Losing Regulation of the Extracellular Matrix is Strongly Predictive of Unfavorable Prognostic Outcome after Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
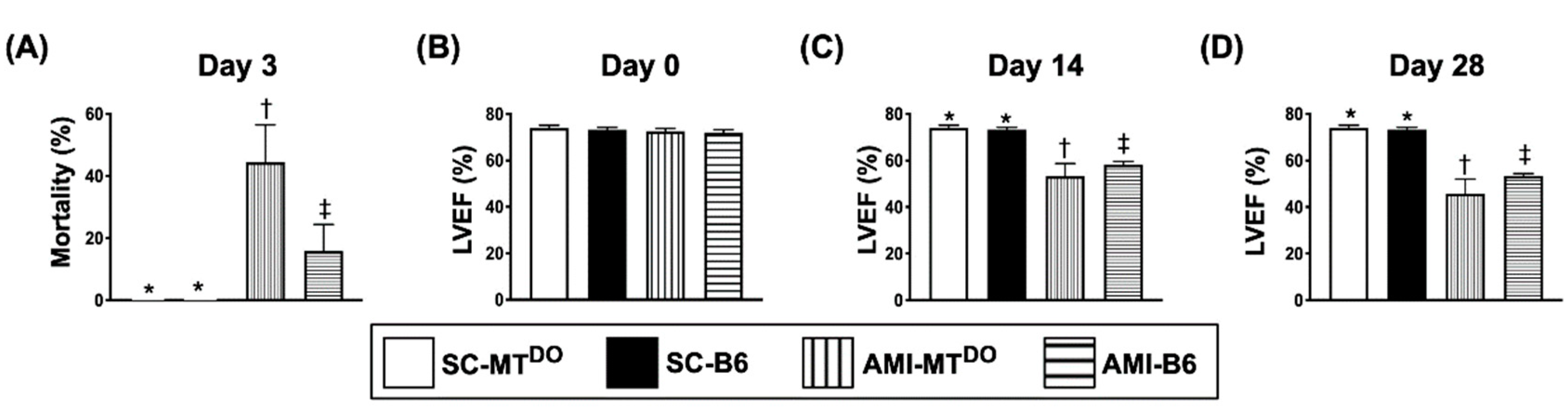
2.1. Day-3 Mortality Rate and LVEF at Days 0, 14 and 28 after AMI Induction
2.2. The Ratio of Heart Weight to Tibial Length and the Anatomic Infarct Size by Day 60 after AMI Induction
2.3. The Protein Expressions of Apoptosis and Fibrosis, and the Lung Injury Score in LV Myocardium by 60 Days after AMI Induction
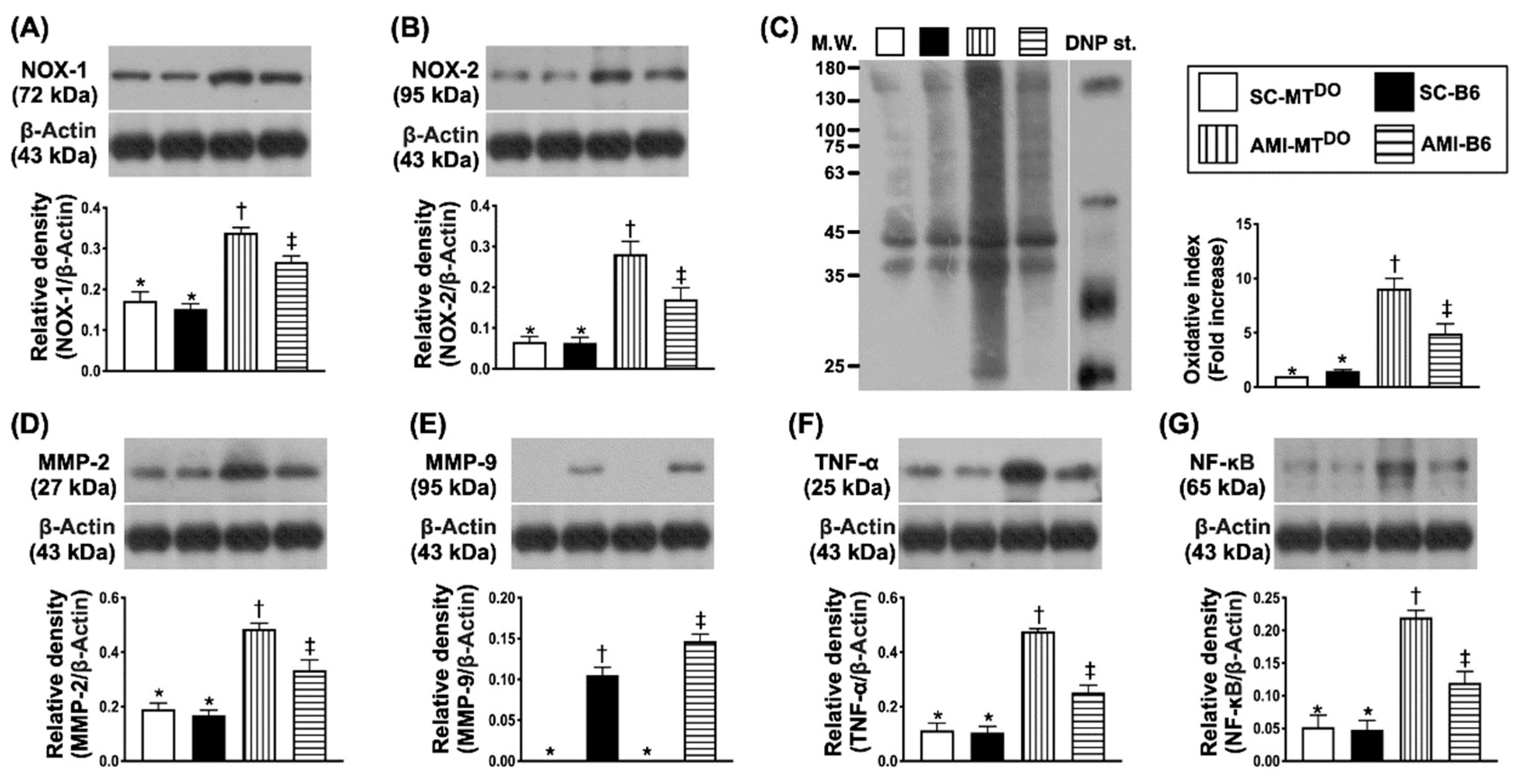
2.4. The Protein Expressions of Oxidative-Stress and Inflammatory Biomarkers in LV Myocardium by 60 Days after AMI Induction
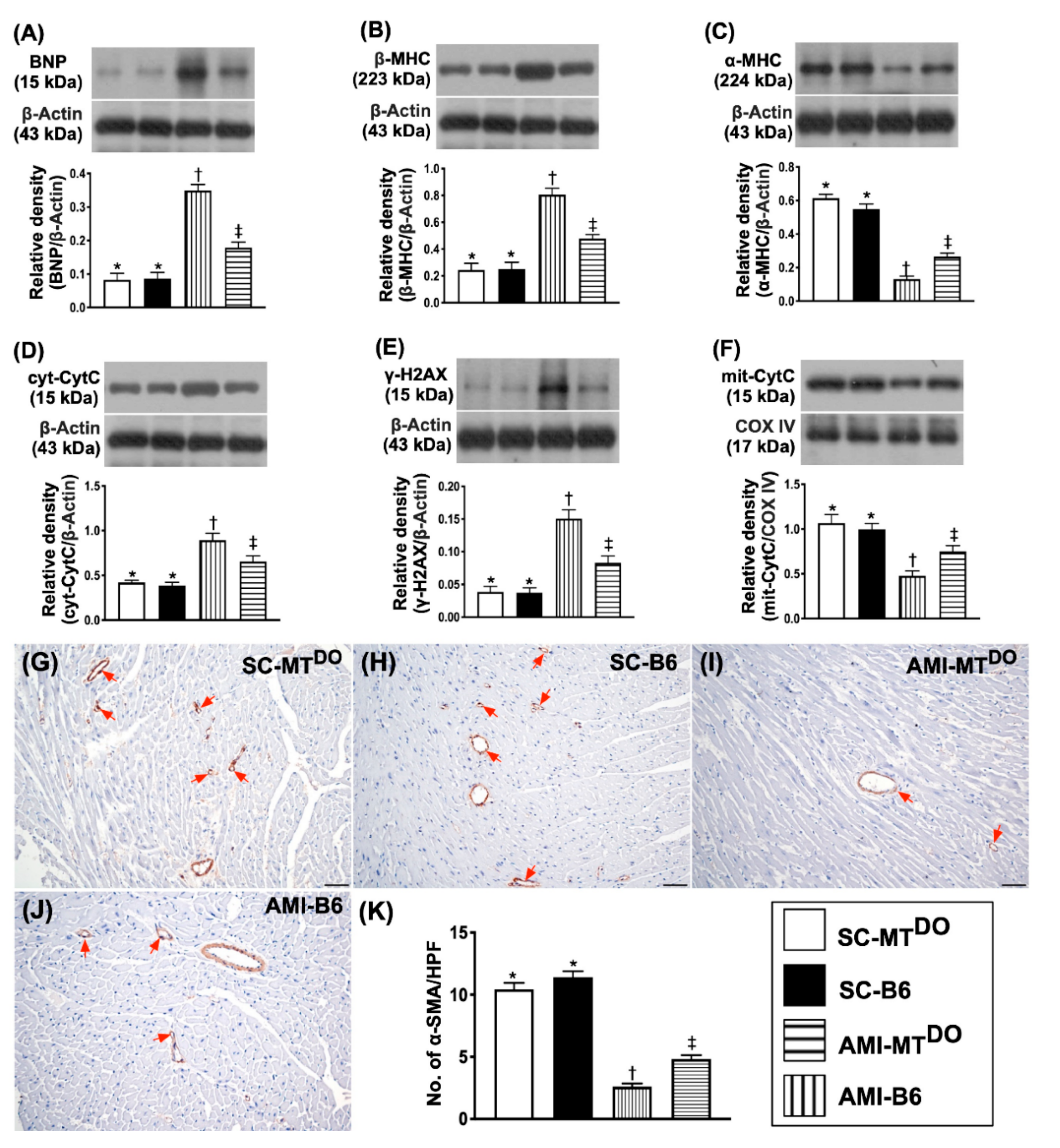
2.5. The Protein Expressions of Heart Failure/Pressure Overload and Mitochondrial/DNA Damage Biomarkers, and Small Vessel Density in LV Myocardium by 60 Days after AMI Induction
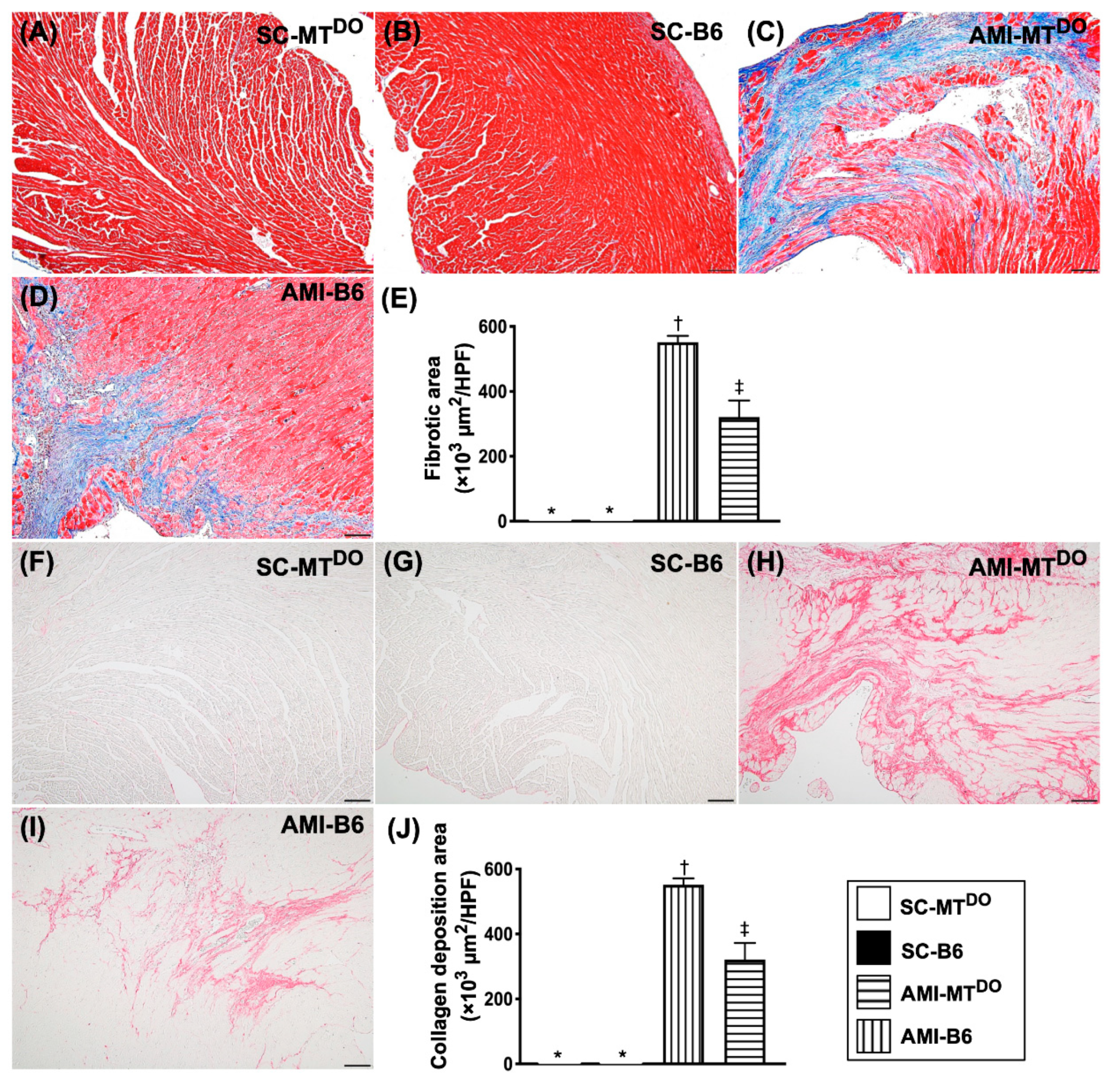
2.6. The Fibrosis and Collagen Deposition area, Infarct Area and Cellular Level of DNA Damage Biomarkers in LV Myocardium by 60 Days after AMI Induction
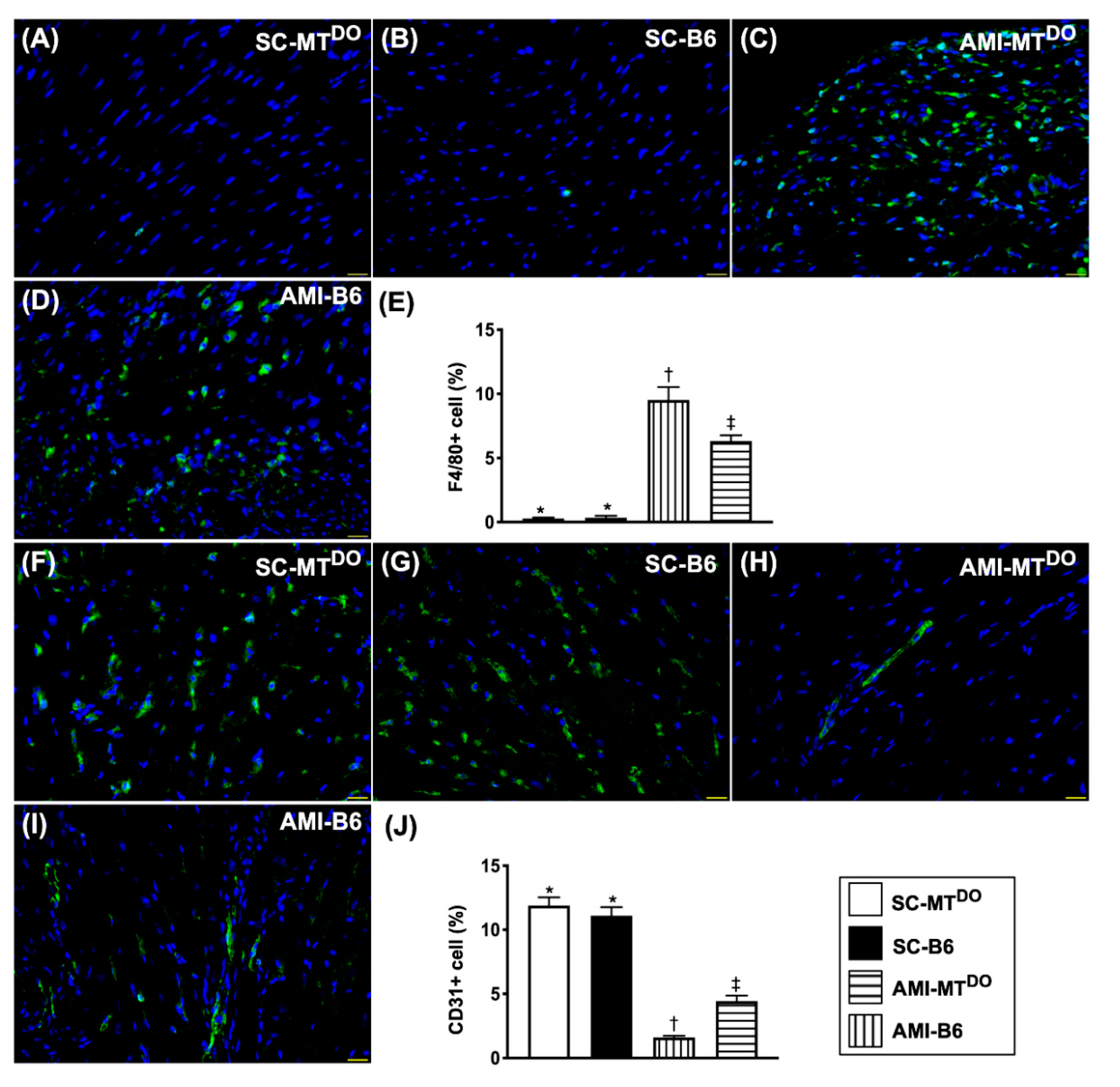
2.7. Inflammatory Cell Infiltration and Endothelial Cell Distribution in LV Myocardium by 60 Days after AMI Induction
