Synergistic Interactions of 5-Fluorouracil with Inhibitors of Protein Kinase CK2 Correlate with p38 MAPK Activation and FAK Inhibition in the Triple-Negative Breast Cancer Cell Line
Abstract
1. Introduction
2. Results
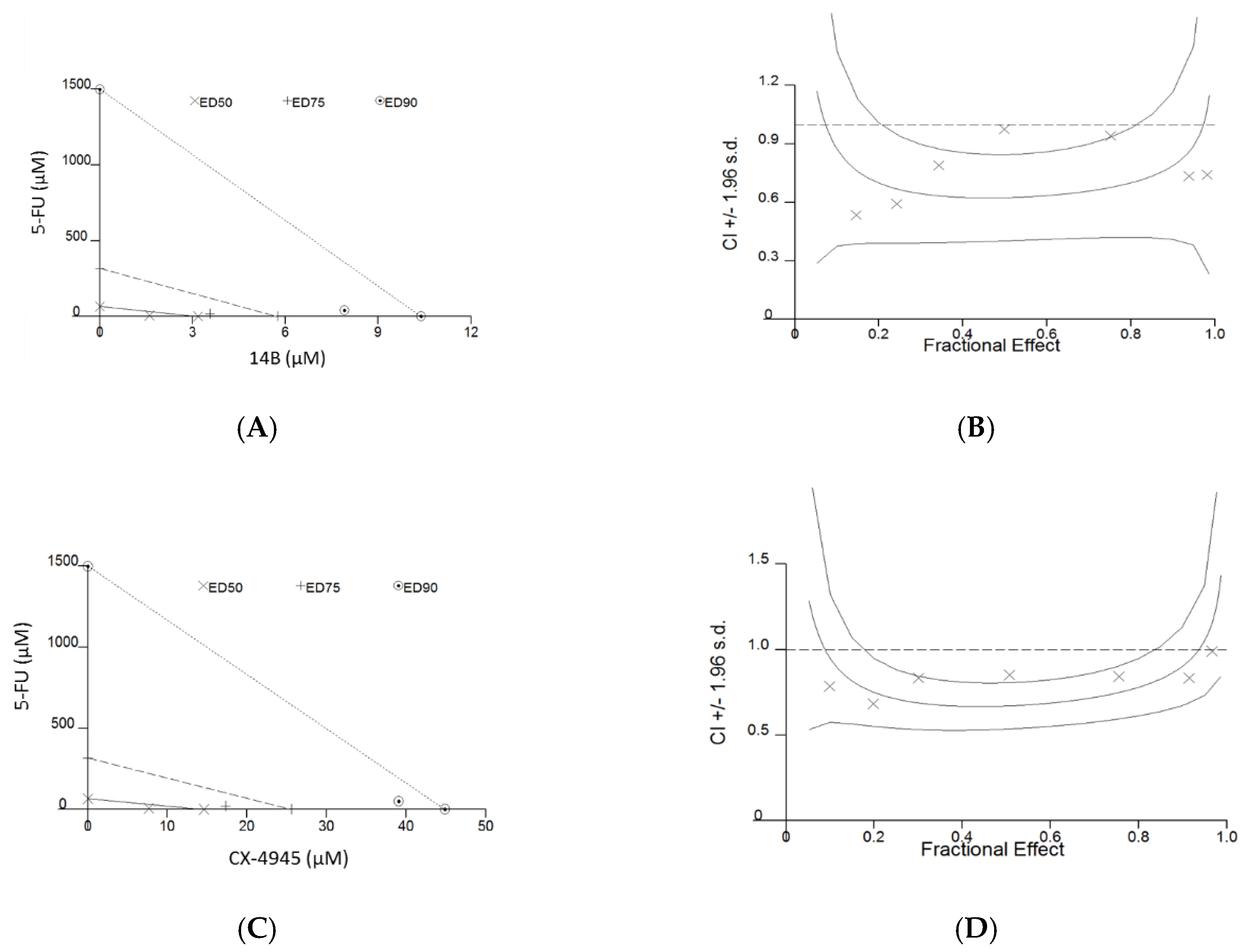
2.1. Compounds’ Influence on the Viability of MDA-MB-231 and MCF-7 Cell Lines
2.2. The Effect of Drug Combinations on TS, CK2α, and NF-κB-p65 in MDA-MB-231 Cells
2.3. The Effect of Drug Combinations on p38 MAPK, FAK, and ERK1/2 Kinases in MDA-MB-231 Cells
2.4. Molecular Docking of 14B to the Activity Site of FAK
2.5. Confocal Laser Scanning Microscopy Examinations of MDA-MB-231 Cells
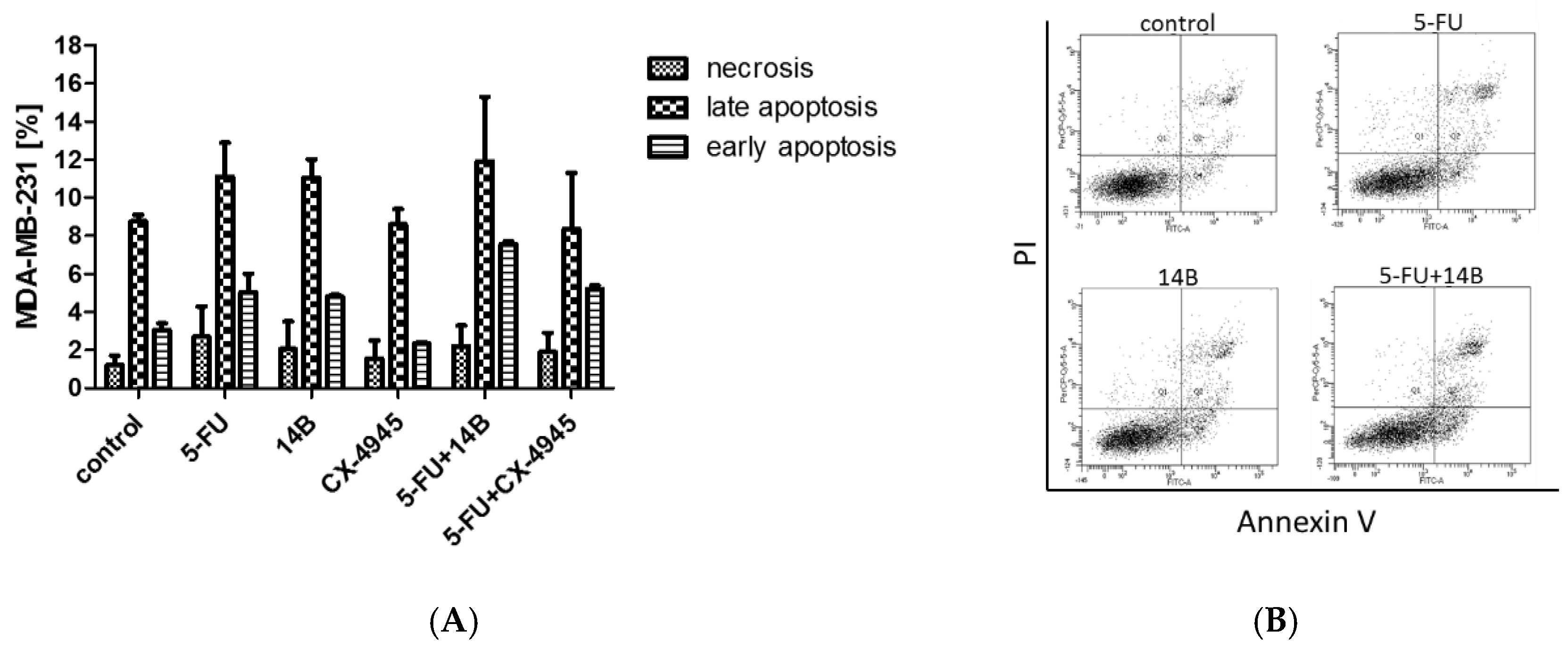
2.6. Induction of Apoptosis in MDA-MB-231 Cells
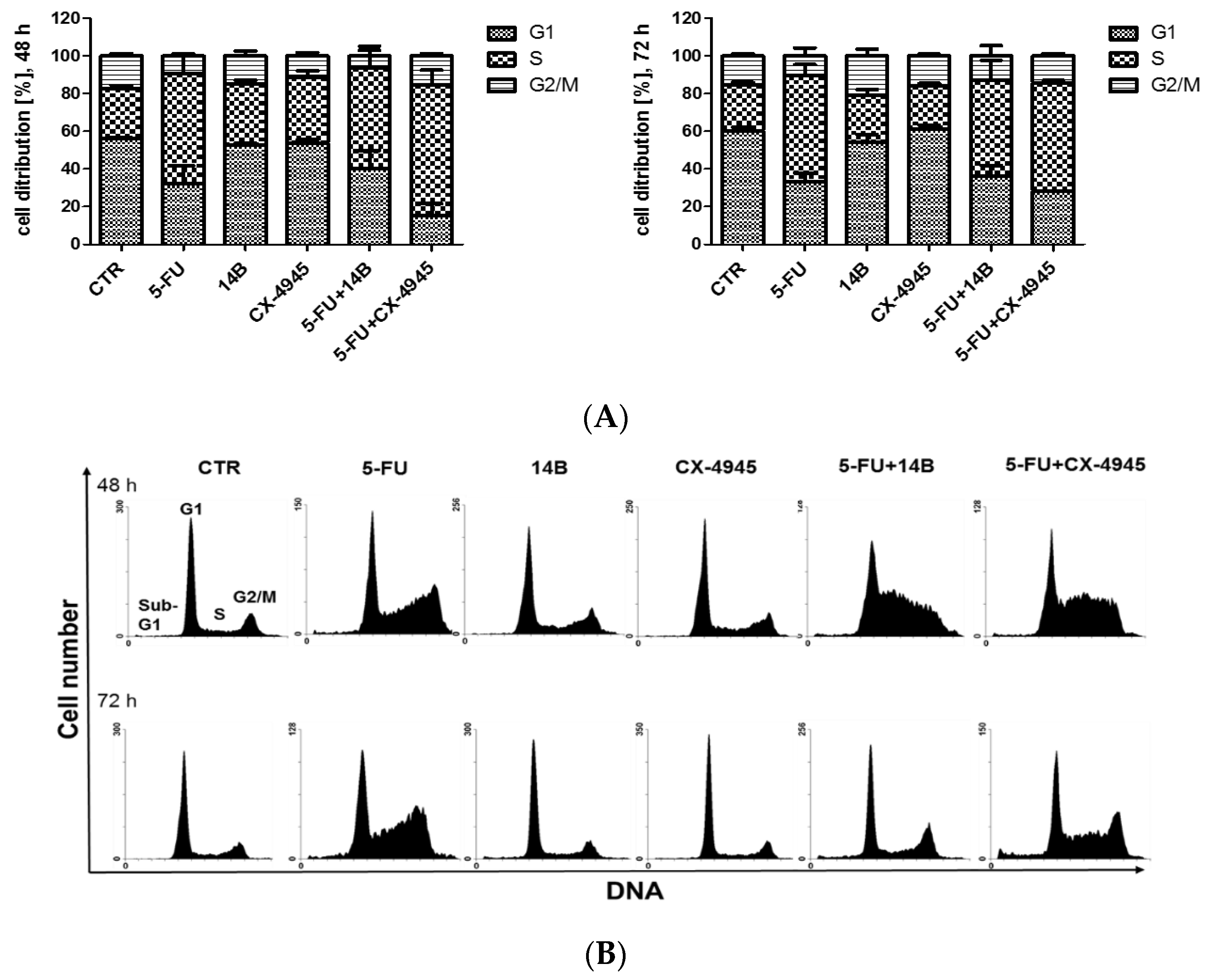
2.7. The Effect of the Synergistically Acting Combinations on Cell Cycle Progression in MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Agent Treatment
4.3. MTT-Based Viability Assay
4.4. Cell Cycle Analysis
4.5. Detection of Apoptosis
4.6. Western Blotting
4.7. Densitometry
4.8. Statistical Evaluation
4.9. Immunocytochemical Staining and Microscopy Analysis
4.10. Molecular Docking
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Muleya, H.; Fadóa, R.; Rodríguez-Rodrígueza, R.; Casalsa, N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem. Pharmacol. 2020, 177, 113959. [Google Scholar] [CrossRef]
- Sagara, A.; Igarashi, K.; Otsuka, M.; Karasawa, T.; Gotoh, N.; Narita, M.; Kuzumaki, N.; Narita, M.; Kato, Y. Intrinsic Resistance to 5-Fluorouracil in a Brain Metastatic Variant of Human Breast Cancer Cell Line, MDA-MB-231BR. PLoS ONE 2016, 11, e0164250. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.G.; Hernández Hernández, J.M.; Rotello, V.M.; Tapia Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public Health 2020, 17, 2078. [Google Scholar] [CrossRef]
- Saleh, E.M.; El-Awady, R.A.; Anis, N. Predictive markers for the response to 5-fluorouracil therapy in cancer cells: Constant-field gel electrophoresis as a tool for prediction of response to 5-fluorouracil-based chemotherapy. Oncol. Lett. 2013, 5, 321–327. [Google Scholar] [CrossRef]
