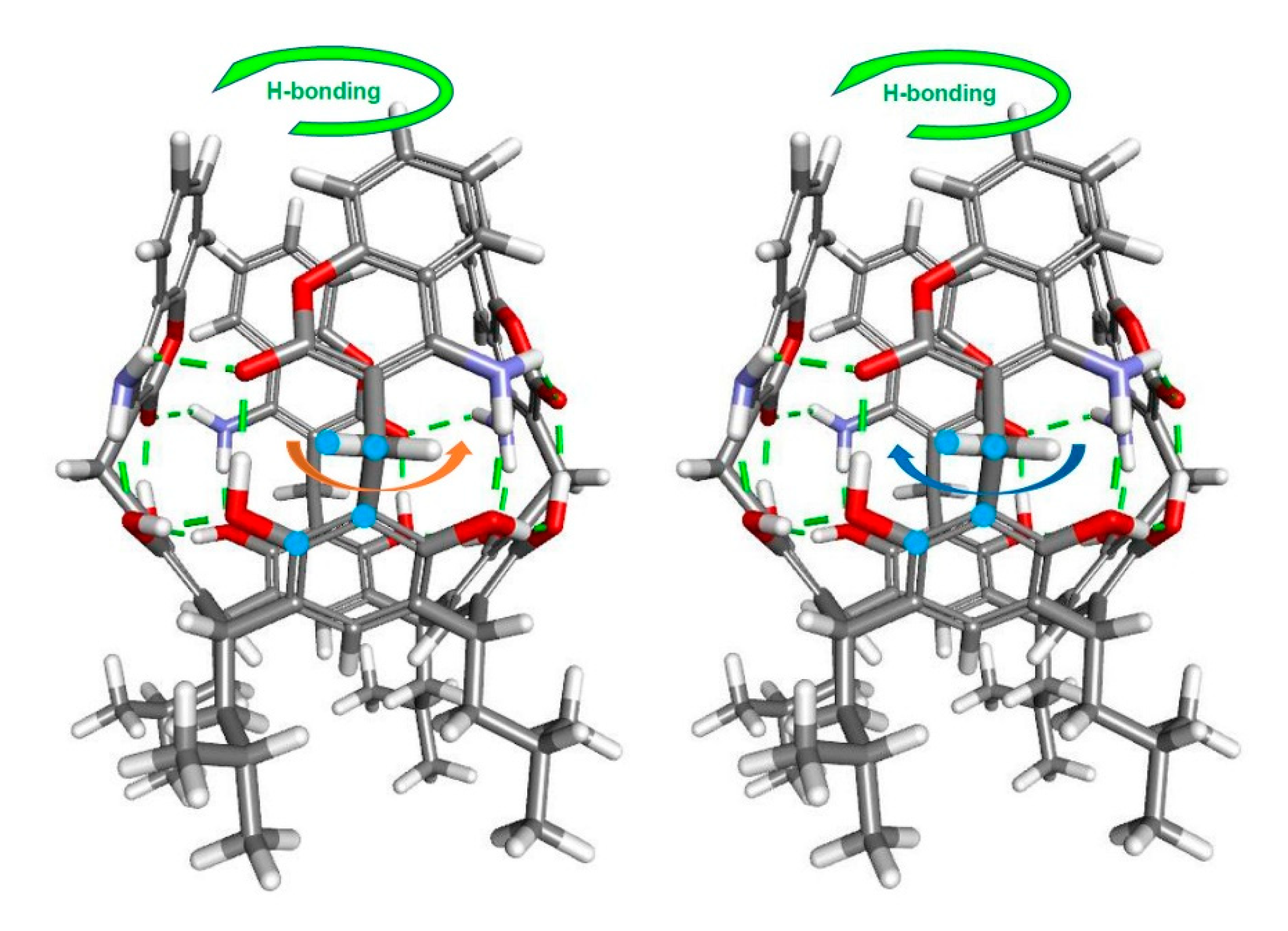
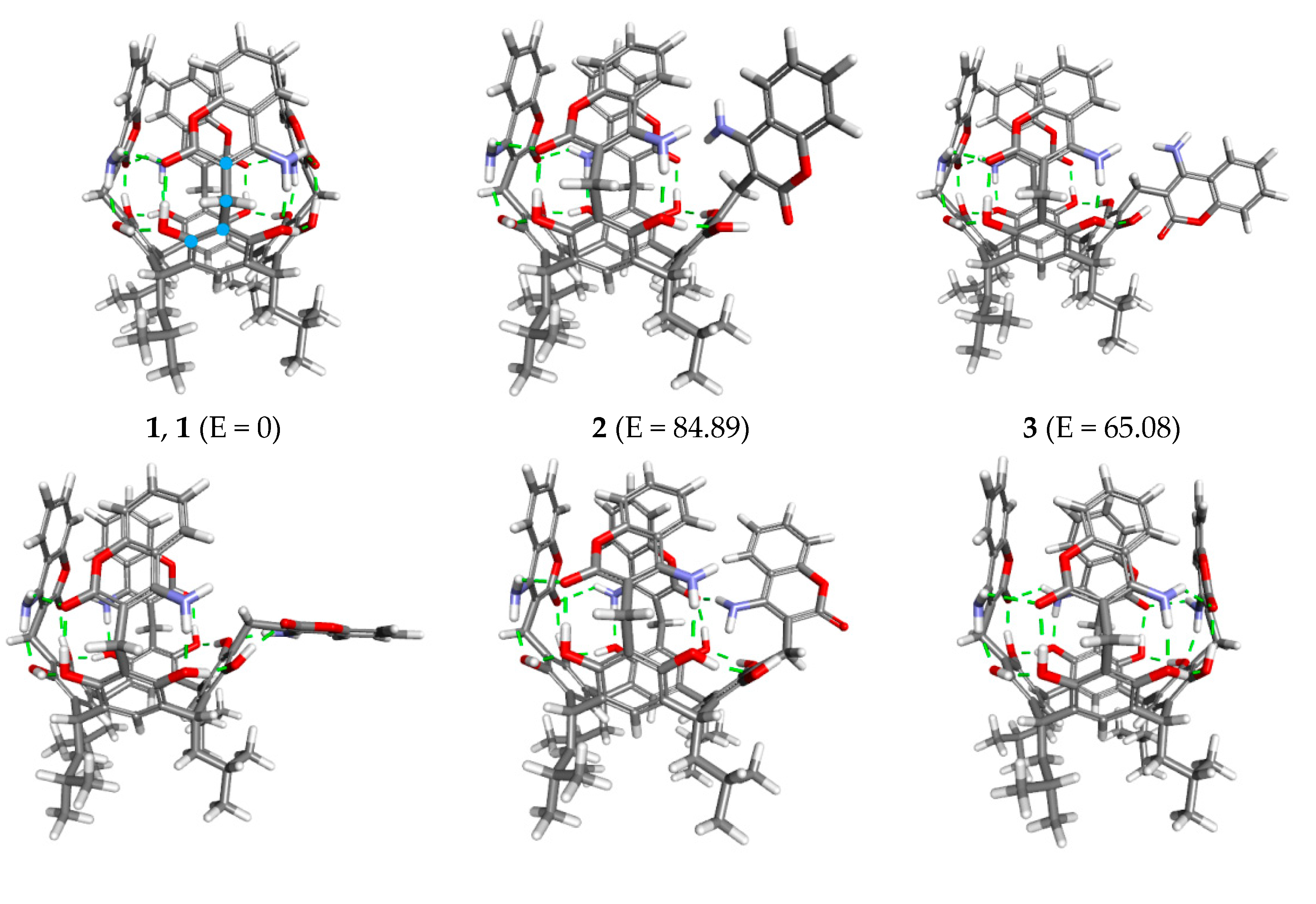
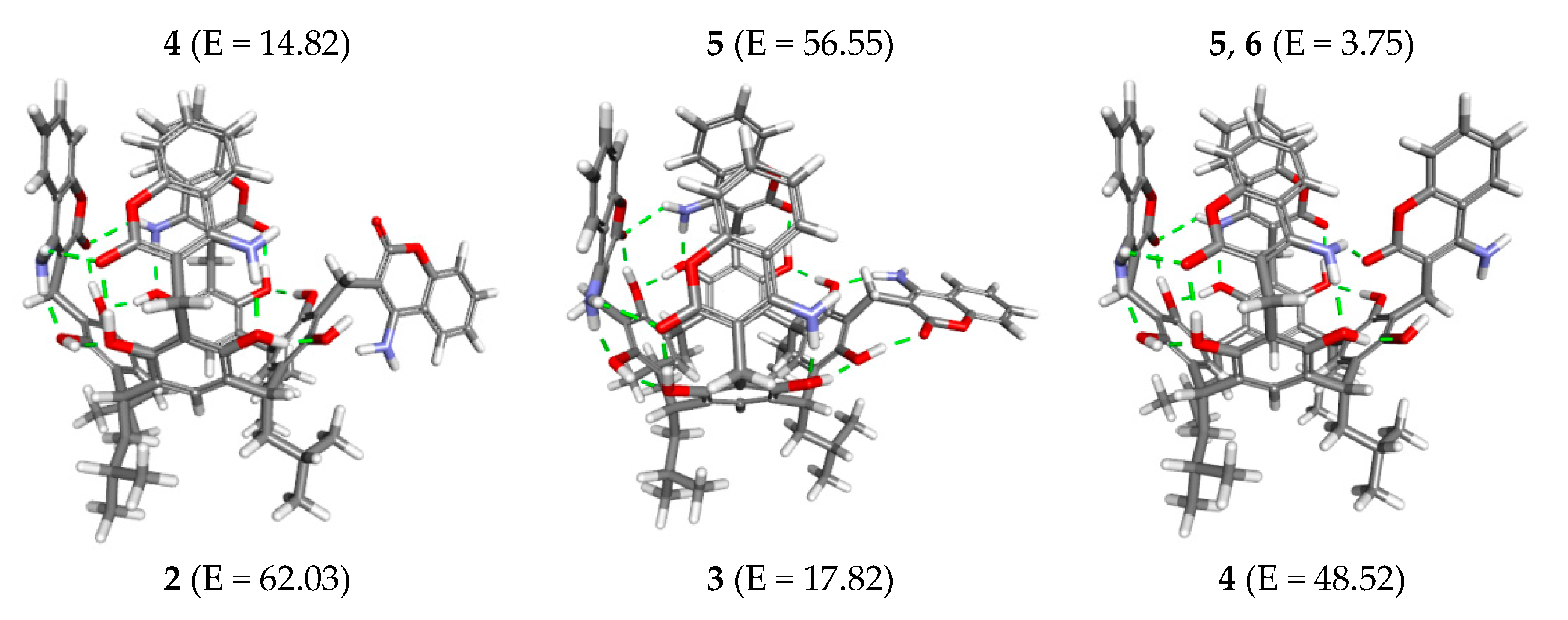
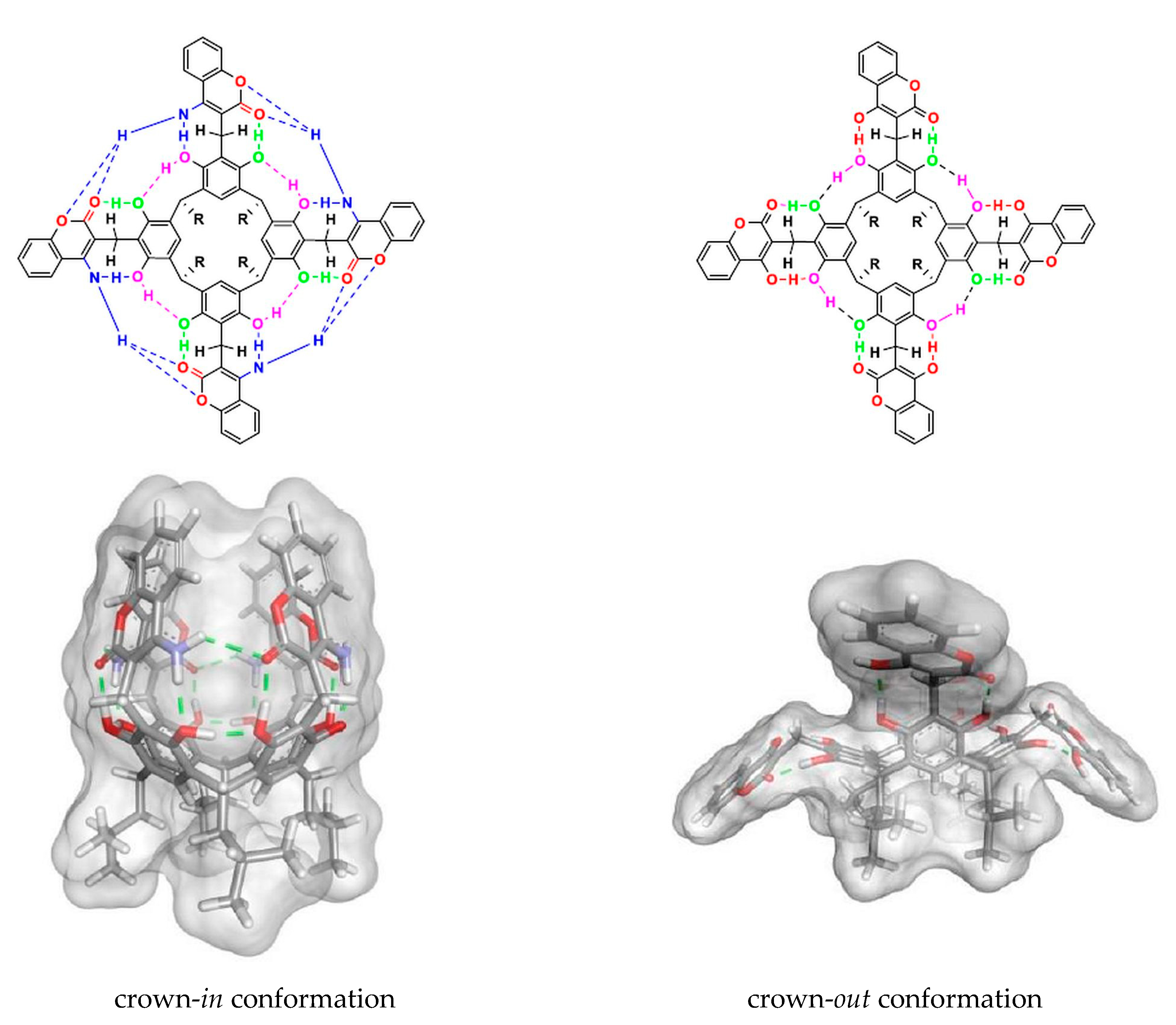
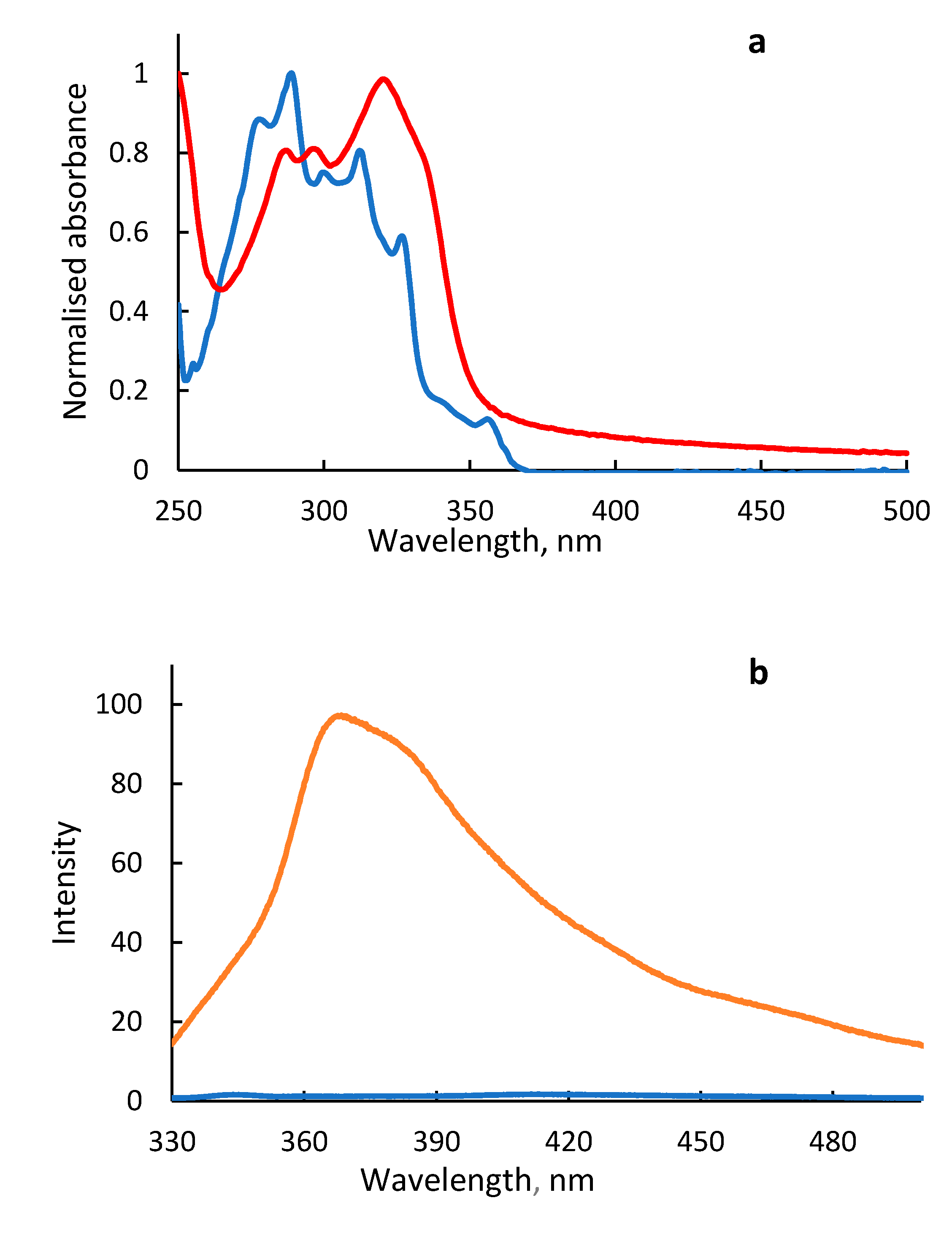
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.; Nakamoto, Y. Para-Bridged symmetrical pillar[5]arenes: Their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Cram, D.J.; Cram, J.M. Container Molecules and Their Guests, Monographs in Supramolecular Chemistry; Stoddart, J.F., Ed.; The Royal Society of Chemistry: Cambridge, UK, 1994. [Google Scholar]
- Timmerman, P.; Verboom, W.; Reinhoudt, D.N. Resorcinarenes. Tetrahedron 1996, 52, 2663–2704. [Google Scholar] [CrossRef] [Green Version]
- Neri, P.; Sessler, J.L.; Wang, M.X. Calixarenes and Beyond; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Beyeh, N.K.; Díez, I.; Taimoory, S.M.; Meister, D.; Feig, A.I.; Trant, J.F.; Ras, R.H.A.; Rissanen, K. High-Affinity and Selective Detection of Pyrophosphate in Water by a Resorcinarene Salt Receptor. Chem. Sci. 2018, 9, 1358–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chen, M.; Diao, G. Assembling Gold and Platinum Nanoparticles on Resorcinarene Modified Graphene and Their Electrochemical Applications. J. Mater. Chem. A 2013, 1, 2278–2285. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.; Wu, W.; Gao, H.; Xu, S.; Guo, Q.; Liu, Y.; Xu, S.; Cao, S. Calix[4]Resorcinarene-Based Branched Macromolecules for All-Optical Photorefractive Applications. J. Mater. Chem. C 2016, 4, 10684–10690. [Google Scholar] [CrossRef]
- Qadri, T.; Ali, I.; Hussain, M.; Ahmed, F.; Shah, M.R.; Hussain, Z. Synthesis of New Tetra Triazole Functionalized Calix[4]resorcinarene and Chemosensing of Copper Ions in Aqueous Medium. Curr. Org. Chem. 2020, 24, 332–337. [Google Scholar] [CrossRef]
- Li, N.; Harrison, R.G.; Lamb, J.D. Application of Resorcinarene Derivatives in Chemical Separations. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 39–60. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yamanaka, M. Self-Assembled Capsules Based on Tetrafunctionalized Calix [4] Resorcinarene Cavitands. Chem. Soc. Rev. 2015, 44, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Jasat, A.; Sherman, J.C. Carceplexes and Hemicarceplexes. Chem. Rev. 1999, 99, 931–968. [Google Scholar] [CrossRef] [PubMed]
- Rebek, J., Jr. Hydrogen-Bonded Capsules: Molecular Behavior in Small Spaces; World Scientific Publishing: Singapore, 2016. [Google Scholar]