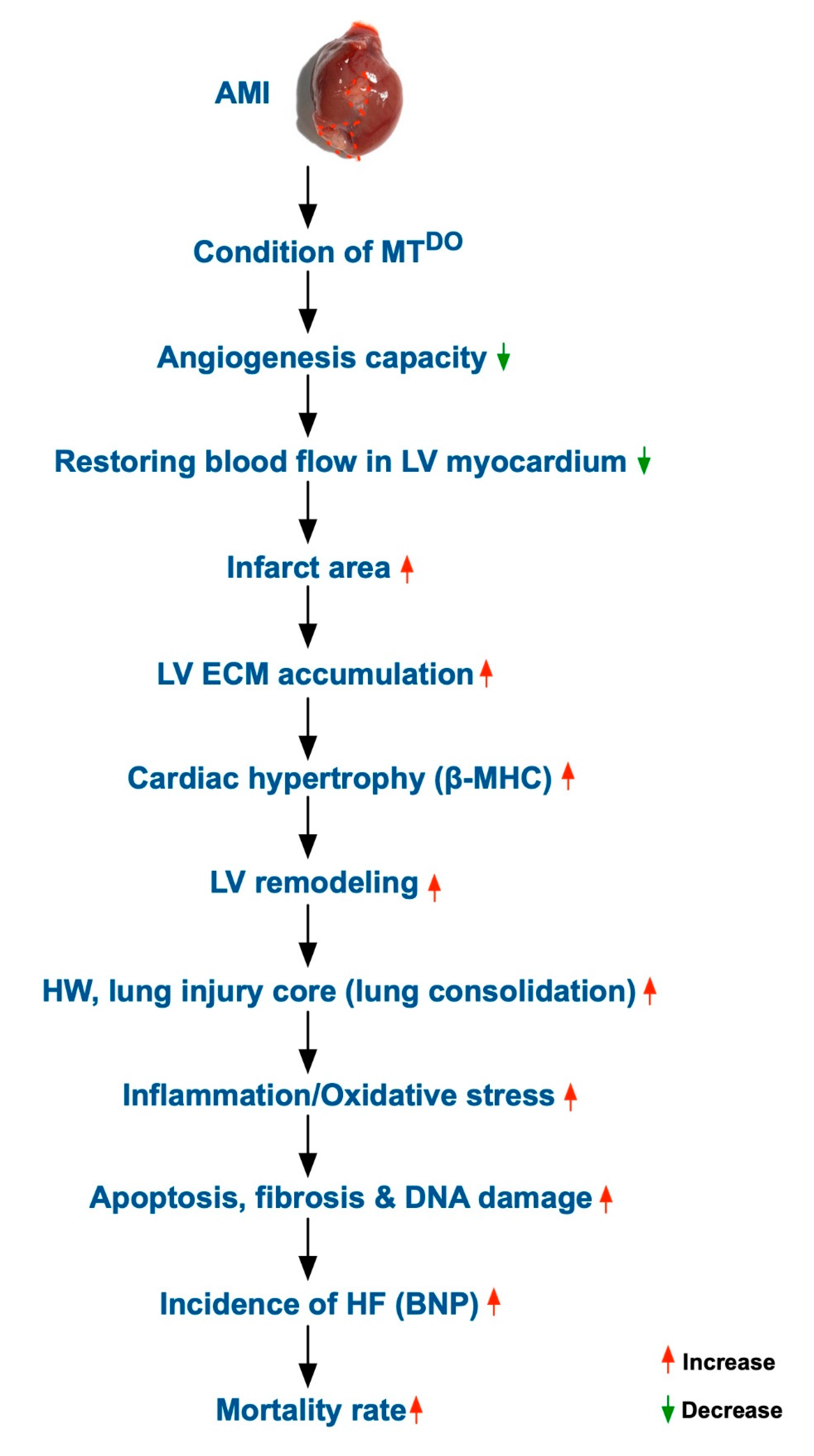
2.8. Schematically Proposed Mechanism of tPA/MMP9 Deficiency on the Prognostic Outcome in MTDKO Mice after AMI Induction
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Animal Grouping and Source of Double Knockout Mice (MMP-9−/−-tPA−/−)
4.3. Animal Model of Acute Myocardial Infarction (AMI) Induction
4.4. Functional Assessment by Echocardiography
4.5. Immunohistochemical (IHC) and Immunofluorescent (IF) Staining
4.6. Western Blot Analysis
4.7. Histological Study of Fibrosis and Collagen-Deposition Area
4.8. Vessel Density in Myocardial and Limb Ischemic Areas
4.9. Assessment of Oxidative Stress
4.10. Pathological Assessment of Lung Injury
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harvey, P.A.; Leinwand, L.A. The cell biology of disease: Cellular mechanisms of cardiomyopathy. J. Cell Biol. 2011, 194, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Kapelko, V.I. Extracellular matrix alterations in cardiomyopathy: The possible crucial role in the dilative form. Exp. Clin. Cardiol. 2001, 6, 41–49. [Google Scholar] [PubMed]
- Liew, C.C.; Dzau, V.J. Molecular genetics and genomics of heart failure. Nat. Rev. Genet. 2004, 5, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current Understanding of the Pathophysiology of Myocardial Fibrosis and Its Quantitative Assessment in Heart Failure. Front. Physiol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef]