- Li, L.S.; Morales, J.C.; Veigl, M.; Sedwick, D.; Greer, S.; Meyers, M.; Wagner, M.; Fishel, R.; Boothman, D.A. DNA mismatch repair (MMR)-dependent 5-fluorouracil cytotoxicity and the potential for new therapeutic targets. Br. J. Pharmacol. 2009, 158, 679–692. [Google Scholar] [CrossRef]
- Botticelli, A.; Scagnoli, S.; Roberto, M.; Lionetto, L.; Cerbelli, B.; Simmaco, M.; Marchetti, P. 5-Fluorouracil degradation rate as a predictive biomarker of toxicity in breast cancer patients treated with capecitabine. J. Oncol. Pharm. Pract. 2020, in press. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Fernández-Carballido, A.; Torres-Suárez, A.I. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother. Pharmacol. 2019, 84, 689–706. [Google Scholar] [CrossRef]
- Wu, D.; Pusuluri, A.; Vogus, D.; Krishnan, V.; Wyatt, S., IV; Kim, J.; Razmi, A.; Mitragotri, S. Design principles of drug combinations for chemotherapy. J. Control Release 2020, 323, 36–46. [Google Scholar] [CrossRef]
- Wińska, P.; Skierka, K.; Łukowska-Chojnacka, E.; Koronkiewicz, M.; Cieśla, J.; Bretner, M. Effect of Simultaneous Inhibition of Protein Kinase CK2 and Thymidylate Synthase in Leukemia and Breast Cancer Cells. Anticancer Res. 2018, 38, 4617–4627. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Su, M.; Zhu, Y.; Zhou, Y.; Soomro, S.H.; Fu, H. Protein Kinase CK2, a Potential Therapeutic Target in Carcinoma Management. Asian Pac. J. Cancer Prev. 2018, 20, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.E.; Seidner, Y.; Dominguez, I. Mining CK2 in cancer. PLoS ONE 2014, 9, e115609. [Google Scholar] [CrossRef]
- Borgo, C.; Ruzzene, M. Role of protein kinase CK2 in antitumor drug resistance. J. Exp. Clin. Cancer Res. 2019, 38, 287. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.K.; McFarland, B.C.; Rowse, A.L.; Gibson, S.A.; Benveniste, E.N. Therapeutic CK2 inhibition attenuates diverse prosurvival signaling cascades and decreases cell viability in human breast cancer cells. Oncotarget 2014, 5, 6484–6496. [Google Scholar] [CrossRef]
