Lipid Dynamics, Identification, and Expression Patterns of Fatty Acid Synthase Genes in an Endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae)
Abstract
:1. Introduction
2. Results
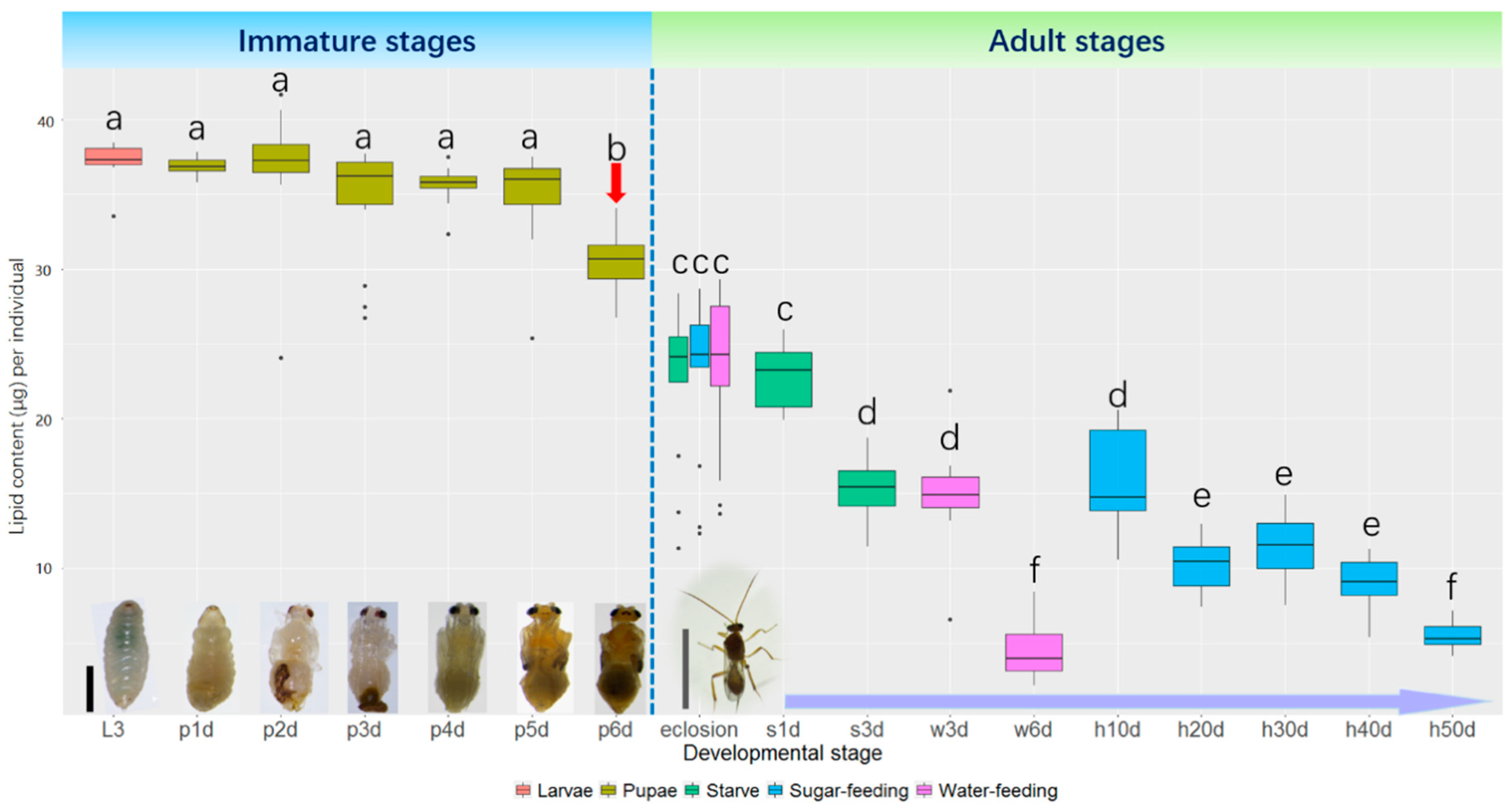
2.1. Lipid Content at Different Stages
2.2. Identification of Fatty Acid Synthase Genes
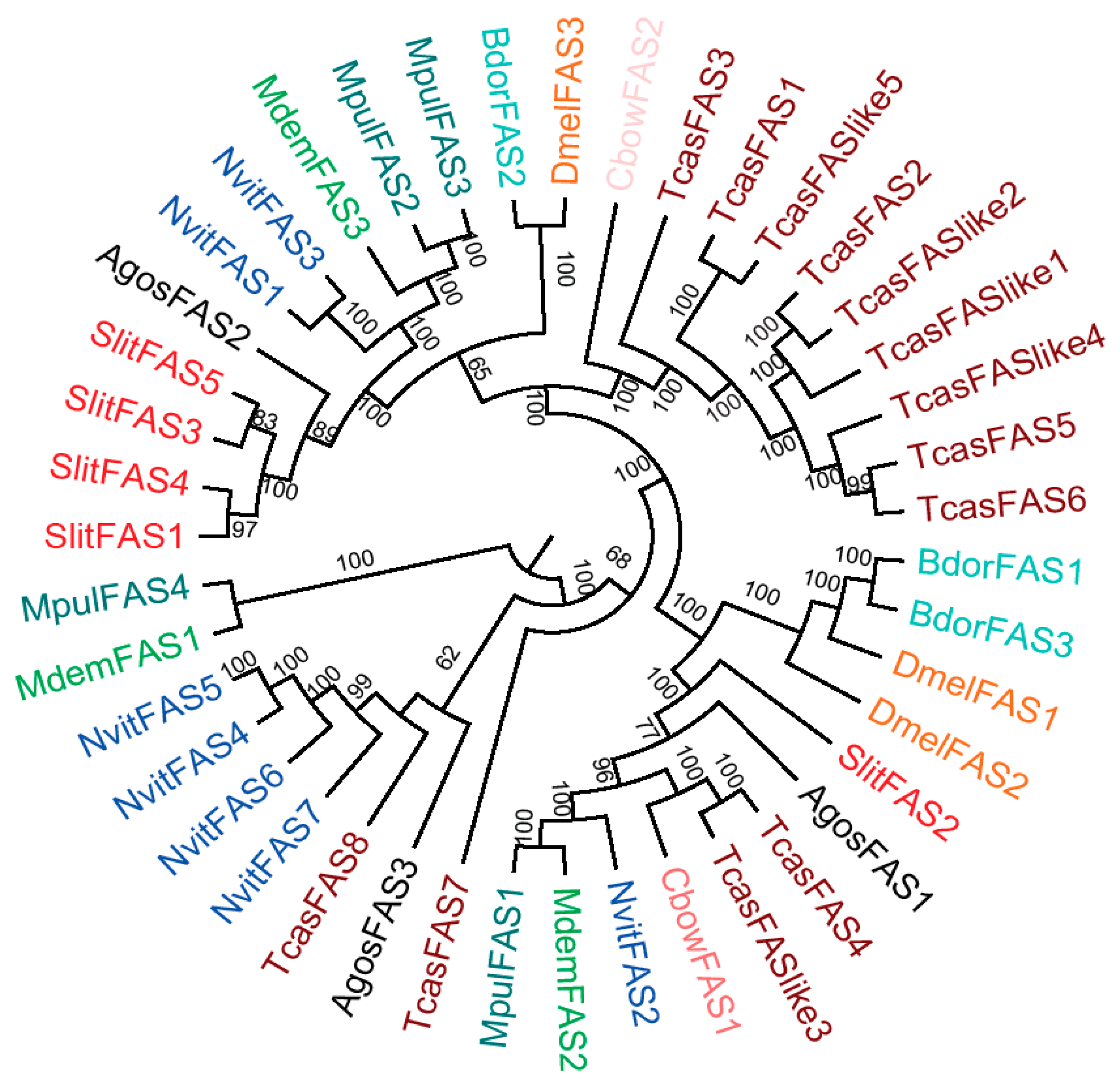
2.3. Components Phylogenetic Analysis
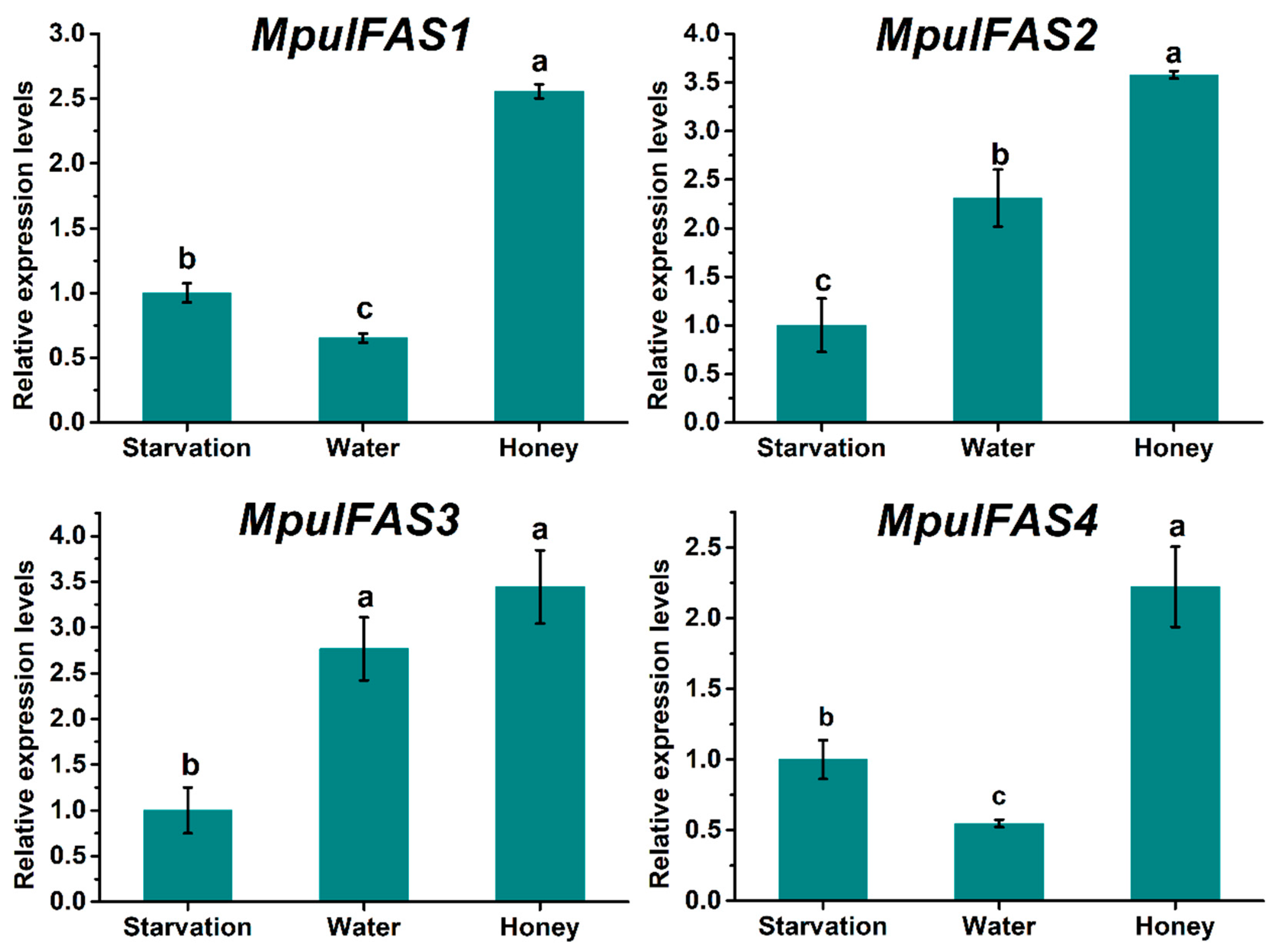
2.4. Expression Patterns of MpulFASs under Different Feeding Conditions
2.5. Expression Patterns of MpulFASs among Different Life-History Stages
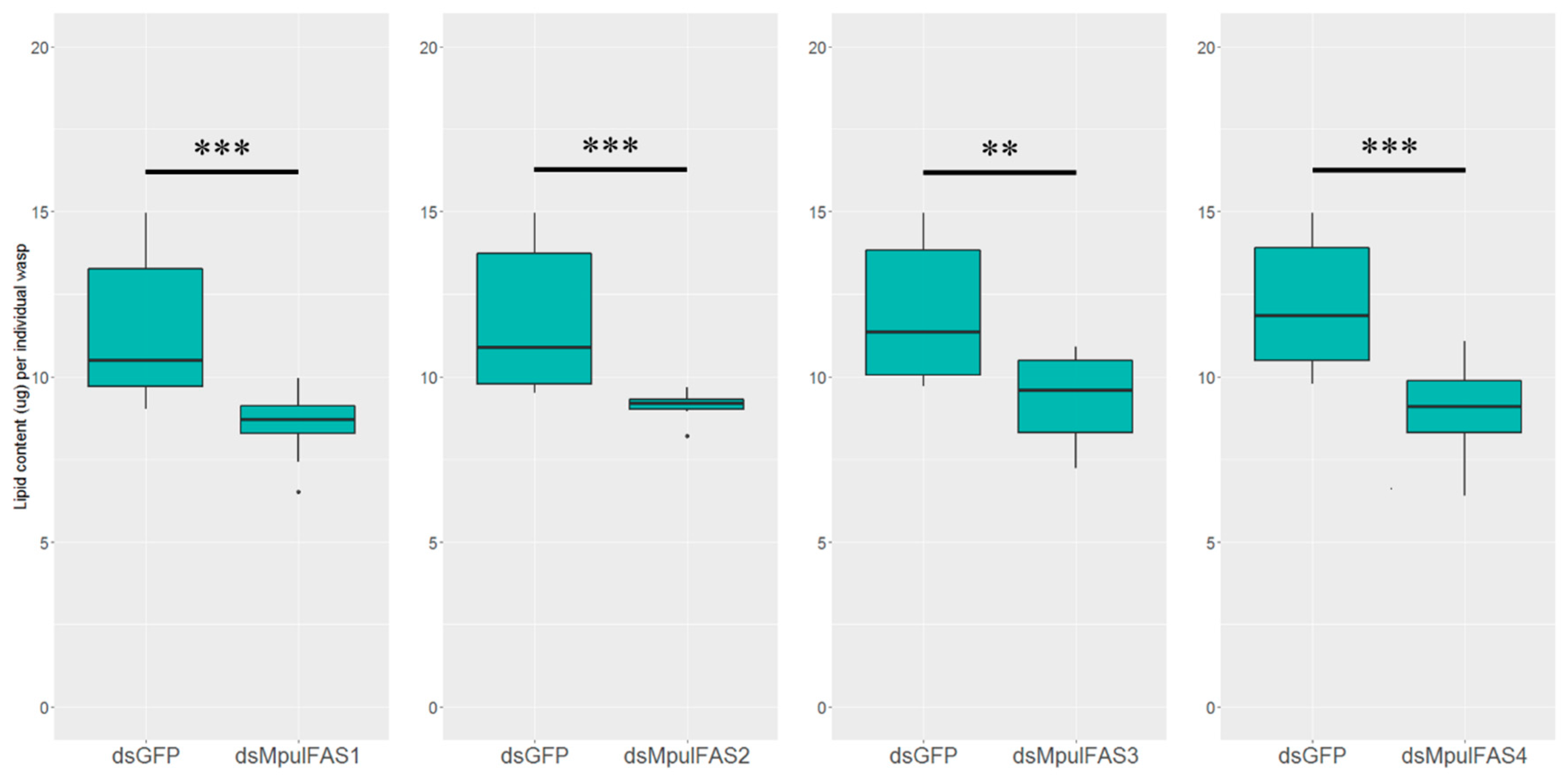
2.6. The Analysis of the Function of MpulFASs Using dsRNA
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Lipid Analysis
4.3. Identification and Bioinformatics Analysis of FASs in M. pulchricornis
4.4. RNA Isolation and Reverse Transcription
4.5. qRT-PCR Validation
4.6. The Synthesis of dsRNA Synthesis and Injection
4.7. Data Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zinke, I.; Schütz, C.S.; Katzenberger, J.R.D.; Bauer, M.; Pankratz, M.J. Nutrient control of gene expression in Drosophila: Microarray analysis of starvation and sugar-dependent response. EMBO J. 2002, 21, 6162–6173. [Google Scholar] [CrossRef] [Green Version]
- Visser, B.; Ellers, J. Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. J. Insect Physiol. 2008, 54, 1315–1322. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Lei, D.; Yiping, L.; Michiyo, G. Physiological and biochemical changes in summer and winter diapause and non-diapause pupae of the cabbage armyworm, Mamestra brassicae L. during long-term cold acclimation. J. Insect Physiol. 2003, 49, 1153–1159. [Google Scholar] [CrossRef]