- Francia, P.; Uccellini, A.; Frattari, A.; Modestino, A.; Ricotta, A.; Balla, C.; Scialla, L.; Volpe, M. Extracellular matrix remodelling in myocardial hypertrophy and failure: Focus on osteopontin. High Blood Press. Cardiovasc. Prev. 2009, 16, 195–199. [Google Scholar] [CrossRef]
- Ingle, K.A.; Kain, V.; Goel, M.; Prabhu, S.D.; Young, M.E.; Halade, G.V. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1827–H1836. [Google Scholar] [CrossRef]
- Kitaoka, H.; Kubo, T.; Okawa, M.; Hayato, K.; Yamasaki, N.; Matsumura, Y.; Doi, Y.L. Impact of metalloproteinases on left ventricular remodeling and heart failure events in patients with hypertrophic cardiomyopathy. Circ. J. 2010, 74, 1191–1196. [Google Scholar] [CrossRef]
- Prinz, C.; Farr, M.; Laser, K.T.; Esdorn, H.; Piper, C.; Horstkotte, D.; Faber, L. Determining the role of fibrosis in hypertrophic cardiomyopathy. Expert Rev. Cardiovasc. Ther. 2013, 11, 495–504. [Google Scholar] [CrossRef]
- Tsoutsman, T.; Wang, X.; Garchow, K.; Riser, B.; Twigg, S.; Semsarian, C. CCN2 plays a key role in extracellular matrix gene expression in severe hypertrophic cardiomyopathy and heart failure. J. Mol. Cell. Cardiol. 2013, 62, 164–178. [Google Scholar] [CrossRef]