- Łukowska-Chojnacka, E.; Wińska, P.; Wielechowska, M.; Poprzeczko, M.; Bretner, M. Synthesis of novel polybrominated benzimidazole derivatives—Potential CK2 inhibitors with anticancer and proapoptotic activity. Bioorg. Med. Chem. 2016, 24, 735–741. [Google Scholar] [CrossRef]
- Siddiqui-Jain, A.; Bliesath, J.; Macalino, D.; Omori, M.; Huser, N.; Streiner, N.; Ho, C.B.; Anderes, K.; Proffitt, C.; O’Brien, S.E.; et al. CK2 inhibitor CX-4945 suppresses DNA repair response triggered by DNA-targeted anticancer drugs and augments efficacy: Mechanistic rationale for drug combination therapy. Mol. Cancer Ther. 2012, 11, 994–1005. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, Y.-S.; Lee, J.H.; Lee, S.H. Casein Kinase 2α Enhances 5-Fluorouracil Resistance in Colorectal Cancer Cells by Inhibiting Endoplasmic Reticulum Stress. Anticancer Res. 2020, 40, 1419–1426. [Google Scholar] [CrossRef]
- Chojnacki, K.; Wińska, P.; Wielechowska, M.; Łukowska-Chojnacka, E.; Tölzer, C.; Niefind, K.; Bretner, M. Biological properties and structural study of new aminoalkyl derivatives of benzimidazole and benzotriazole, dual inhibitors of CK2 and PIM1 kinases. Bioorg. Chem. 2018, 80, 266–275. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Han, J.; Sun, P. The pathways to tumor suppression via route p38. Trends Biochem. Sci. 2007, 32, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mazivey, M.; Alahari, S.K. Cell matrix adhesions in cancer: The proteins that form the glue. Oncotarget 2017, 8, 48471–48487. [Google Scholar] [CrossRef]
- Olsen, B.B.; Tvenstrup, T.H.; Guerra, B. Downregulation of protein kinase CK2 induces autophagic cell death through modulation of the mTOR and MAPK signaling pathways in human glioblastoma cells. Int. J. Oncol. 