- Shirai, Y. Flight activity, reproduction, and adult nutrition of the beet webworm, Spoladea recurvalis (Lepidoptera: Pyralidae). Appl. Entomol. Zool. 2006, 41, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Eijs, I.E.; Ellers, J.; van Duinen, G.J. Feeding strategies in drosophilid parasitoids: The impact of natural food resources on energy reserves in females. Ecol. Entomol. 1998, 23, 133–138. [Google Scholar] [CrossRef]
- Gianoli, E.; Suárez, L.H.; Gonzáles, W.L.; Teuber, M.G.; Rodríguez, I.S.A. Host-associated variation in sexual size dimorphism and fitness effects of adult feeding in a bruchid beetle. Entomol. Exp. Appl. 2007, 122, 222–223. [Google Scholar] [CrossRef]
- Ellers, J. Fat and eggs: An alternative method to measure the trade-off between survival and reproduction in insect parasitoids. Neth. J. Zool. 1995, 3, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Sheng, S.; Zhang, X.; Zheng, Y.; Wang, J.; Zhou, Y.; Liao, C.; Wang, J.; Wu, F. Effect of six sugars on the longevity, oviposition performance and nutrition accumulation in an endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae). J. Asia-Pac. Entomol. 2019, 22, 263–268. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E.; Leibee, G.L. Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol. Exp. Appl. 2004, 111, 189–199. [Google Scholar] [CrossRef]