- Yip, H.K.; Yuen, C.M.; Chen, K.H.; Chai, H.T.; Chung, S.Y.; Tong, M.S.; Chen, S.Y.; Kao, G.S.; Chen, C.H.; Chen, Y.L.; et al. Tissue plasminogen activator deficiency preserves neurological function and protects against murine acute ischemic stroke. Int. J. Cardiol. 2016, 205, 133–141. [Google Scholar] [CrossRef]
- de Haas, H.J.; Arbustini, E.; Fuster, V.; Kramer, C.M.; Narula, J. Molecular imaging of the cardiac extracellular matrix. Circ. Res. 2014, 114, 903–915. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.L.; Blaxall, B.C. Cardiac intercellular communication: Are myocytes and fibroblasts fair-weather friends? J. Cardiovasc. Transl. Res. 2012, 5, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.K.; Sun, C.K.; Tsai, T.H.; Sheu, J.J.; Kao, Y.H.; Lin, Y.C.; Shiue, Y.L.; Chen, Y.L.; Chai, H.T.; Chua, S.; et al. Tissue plasminogen activator enhances mobilization of endothelial progenitor cells and angiogenesis in murine limb ischemia. Int. J. Cardiol. 2013, 168, 226–236. [Google Scholar] [CrossRef]
- Leu, S.; Lu, H.I.; Sun, C.K.; Sheu, J.J.; Chen, Y.L.; Tsai, T.H.; Yeh, K.H.; Chai, H.T.; Chua, S.; Tsai, C.Y.; et al. Retention of endothelial progenitor cells in bone marrow in a murine model of endogenous tissue plasminogen activator (tPA) deficiency in response to critical limb ischemia. Int. J. Cardiol. 2014, 170, 394–405. [Google Scholar] [CrossRef]
- Sun, C.K.; Zhen, Y.Y.; Leu, S.; Tsai, T.H.; Chang, L.T.; Sheu, J.J.; Chen, Y.L.; Chua, S.; Chai, H.T.; Lu, H.I.; et al. Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. Int. J. Cardiol. 2014, 173, 410–423. [Google Scholar] [CrossRef]
- Sun, C.K.; Yen, C.H.; Lin, Y.C.; Tsai, T.H.; Chang, L.T.; Kao, Y.H.; Chua, S.; Fu, M.; Ko, S.F.; Leu, S.; et al. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J. Transl. Med. 2011, 9, 118. [Google Scholar] [CrossRef]
- Yip, H.K.; Chang, Y.C.; Wallace, C.G.; Chang, L.T.; Tsai, T.H.; Chen, Y.L.; Chang, H.W.; Leu, S.; Zhen, Y.Y.; Tsai, C.Y.; et al. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J. Pineal. Res. 2013, 54, 207–221. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Hou, L.; Huang, N.F. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv. Healthc. Mater. 2014, 3, 628–641. [Google Scholar] [CrossRef]
- Aoki, T.; Fukumoto, Y.; Sugimura, K.; Oikawa, M.; Satoh, K.; Nakano, M.; Nakayama, M.; Shimokawa, H. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. -Comparison between preserved and reduced ejection fraction heart failure. Circ. J. 2011, 75, 2605–2613. [Google Scholar] [CrossRef]
