Anti-Fibrotic Effect of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells on Skeletal Muscle Cells, Mediated by Secretion of MMP-1
Abstract
:1. Introduction
2. Results
2.1. Anti-Fibrotic Effects of WJ-MSCs on an Mdx Model
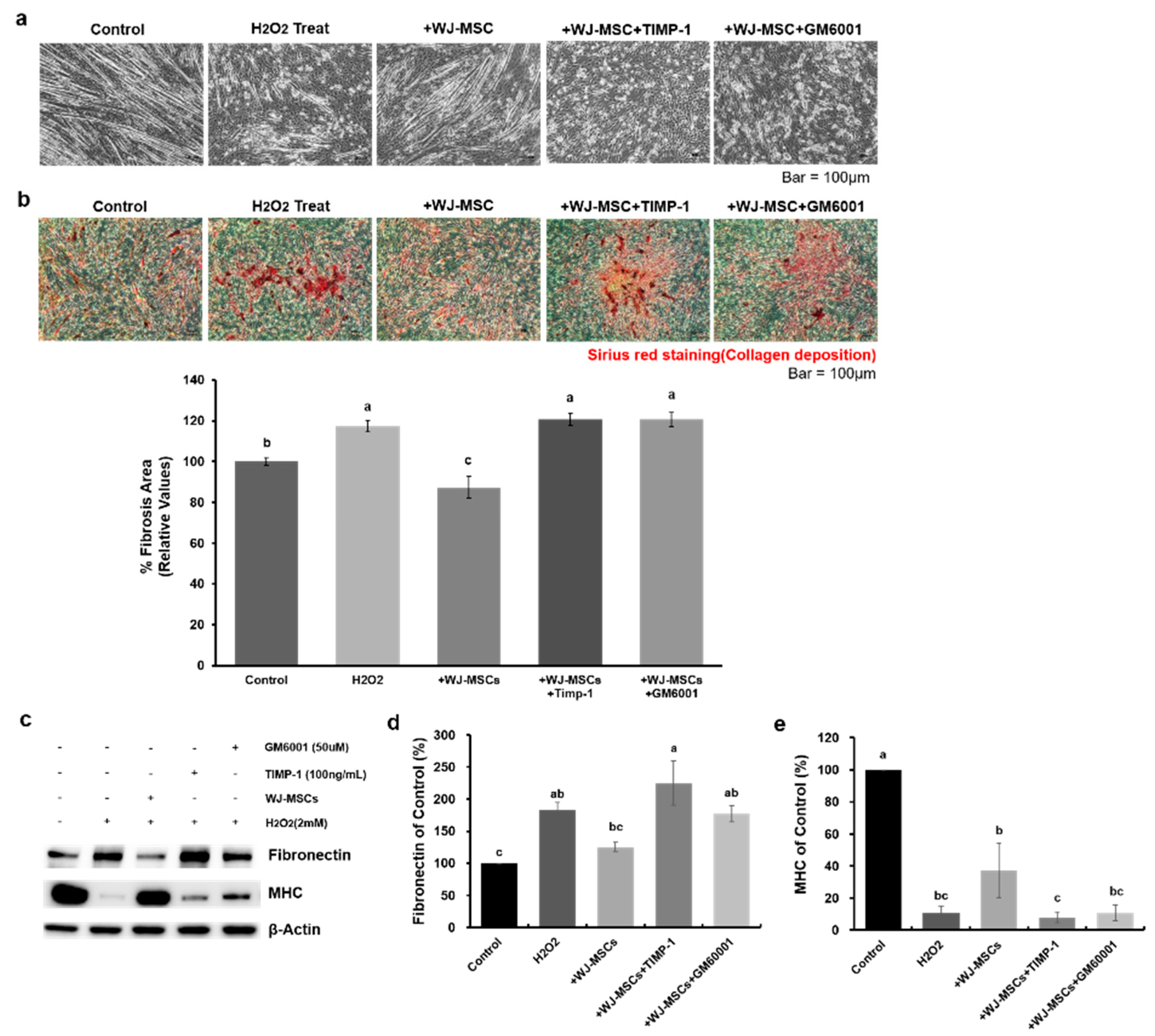
2.2. Effects of WJ-MSCs on an In-Vitro H2O2-Induced Fibrosis Model
2.3. Identification of Proteins Secreted from WJ-MSCs in a Co-Culture System
2.4. Human Recombinant MMP-1 Protein Has Protective Effects in an In-Vitro H2O2-Induced Fibrosis Model
2.5. MMP-1 Is a Key Factor for the Anti-Fibrotic Effect of Human WJ-MSCs
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Cell Culture
4.3. MMP Inhibitors Application
4.4. MSCs Administration
4.5. Protein Extraction and Immunoblotting
4.6. Antibody Arrays and Enzyme-Linked Immunosorbent Assay
4.7. Histological Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| DMD | Duchenne muscular dystrophy |
| ECM | extracellular matrix |
| ELISA | enzyme-linked immunosorbent assay |
| H2O2 | hydrogen peroxide |
| MHC | myosin heavy chain |
| MMP-1 | matrix metalloproteinase-1 |
| MSCs | mesenchymal stem cells |
| PBS | phosphate-buffered saline |
| ROS | reactive oxygen species |
| SBRI | Samsung Biomedical Research Institute |
| TIMP-1 | tissue inhibitor of metallopeptidase-1 |
| WJ | Wharton’s Jelly |
Appendix A


References
- Serrano, A.L.; Munoz-Canoves, P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp. Cell Res. 2010, 316, 3050–3058. [Google Scholar] [CrossRef] [PubMed]
- Klingler, W.; Jurkat-Rott, K.; Lehmann-Horn, F.; Schleip, R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012, 31, 184–195. [Google Scholar] [PubMed]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. TGF-beta-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdy, M.A.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Cholok, D.; Lee, E.; Lisiecki, J.; Agarwal, S.; Loder, S.; Ranganathan, K.; Qureshi, A.T.; Davis, T.A.; Levi, B. Traumatic muscle fibrosis: From pathway to prevention. J. Trauma Acute Care Surg. 2017, 82, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Kharraz, Y.; Guerra, J.; Pessina, P.; Serrano, A.L.; Munoz-Canoves, P. Understanding the process of fibrosis in Duchenne muscular dystrophy. Biomed. Res. Int. 2014, 2014, 965631. [Google Scholar] [CrossRef]
- Forcina, L.; Pelosi, L.; Miano, C.; Musarò, A. Insights into the Pathogenic Secondary Symptoms Caused by the Primary Loss of Dystrophin. J. Funct. Morphol. Kinesiol. 2017, 2, 44. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kwak, H.B.; Thompson, L.V.; Lawler, J.M. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 2013, 34, 1–13. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, H. Targeting fibrosis in Duchenne muscular dystrophy. J. Neuropathol. Exp. Neurol. 2010, 69, 771–776. [Google Scholar] [CrossRef]