- Ohlrogge, J.B.; Browse, J.G. Lipid Biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, M.A.; Gramates, L.S.; Santos, G.D.; Matthews, B.; Pierre, S.E.S.; Zhou, P.L.; Schroeder, A.J.; Falls, K.; Emmert, D.B.; Russo, S.M.; et al. Gene model annotations for Drosophila melanogaster: The rule-benders. G3-Genes Genomes Genet. 2015, 5, 1737–1749. [Google Scholar] [CrossRef] [Green Version]
- Visser, B.; Roelofs, D.; Hahn, D.A.; Teal, P.E.A.; Mariën, J.; Ellers, J. Transcriptional changes associated with lack of lipid synthesis in parasitoids. Genome Biol. Evol. 2012, 4, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Ye, Z.; Huang, S.; Ding, J.; Luo, R. The correlations of the different host plants with preference level, life duration and survival rate of Spodoptera litura Fabricius. Chin. J. Eco-Agric. 2004, 12, 40–42. [Google Scholar]
- Sheng, S.; Feng, S.; Meng, L.; Li, B. Departure mechanisms for host search on high-density patches by the parasitoid, Meteorus pulchricornis. J. Insect Sci. 2014, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, B. Developmental interactions between Spodoptera exigua (Noctuidae: Lepidoptera) and its uniparental endoparasitoid, Meteorus pulchricornis (Braconidae: Hymenoptera). Biol. Control. 2006, 38, 264–269. [Google Scholar] [CrossRef]
- Sheng, S.; Meng, L.; Wu, F.; Li, B. Patch time allocation and oviposition behavior in response to patch quality and the presence of a generalist predator in Meteorus pulchricornis (Hymenoptera: Braconidae). J. Insect Sci. 2015, 15, 1–4. [Google Scholar] [CrossRef]