- Gerkensmeier, T.; Iwanek, W.; Agena, C.; Fröhlich, R.; Kotila, S.; Näther, C.; Mattay, J. Self-Assembly of 2,8,14,20-Tetraisobutyl-5,11,17,23-tetrahydroxyresorc[4]arene. Eur. J. Org. Chem. 1999, 9, 2257–2262. [Google Scholar] [CrossRef]
- Iwanek, W. Chiral calixarene derived from resorcinol. Part 4: Diastereoselective closure of the oxazine ring. Tetrahedron Asymmetry 1998, 9, 4289–4290. [Google Scholar] [CrossRef]
- Stefańska, K.; Jędrzejewska, H.; Wierzbicki, M.; Szumna, A.; Iwanek, W. The inverse demand oxa-Diels—Alder reaction of resorcinarenes: An experimental and theoretical analysis of regioselectivity and diastereoselectivity. J. Org. Chem. 2016, 81, 6018. [Google Scholar] [CrossRef] [PubMed]
- Iwanek, W.; Fröhlich, R.; Schwab, P.; Schurig, V. The synthesis and crystallographic structures of novel bora-oxazino-oxazolidine derivatives of resorcarene. Chem. Commun. 2002, 21, 2516–2517. [Google Scholar] [CrossRef]
- Poul, L.; Le Mest, Y.; Jabin, I.; Reinaud, O. Supramolecular Modeling of Mono-Copper Enzyme Active Sites with Funnel-Complexes. Acc. Chem. Res. 2015, 48, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Twum, K.; Rautiainen, J.; Yu, S.; Truong, K.-N.; Feder, J.; Rissanen, K.; Puttreddy, R.; Beyeh, N.K. Host-Guest Interactions of Sodium sulfonate methyleneresorcinarene and Quaternary Ammonium Halides: An Experimental-Computational Analysis of the Guest Inclusion Properties. Cryst. Growth Des. 2020. [Google Scholar] [CrossRef]