- Weber, K.T. Extracellular matrix remodeling in heart failure: A role for de novo angiotensin II generation. Circulation 1997, 96, 4065–4082. [Google Scholar] [CrossRef]
- Lee, F.Y.; Luo, C.W.; Wallace, C.G.; Chen, K.H.; Sheu, J.J.; Yin, T.C.; Chai, H.T.; Yip, H.K. Direct implantations of erythropoietin and autologous EPCs in critical limb ischemia (CLI) area restored CLI area blood flow and rescued remote AMI-induced LV dysfunction. Biomed. Pharmacother. 2019, 118, 109296. [Google Scholar] [CrossRef]
- Yip, H.K.; Chang, L.T.; Wu, C.J.; Sheu, J.J.; Youssef, A.A.; Pei, S.N.; Lee, F.Y.; Sun, C.K. Autologous bone marrow-derived mononuclear cell therapy prevents the damage of viable myocardium and improves rat heart function following acute anterior myocardial infarction. Circ. J. 2008, 72, 1336–1345. [Google Scholar] [CrossRef][Green Version]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Pina, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef]
- Nadar, S.K.; Shaikh, M.M. Biomarkers in Routine Heart Failure Clinical Care. Card. Fail. Rev. 2019, 5, 50–56. [Google Scholar] [CrossRef]