- Maeto, K. Polyphagous koinobiosis: The biology and biocontrol potential of a braconid endoparasitoid of exophytic caterpillars. Appl. Entomol. Zool. 2018, 53, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Miura, K.; Tanaka, T. Effects of the virus-like particles of a braconid endoparasitoid, Meteorus pulchricornis, on hemocytes and hematopoietic organs of its noctuid host, Pseudaletia separata. Appl. Entomol. Zool. 2009, 44, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Tanaka, T. Virus-like particles in venom of Meteorus pulchricornis induce host hemocyte apoptosis. J. Insect Physiol. 2006, 52, 602–613. [Google Scholar] [CrossRef]
- Harvey, J.A.; Malcicka, M. Nutritional integration between insect hosts and koinobiont parasitoids in an evolutionary framework. Entomol. Exp. Appl. 2016, 159, 181–188. [Google Scholar] [CrossRef]
- Visser, B.; Lann, C.L.; Blanken, F.J.D.; Harvey, J.A.; van Alphen, J.J.M.; Jacintha, E. Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2010, 107, 86777–88682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Lu, S.; Liu, W.; Cheng, L.; Zhang, Y.; Wan, F. Effects of five naturally occurring sugars on the longevity, oogenesis, and nutrient accumulation pattern in adult females of the synovigenic parasitoid, Neochrysocharis formosa (Hymenoptera: Eulophidae). Neotrop. Entomol. 2014, 43, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.; Grisham, C. Biochemistry; Saunders: Orlando, FL, USA, 1999. [Google Scholar]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Ellers, J.; Jervis, M.A. Why are so few parasitoid wasp species pro-ovigenic? Evol. Ecol. Res. 2004, 6, 993–1002. [Google Scholar] [CrossRef]