- Kim, H.J.; Seo, S.W.; Chang, J.W.; Lee, J.I.; Kim, C.H.; Chin, J.; Choi, S.J.; Kwon, H.; Yun, H.J.; Lee, J.M.; et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimers Dement. 2015, 1, 95–102. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolucci, J.; Verdugo, F.J.; Gonzalez, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, S.; Liu, X.; Song, B.; Shi, L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn’s Disease: A Randomized Controlled Clinical Trial. Gut Liver 2018, 12, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelek, J.; Zuba-Surma, E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol. Sci. 2020, 21, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, C.; Yang, C.; Zhang, J.; Dong, Q.; Wang, W. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018, 215, 113–118. [Google Scholar] [CrossRef]
- Lee, N.K.; Na, D.L.; Chang, J.W. Killing two birds with one stone: The multifunctional roles of mesenchymal stem cells in the treatment of neurodegenerative and muscle diseases. Histol. Histopathol. 2018, 33, 629–638. [Google Scholar] [CrossRef]
- Kim, D.H.; Lim, H.; Lee, D.; Choi, S.J.; Oh, W.; Yang, Y.S.; Oh, J.S.; Hwang, H.H.; Jeon, H.B. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer’s disease model. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.K.; Park, S.E.; Kwon, S.J.; Shim, S.; Byeon, Y.; Kim, J.H.; Na, D.L.; Chang, J.W. Agouti Related Peptide Secreted Via Human Mesenchymal Stem Cells Upregulates Proteasome Activity in an Alzheimer’s Disease Model. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Lee, J.; Chang, E.H.; Kim, J.H.; Sung, J.H.; Na, D.L.; Chang, J.W. Activin A secreted by human mesenchymal stem cells induces neuronal development and neurite outgrowth in an in vitro model of Alzheimer’s disease: Neurogenesis induced by MSCs via activin A. Arch. Pharm. Res. 2016, 39, 1171–1179. [Google Scholar] [CrossRef]
- Cordova, G.; Negroni, E.; Cabello-Verrugio, C.; Mouly, V.; Trollet, C. Combined Therapies for Duchenne Muscular Dystrophy to Optimize Treatment Efficacy. Front. Genet. 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.H.; Wang, C.J.; Fu, H.C.; Sheng, C.M.; Tsai, C.C.; Cheng, J.H.; Chuang, P.C. Human Umbilical Cord Mesenchymal Stem Cells Extricate Bupivacaine-Impaired Skeletal Muscle Function via Mitigating Neutrophil-Mediated Acute Inflammation and Protecting against Fibrosis. Int. J. Mol. Sci. 2019, 20, 4312. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.; Ki, S.M.; Park, S.E.; Kim, M.J.; Hyung, B.; Lee, N.K.; Shim, S.; Choi, B.O.; Na, D.L.; Lee, J.E.; et al. Anti-apoptotic Effects of Human Wharton’s Jelly-derived Mesenchymal Stem Cells on Skeletal Muscle Cells Mediated via Secretion of XCL1. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1550–1560. [Google Scholar] [CrossRef] [Green Version]
- Pessina, P.; Cabrera, D.; Morales, M.G.; Riquelme, C.A.; Gutierrez, J.; Serrano, A.L.; Brandan, E.; Munoz-Canoves, P. Novel and optimized strategies for inducing fibrosis in vivo: Focus on Duchenne Muscular Dystrophy. Skelet. Muscle 2014, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Shen, L.; Zhang, Z.; Xie, X. Therapeutic Strategies for Duchenne Muscular Dystrophy: An Update. Genes 2020, 11, 837. [Google Scholar] [CrossRef]
- Gillis, J.M. Membrane abnormalities and Ca homeostasis in muscles of the mdx mouse, an animal model of the Duchenne muscular dystrophy: A review. Acta Physiol. Scand. 1996, 156, 397–406. [Google Scholar] [CrossRef]