2012, 41, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, T.; Seenisamy, J.; Emmanuvel, L.; Kulkarni, S.S.; Bomke, J.; Rohdich, F.; Greiner, H.; Esdar, C.; Krier, M.; Grädler, U.; et al. Fragment-Based Discovery of New Highly Substituted1H-Pyrrolo [2,3-b]—and 3H-Imidazolo[4,5-b]-Pyridines as Focal Adhesion Kinase Inhibitors. J. Med. Chem. 2013, 56, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Wu, F.; Xu, T.; He, G.; Ouyang, L.; Han, B.; Peng, C.; Song, X.; Xiang, M. Discovery of Novel Focal Adhesion Kinase Inhibitors Using a Hybrid Protocol of Virtual Screening Approach Based on Multicomplex-Based Pharmacophore and Molecular Docking. Int. J. Mol. Sci. 2012, 13, 15668–15678. [Google Scholar] [CrossRef] [PubMed]
- Yen-Pon, E.; Li, B.; Acebrón-Garcia-de-Eulate, M.; Tomkiewicz-Raulet, C.; Dawson, J.; Lietha, D.; Frame, M.C.; Coumoul, X.; Garbay, C.; Etheve-Quelquejeu, M.; et al. Structure-Based Design, Synthesis, and Characterization of the First Irreversible Inhibitor of Focal Adhesion Kinase. ACS Chem. Biol. 2018, 13, 2067–2073. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, Y.; He, L.-Q.; Liu, Z.-J.; Jiang, A.-Q.; Yang, Y.-H.; Zhu, H.-L. Synthesis, biological evaluation, and molecular docking studies of novel 1,3,4-oxadiazole derivatives possessing benzotriazole moiety as FAK inhibitors with anticancer activity. Bioorg. Med. Chem. 2013, 21, 3723–3729. [Google Scholar] [CrossRef]
- Dao, P.; Jarray, R.; Le Coq, J.; Lietha, D.; Loukaci, A.; Lepelletier, Y.; Hadj-Slimane, R.; Garbay, C.; Raynaud, F.; Chen, H. Synthesis of novel diarylamino-1,3,5-triazine derivatives as FAK inhibitors with anti-angiogenic activity. Bioorg. Med. Chem. Lett. 2013, 23, 4552–4556. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Wińska, P.; Widło, Ł.; Skierka, K.; Krzyśko, A.; Koronkiewicz, M.; Cieśla, J.M.; Cieśla, J.; Bretner, M. Simultaneous Inhibition of Protein Kinase CK2 and Dihydrofolate Reductase Results in Synergistic Effect on Acute Lymphoblastic Leukemia Cells. Anticancer Res. 2019, 39, 3531–3542. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.G.; Drake, J.C.; Trepel, J.; Allegra, C.J. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res. 1992, 52, 4306–4312. [Google Scholar]