- Yip, H.K.; Chen, M.C.; Chang, H.W.; Hang, C.L.; Hsieh, Y.K.; Fang, C.Y.; Wu, C.J. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: Predictors of slow-flow and no-reflow phenomenon. Chest 2002, 122, 1322–1332. [Google Scholar] [CrossRef]
- Yip, H.K.; Wu, C.J.; Chang, H.W.; Hsieh, Y.K.; Fang, C.Y.; Chen, S.M.; Chen, M.C. Impact of tirofiban on angiographic morphologic features of high-burden thrombus formation during direct percutaneous coronary intervention and short-term outcomes. Chest 2003, 124, 962–968. [Google Scholar] [CrossRef]
- Sheu, J.J.; Chang, M.W.; Wallace, C.G.; Chiang, H.J.; Sung, P.H.; Tsai, T.H.; Chung, S.Y.; Chen, Y.L.; Chua, S.; Chang, H.W.; et al. Exendin-4 protected against critical limb ischemia in obese mice. Am. J. Transl. Res. 2015, 7, 445–459. [Google Scholar]
- Sung, P.H.; Chiang, H.J.; Chen, C.H.; Chen, Y.L.; Huang, T.H.; Zhen, Y.Y.; Chang, M.W.; Liu, C.F.; Chung, S.Y.; Chen, Y.L.; et al. Combined Therapy With Adipose-Derived Mesenchymal Stem Cells and Ciprofloxacin Against Acute Urogenital Organ Damage in Rat Sepsis Syndrome Induced by Intrapelvic Injection of Cecal Bacteria. Stem Cells Transl. Med. 2016, 5, 782–792. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, P.-H.; Lin, K.-C.; Chai, H.-T.; Chiang, J.Y.; Shao, P.