- Law, M.L.; Cohen, H.; Martin, A.A.; Angulski, A.B.B.; Metzger, J.M. Dysregulation of Calcium Handling in Duchenne Muscular Dystrophy-Associated Dilated Cardiomyopathy: Mechanisms and Experimental Therapeutic Strategies. J. Clin. Med. 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameen, V.; Robson, L.G. Experimental models of duchenne muscular dystrophy: Relationship with cardiovascular disease. Open Cardiovasc. Med. J. 2010, 4, 265–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutdibi, O.; Brinkmeier, H.; Rudel, R.; Fohr, K.J. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J. Physiol. 1999, 515 Pt 3, 859–868. [Google Scholar] [CrossRef]
- Burr, A.R.; Molkentin, J.D. Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy. Cell Death Differ. 2015, 22, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Bozycki, L.; Lukasiewicz, K.; Matryba, P.; Pikula, S. Whole-body clearing, staining and screening of calcium deposits in the mdx mouse model of Duchenne muscular dystrophy. Skelet. Muscle 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Dulak, J. Muscle and cardiac therapeutic strategies for Duchenne muscular dystrophy: Past, present, and future. Pharmacol. Rep. 2020. [Google Scholar] [CrossRef]
- Choi, M.H.; Ow, J.R.; Yang, N.D.; Taneja, R. Oxidative Stress-Mediated Skeletal Muscle Degeneration: Molecules, Mechanisms, and Therapies. Oxid. Med. Cell. Longev. 2016, 2016, 6842568. [Google Scholar] [CrossRef] [Green Version]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; He, H.; Xu, Q.; Yu, Y.; Zheng, N.; Liu, W.; Hayashi, T.; Tashiro, S.; Onodera, S.; Ikejima, T. Reactive oxygen species H2O2 and *OH, but not O2*(-) promote oridonin-induced phagocytosis of apoptotic cells by human histocytic lymphoma U937 cells. Int. Immunopharmacol. 2013, 15, 414–423. [Google Scholar] [CrossRef]
- Oba, T.; Koshita, M.; Yamaguchi, M. H2O2 modulates twitch tension and increases Po of Ca2+ release channel in frog skeletal muscle. Am. J. Physiol. 1996, 271, C810–C818. [Google Scholar] [CrossRef]
- Østdal, H.; Skibsted, L.H.; Andersen, H.J. Formation of Long-Lived Protein Radicals in the Reaction between H2O2-Activated Metmyoglobin and Other Proteins. Free Radic. Biol. Med. 1997, 23, 754–761. [Google Scholar] [CrossRef]
- Abdel, S.E.; Abdel-Meguid, I.; Korraa, S. Markers of oxidative stress and aging in Duchene muscular dystrophy patients and the possible ameliorating effect of He:Ne laser. Acta Myol. 2007, 26, 14–21. [Google Scholar] [PubMed]
- Vielma, A.Z.; Boric, M.P.; Gonzalez, D.R. Apocynin Treatment Prevents Cardiac Connexin 43 Hemichannels Hyperactivity by Reducing Nitroso-Redox Stress in Mdx Mice. Int. J. Mol. Sci. 2020, 21, 5415. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, Y. Role of matrix metalloproteinases in skeletal muscle: Migration, differentiation, regeneration and fibrosis. Cell Adhes. Migr. 2009, 3, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, H.; Murray, K.; Jefferson, B.S.; Li, Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am. J. Pathol. 2009, 174, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Kaar, J.L.; Li, Y.; Blair, H.C.; Asche, G.; Koepsel, R.R.; Huard, J.; Russell, A.J. Matrix metalloproteinase-1 treatment of muscle fibrosis. Acta Biomater. 2008, 4, 1411–1420. [Google Scholar] [CrossRef]