- Bowen, J.M.; Gibson, R.J.; Keefe, D.M. Animal models of mucositis: Implications for therapy. J. Support Oncol. 2011, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.V.S.; Bon-Frauches, A.C.; Silva, A.M.H.P.; Lima-Júnior, R.C.P.; Martins, C.S.; Leitão, R.F.C.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway. Sci Rep. 2019, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Frączyk, T.; Kubiński, K.; Masłyk, M.; Cieśla, J.; Hellman, U.; Shugar, D.; Rode, W. Phosphorylation of thymidylate synthase from various sources by human protein kinase CK2 and its catalytic subunits. Bioorg. Chem. 2010, 38, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.H. CK2 inhibitor CX-4945 blocks TGF-β1-induced epithelial-to-mesenchymal transition in A549 human lung adenocarcinoma cells. PLoS ONE 2013, 8, e74342. [Google Scholar] [CrossRef]
- Martínez, P.T.; Navajas, P.L.; Lietha, D. FAK Structure and Regulation by Membrane Interactions and Force in Focal Adhesions. Biomolecules 2020, 10, 179. [Google Scholar] [CrossRef]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef]
- Luo, M.; Guan, J.L. Focal Adhesion Kinase: A Prominent Determinant in Breast Cancer Initiation, Progression and Metastasis. Cancer Lett. 2010, 289, 127–139. [Google Scholar] [CrossRef]
- Wu, H.J.; Hao, M.; Yeo, S.K.; Guan, J.L. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 2020, 39, 2539–2549. [Google Scholar] [CrossRef]
- Pan, M.R.; Wu, C.C.; Kan, J.Y.; Li, Q.L.; Chang, S.J.; Wu, C.C.; Li, C.L.; Yang, F.O.; Hou, M.F.; Yip, H.K.; et al. Impact of FAK Expression on the Cytotoxic Effects of CIK Therapy in Triple-Negative Breast Cancer. Cancers 2020, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Tan, Y.; Su, C.; Wang, T.; Gao, Z.; Song, D.; Zhao, J.; Liao, Y.; Liu, X.; Jiang, Y.; et al. Inhibition of protein FAK enhances 5-FU chemosensitivity to gastric carcinoma via p53 signaling pathways. Comput Struct. Biotechnol. J. 2019, 18, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, M.C.; Morrot, A.; Soares, P.M.; Costa, M.L.; Mermelstein, C. Effects of 5-fluorouracil in nuclear and cellular morphology, proliferation, cell cycle, apoptosis, cytoskeletal and caveolar distribution in primary cultures of smooth muscle cells. PLoS ONE 2013, 8, e63177. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, C.; Salizzato, V.; Borgo, C.; Cesaro, L.; Pinna, L.A.; Salvia, M. A journey through the Cytoskeleton with Protein Kinase CK2. Curr. Protein Pept. Sci. 2019, 20, 547–562. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Morcillo, M.A.; Valero, M.L.; Callejas-Valera, J.L.; Arias-González, L.; Melgar-Rojas, P.; Galán-Moya, E.M.; García-Gil, E.; García-Cano, J.; Sánchez-Prieto, R. P38MAPK is a major determinant of the balance between apoptosis and autophagy triggered by 5-fluorouracil: Implication in resistance. Oncogene 2012, 31, 1073–1085. [Google Scholar] [CrossRef]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic reticulum stress confers 5-fluorouracil resistance in breast cancer cell via the GRP78/OCT4/lncRNA MIAT/AKT pathway. Am. J. Cancer Res. 2020, 10, 838–855. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Becherel, O.J.; Jakob, B.; Cherry, A.L.; Gueven, N.; Fusser, M.; Kijas, A.W.; Peng, C.; Katyal, S.; McKinnon, P.J.; Chen, J.; et al. CK2 phosphorylation-dependent interaction between aprataxin and MDC1 in the DNA damage response. Nucleic Acids Res. 2010, 38, 1489–1503. [Google Scholar] [CrossRef]
- Nair, S.K.; Verma, A.; Thomas, T.J.; Chou, T.C.; Gallo, M.A.; Shirahata, A.; Thomas, T. Synergistic Apoptosis of MCF-7 Breast Cancer Cells by 2- Methoxyestradiol and Bis(ethyl)norspermine. Cancer Lett. 2007, 250, 311–322. [Google Scholar] [CrossRef]





| Compound | Molecular Target | Dm * ± SD (µM) | |
|---|---|---|---|
| MDA-MB-231 | MCF-7 ** | ||
| 5-Fluorouracil (5-FU) | TS | 61.84 ± 7.64 | 14.88 ± 2.56 |
| 4,5,6,7-tetrabromo-1-(3-bromopropyl)-2-methyl-1H-benzimidazole (14B) | CK2 | 3.94 ± 1.08 | 4.28 ± 0.56 |
| CX-4945 (silmitasertib) | CK2 | 12.47 ± 2.99 | 8.36 ± 0.35 |
| Cell Line | Drugs in Combination | Combination Index at | Dm | DRI for 5-FU at Fa = 0.95 | Type of Drug Interaction ** | ||