- Jędrzejewska, H.; Kwit, M.; Szumna, A. Switching of inherent chirality driven by self-assembly. Chem. Commun. 2015, 51, 13799–13801. [Google Scholar] [CrossRef]
- Roncucci, P.; Pirondini, L.; Paderni, G.; Massera, C.; Dalcanale, E.; Azov, V.A.; Diederich, F. Conformational Behavior of Pyrazine-Bridged and Mixed-Bridged Cavitands: A General Model for Solvent Effects on Thermal “Vase–Kite” Switching. Chem. Eur. J. 2006, 12, 4775–4784. [Google Scholar] [CrossRef]
- Azov, V.A.; Beeby, A.; Cacciarini, M.; Cheetham, A.G.; Diederich, F.; Frei, M.; Gimzewski, J.K.; Gramlich, V.; Hecht, B.; Jaun, B.; et al. Resorcin[4]arene Cavitand-Based Molecular Switches. Adv. Funct. Mater. 2006, 16, 147–156. [Google Scholar] [CrossRef]
- Skinner, P.J.; Cheetham, A.G.; Beeby, A.; Gramlich, V.; Diederich, F. Conformational switching of resorcin[4]arene cavitands by protonation, preliminary communication. Helv. Chim. Acta 2001, 84, 2146–2153. [Google Scholar] [CrossRef]