- Ferns, M.A.J.A. The timing of egg maturation in insects: Ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos 2004, 107, 449–460. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Ann. Rev. Entomol. 2007, 54, 361. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, J.; Boivin, G. Functional ecology of immature parasitoids. Annu. Rev. Entomol. 2004, 49, 27. [Google Scholar] [CrossRef]
- Lammers, M.; Kraaijeveld, K.; Mariën, J.; Ellers, J. Gene expression changes associated with the evolutionary loss of a metabolic trait: Lack of lipogenesis in parasitoids. BMC Genom. 2019, 20, 309. [Google Scholar] [CrossRef] [Green Version]
- Burke, G.R.; Strand, M.R. Deep sequencing identifies viral and wasp genes with potential roles in replication of Microplitis demolitor Bracovirus. J. Virol. 2012, 86, 3293. [Google Scholar] [CrossRef] [Green Version]
- Nakamatsu, Y.; Fujii, S.; Tanaka, T. Larvae of an endoparasitoid, Cotesia kariyai (Hymenoptera: Braconidae), feed on the host fat body directly in the second stadium with the help of teratocytes. J. Insect Physiol. 2002, 48, 1041–1052. [Google Scholar] [CrossRef]
- Prager, L.; Bruckmann, A.; Ruther, J. De novo biosynthesis of fatty acids from α-D-glucose in parasitoid wasps of the Nasonia group. Insect Biochem. Mol. Biol. 2019, 115, 103256. [Google Scholar] [CrossRef]
- Rivers, D.B.; Denlinger, D.L. Developmental fate of the flesh fly, Sarcophaga bullata, envenomated by the pupal ectoparasitoid, Nasonia vitripennis. J. Insect Physiol. 1994, 40, 121–127. [Google Scholar] [CrossRef]
- Tan, Q.Q.; Liu, W.; Zhu, F.; Lei, C.L.; Wang, X.P. Fatty acid synthase 2 contributes to diapause preparation in a beetle by regulating lipid accumulation and stress tolerance genes expression. Sci. Rep. 2017, 7, 40509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Elheiga, L.; Matzuk, M.M.; Abo-Hashema, K.A.; Wakil, S.L. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001, 291, 2613–2616. [Google Scholar] [CrossRef]