|---|---|---|---|---|---|---|---|
| ED50 | ED75 | ED90 | |||||
| MDA-MB-231 | 5-FU:14B | 0.69 ± 0.11 | 0.66 ± 0.09 | 0.76 ± 0.14 | 3.89 ± 0.54 | 43.42 | Synergism |
| 5-FU:CX-4945 | 0.70 ± 0.06 | 0.65 ± 0.10 | 0.64 ± 0.23 | 11.93 ± 2.54 | 46.27 | Synergism | |
| MCF-7 | 5-FU:14B | 2.94 ± 0.48 | 1.16 ± 0.39 | 2.38 ± 0.54 | 1.86 ± 1.15 | - | Antagonism |
| 5-FU:CX-4945 * | 0.92 ± 0.11 | 0.74 ± 0.07 | 0.62 ± 0.08 | 11.31 ± 3.68 | 3.25 | Synergism | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wińska, P.; Karatsai, O.; Staniszewska, M.; Koronkiewicz, M.; Chojnacki, K.; Rędowicz, M.J. Synergistic Interactions of 5-Fluorouracil with Inhibitors of Protein Kinase CK2 Correlate with p38 MAPK Activation and FAK Inhibition in the Triple-Negative Breast Cancer Cell Line. Int. J. Mol. Sci. 2020, 21, 6234. https://doi.org/10.3390/ijms21176234
Wińska P, Karatsai O, Staniszewska M, Koronkiewicz M, Chojnacki K, Rędowicz MJ. Synergistic Interactions of 5-Fluorouracil with Inhibitors of Protein Kinase CK2 Correlate with p38 MAPK Activation and FAK Inhibition in the Triple-Negative Breast Cancer Cell Line. International Journal of Molecular Sciences. 2020; 21(17):6234. https://doi.org/10.3390/ijms21176234
Chicago/Turabian StyleWińska, Patrycja, Olena Karatsai, Monika Staniszewska, Mirosława Koronkiewicz, Konrad Chojnacki, and Maria Jolanta Rędowicz. 2020. "Synergistic Interactions of 5-Fluorouracil with Inhibitors of Protein Kinase CK2 Correlate with p38 MAPK Activation and FAK Inhibition in the Triple-Negative Breast Cancer Cell Line" International Journal of Molecular Sciences 21, no. 17: 6234. https://doi.org/10.3390/ijms21176234
APA StyleWińska, P., Karatsai, O., Staniszewska, M., Koronkiewicz, M., Chojnacki, K., & Rędowicz, M. J. (2020). Synergistic Interactions of 5-Fluorouracil with Inhibitors of Protein Kinase CK2 Correlate with p38 MAPK Activation and FAK Inhibition in the Triple-Negative Breast Cancer Cell Line. International Journal of Molecular Sciences, 21(17), 6234. https://doi.org/10.3390/ijms21176234