- Milic, J.; Zalibera, M.; Pochorovski, I.; Trapp, N.; Nomrowski, J.; Neshchadin, D.; Ruhlmann, L.; Boudon, C.; Wenger, O.S.; Savitsky, A.; et al. Paramagnetic Molecular Grippers: The Elements of Six-State Redox Switches. J. Phys. Chem. Lett. 2016, 7, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Milic’, J.; Zalibera, M.; Talaat, D.; Nomrowski, J.; Trapp, N.; Ruhlmann, L.; Boudon, C.; Wenger, O.S.; Savitsky, A.; Lubitz, W.; et al. Photoredox-Switchable Resorcin[4]arene Cavitands: Radical Control of Molecular Gripping Machinery via Hydrogen Bonding. Chem. Eur. J. 2018, 24, 1431–1440. [Google Scholar] [CrossRef]
- Simpson, J. Organic Structure Determination Using 2-D NMR Spectroscopy: A Problem-Based Approach; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Szafraniec, A.; Iwanek, W. Synthesis of a coumarin derivative of resorcin[4]arene with solvent-controlled chirality. RSC Adv. 2020, 10, 12747–12753. [Google Scholar] [CrossRef] [Green Version]
- Tero, T.-R.; Suhonen, A.; Salorinne, K.; Campos-Barbosa, H.; Nissinen, M. The Missing Member of the Partially O-Alkylated Resorcinarene Family: Synthesis and Conformation of Methyl Tetramethoxy Resorcinarene. Org. Lett. 2013, 15, 1096–1099. [Google Scholar] [CrossRef]