-L.; Luo, C.-W.; Yip, H.-K. Losing Regulation of the Extracellular Matrix is Strongly Predictive of Unfavorable Prognostic Outcome after Acute Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 6219. https://doi.org/10.3390/ijms21176219
Sung P-H, Lin K-C, Chai H-T, Chiang JY, Shao P-L, Luo C-W, Yip H-K. Losing Regulation of the Extracellular Matrix is Strongly Predictive of Unfavorable Prognostic Outcome after Acute Myocardial Infarction. International Journal of Molecular Sciences. 2020; 21(17):6219. https://doi.org/10.3390/ijms21176219
Chicago/Turabian StyleSung, Pei-Hsun, Kun-Chen Lin, Han-Tan Chai, John Y. Chiang, Pei-Lin Shao, Chi-Wen Luo, and Hon-Kan Yip. 2020. "Losing Regulation of the Extracellular Matrix is Strongly Predictive of Unfavorable Prognostic Outcome after Acute Myocardial Infarction" International Journal of Molecular Sciences 21, no. 17: 6219. https://doi.org/10.3390/ijms21176219
APA StyleSung, P.-H., Lin, K.-C., Chai, H.-T., Chiang, J. Y., Shao, P.-L., Luo, C.-W., & Yip, H.-K. (2020). Losing Regulation of the Extracellular Matrix is Strongly Predictive of Unfavorable Prognostic Outcome after Acute Myocardial Infarction. International Journal of Molecular Sciences, 21(17), 6219. https://doi.org/10.3390/ijms21176219