- Abu-Elheiga, L.; Matzuk, M.M.; Kordari, P.; Oh, W.; Shaikenov, T.; Gu, Z.; Wakil, S.J. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc. Natl. Acad. Sci. USA 2005, 102, 12011–12016. [Google Scholar] [CrossRef] [Green Version]
- Sheng, S.; Liao, C.W.; Zheng, Y.; Zhou, Y.; Xu, Y.; Song, W.M.; He, P.; Zhang, J.; Wu, F.A. Candidate chemosensory genes identified in the endoparasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) by antennal transcriptome analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.L.; Wu, Y.D. Pesticide Resistance and Management of Helicoverpa Armigera; China Agricultural Press: Beijing, China, 1995; pp. 94–95. [Google Scholar]
- Foray, V.; Pelisson, P.; Belvenner, M.; Desouhant, E.; Venner, S.; Menu, F.; Giron, D.; Rey, B. A handbook for uncovering the complete energetic budget in insects: The van Handel’s method (1985) revisited. Physiol. Entomol. 2012, 37, 295–302. [Google Scholar] [CrossRef]
- Pinheiro, L.A.; Torres, L.M.; Raimundo, J.; Santos, S.A.P. Effects of pollen, sugars and honeydew on lifespan and nutrient levels of Episyrphus balteatus. BioControl 2015, 60, 47–57. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, J.Q.; Shao, Y.Y.; Xing, X.R.; Wang, J.; Liu, Z.X.; Li, Y.J.C.; Ofori, A.D.; Tu, Q.B.; Wang, J.; et al. Identification of glutathione-S-transferase genes by transcriptome analysis in Meteorus pulchricornis (Hymenoptera: Braconidae) and their expression patterns under stress of phoxim and cypermethrin. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100607. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Li, X.M.; Zhu, X.Y.; Wang, Z.Q.; Wang, Y.; He, P.; Chen, G.; Sun, L.; Deng, D.G.; Zhang, Y.N. Candidate chemosensory genes identified in Colaphellus bowringi by antennal transcriptome analysis. BMC Genom. 2015, 16, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and environment for statistical computing. Computing 2014, 14, 12–21. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shen, L.-W.; Xing, X.-R.; Xie, Y.-Q.; Li, Y.-J.; Liu, Z.-X.; Wang, J.; Wu, F.-A.; Sheng, S. Lipid Dynamics, Identification, and Expression Patterns of Fatty Acid Synthase Genes in an Endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae). Int. J. Mol. Sci. 2020, 21, 6228. https://doi.org/10.3390/ijms21176228
Wang J, Shen L-W, Xing X-R, Xie Y-Q, Li Y-J, Liu Z-X, Wang J, Wu F-A, Sheng S. Lipid Dynamics, Identification, and Expression Patterns of Fatty Acid Synthase Genes in an Endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae). International Journal of Molecular Sciences. 2020; 21(17):6228. https://doi.org/10.3390/ijms21176228
Chicago/Turabian StyleWang, Jiao, Li-Wei Shen, Xiao-Rong Xing, Yu-Qi Xie, Yi-Jiangcheng Li, Zhi-Xiang Liu, Jun Wang, Fu-An Wu, and Sheng Sheng. 2020. "Lipid Dynamics, Identification, and Expression Patterns of Fatty Acid Synthase Genes in an Endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae)" International Journal of Molecular Sciences 21, no. 17: 6228. https://doi.org/10.3390/ijms21176228