- McIldowie, M.J.; Mocerino, M.; Skelton, B.W.; White, A.H. Facile Lewis Acid Catalyzed Synthesis of C4 Symmetric Resorcinarenes. Org. Lett. 2000, 2, 3869–3871. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB an Accurate and Broadly Parametrized Self Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical Extended Tight-Binding Program Package xtb. 2020. Available online: https://github.com/grimme-lab/xtb (accessed on 16 March 2020).
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangili, P.; Kim, J.S.; Lin, W. Correction to coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Jang, Y.J.; Moon, B.S.; Park, M.S.; Kang, B.G.; Kwon, J.Y.; Sung, J.; Hong, J.; Yoon, Y.L.; Leeb, K.D.; Yoona, J. New cavitand derivatives bearing four coumarin groups as fluorescent chemosensors for Cu2+ and recognition of dicarboxylates utilizing Cu2+ complex. Tetrahedron Let. 2006, 47, 2707–2710. [Google Scholar] [CrossRef]
- Krystkowiak, E.; Dobek, K.; Maciejewski, A. Deactivation of 6-Aminocoumarin Intramolecular ChargeTransfer Excited State through Hydrogen Bonding. Int. J. Mol. Sci. 2014, 15, 16628–16648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, C.; Han, J.; Zhou, Q.; Cao, B.; Yin, H.; Shi, Y. Theoretical investigation of intermolecular hydrogen bond induces fluorescence quenching phenomenon for Coumarin-1. J. Lumin. 2020, 221, 117110. [Google Scholar] [CrossRef]