- Pescatori, M.; Broccolini, A.; Minetti, C.; Bertini, E.; Bruno, C.; D’Amico, A.; Bernardini, C.; Mirabella, M.; Silvestri, G.; Giglio, V.; et al. Gene expression profiling in the early phases of DMD: A constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J. 2007, 21, 1210–1226. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Lian, Q.; Tse, H.F. Perspective and challenges of mesenchymal stem cells for cardiovascular regeneration. Expert Rev. Cardiovasc. Ther. 2013, 11, 505–517. [Google Scholar] [CrossRef]
- Siu, C.W.; Liao, S.Y.; Liu, Y.; Lian, Q.; Tse, H.F. Stem cells for myocardial repair. Thromb. Haemost. 2010, 104, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Xiong, G.; Feng, H.; Zhang, Z.; Chen, P.; Yan, B.; Chen, L.; Gandhervin, K.; Ma, C.; Li, C.; et al. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics 2019, 9, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Fan, X.L.; Jiang, D.; Zhang, Y.; Li, X.; Xu, Z.B.; Fang, S.B.; Chiu, S.; Tse, H.F.; Lian, Q.; et al. Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation. Stem Cell Rep. 2018, 11, 1120–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yu, Z.; Jiang, D.; Liang, X.; Liao, S.; Zhang, Z.; Yue, W.; Li, X.; Chiu, S.M.; Chai, Y.H.; et al. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-alpha Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Rep. 2016, 7, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Batiz, L.F.; Contreras, R.A.; Ortuzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Laroye, C.; Boufenzer, A.; Jolly, L.; Cunat, L.; Alauzet, C.; Merlin, J.L.; Yguel, C.; Bensoussan, D.; Reppel, L.; Gibot, S. Bone marrow vs Wharton’s jelly mesenchymal stem cells in experimental sepsis: A comparative study. Stem Cell Res. Ther. 2019, 10, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chan, Y.C.; Ho, J.C.; Siu, C.W.; Lian, Q.; Tse, H.F. Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via ether-a-go-go 1 (hEAG1) potassium channel. Am. J. Physiol. Cell Physiol. 2012, 303, C115–C125. [Google Scholar] [CrossRef]
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.; Kang, S.; Xia, J.C.; Lai, W.H.; et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 2010, 121, 1113–1123. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, A.; Park, S.E.; Jeong, J.B.; Choi, S.-j.; Oh, S.-y.; Ryu, G.H.; Lee, J.; Jeon, H.B.; Chang, J.W. Anti-Fibrotic Effect of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells on Skeletal Muscle Cells, Mediated by Secretion of MMP-1. Int. J. Mol. Sci. 2020, 21, 6269. https://doi.org/10.3390/ijms21176269
Choi A, Park SE, Jeong JB, Choi S-j, Oh S-y, Ryu GH, Lee J, Jeon HB, Chang JW. Anti-Fibrotic Effect of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells on Skeletal Muscle Cells, Mediated by Secretion of MMP-1. International Journal of Molecular Sciences. 2020; 21(17):6269. https://doi.org/10.3390/ijms21176269
Chicago/Turabian StyleChoi, Alee, Sang Eon Park, Jang Bin Jeong, Suk-joo Choi, Soo-young Oh, Gyu Ha Ryu, Jeehun Lee, Hong Bae Jeon, and Jong Wook Chang. 2020. "Anti-Fibrotic Effect of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells on Skeletal Muscle Cells, Mediated by Secretion of MMP-1" International Journal of Molecular Sciences 21, no. 17: 6269. https://doi.org/10.3390/ijms21176269