- Urbaniak, M.; Iwanek, W. Synthesis of alkoxymethyl derivatives of resorcinarene via the Mannich reaction catalysed with iminodiacetic acid. Tetrahedron 2006, 62, 1508–1511. [Google Scholar] [CrossRef]


| Method |
∆E kJ/mol (Ecrown-out gas − Ecrown-in gas) |
∆E kJ/mol (Ecrown-out CHCl3 − Ecrown-in CHCl3) |
∆E kJ/mol (Ecrown-out DMSO − Ecrown-in DMSO) |
|---|---|---|---|
| DFTB/GFN2-xTB | 95.01 | 28.34 | 8.01 |
| DFT/B3LYP/6-311G(d,p) | 44.84 | 19.54 | 10.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafraniec, A.; Iwanek, W. Intramolecular Hydrogen Bond Driven Conformational Selectivity of Coumarin Derivatives of Resorcin[4]arene. Int. J. Mol. Sci. 2020, 21, 6160. https://doi.org/10.3390/ijms21176160
Szafraniec A, Iwanek W. Intramolecular Hydrogen Bond Driven Conformational Selectivity of Coumarin Derivatives of Resorcin[4]arene. International Journal of Molecular Sciences. 2020; 21(17):6160. https://doi.org/10.3390/ijms21176160
Chicago/Turabian StyleSzafraniec, Anna, and Waldemar Iwanek. 2020. "Intramolecular Hydrogen Bond Driven Conformational Selectivity of Coumarin Derivatives of Resorcin[4]arene" International Journal of Molecular Sciences 21, no. 17: 6160. https://doi.org/10.3390/ijms21176160






