GPx8 Expression in Rat Oocytes, Embryos, and Female Genital Organs During Preimplantation Period of Pregnancy
Abstract
:1. Introduction
2. Results
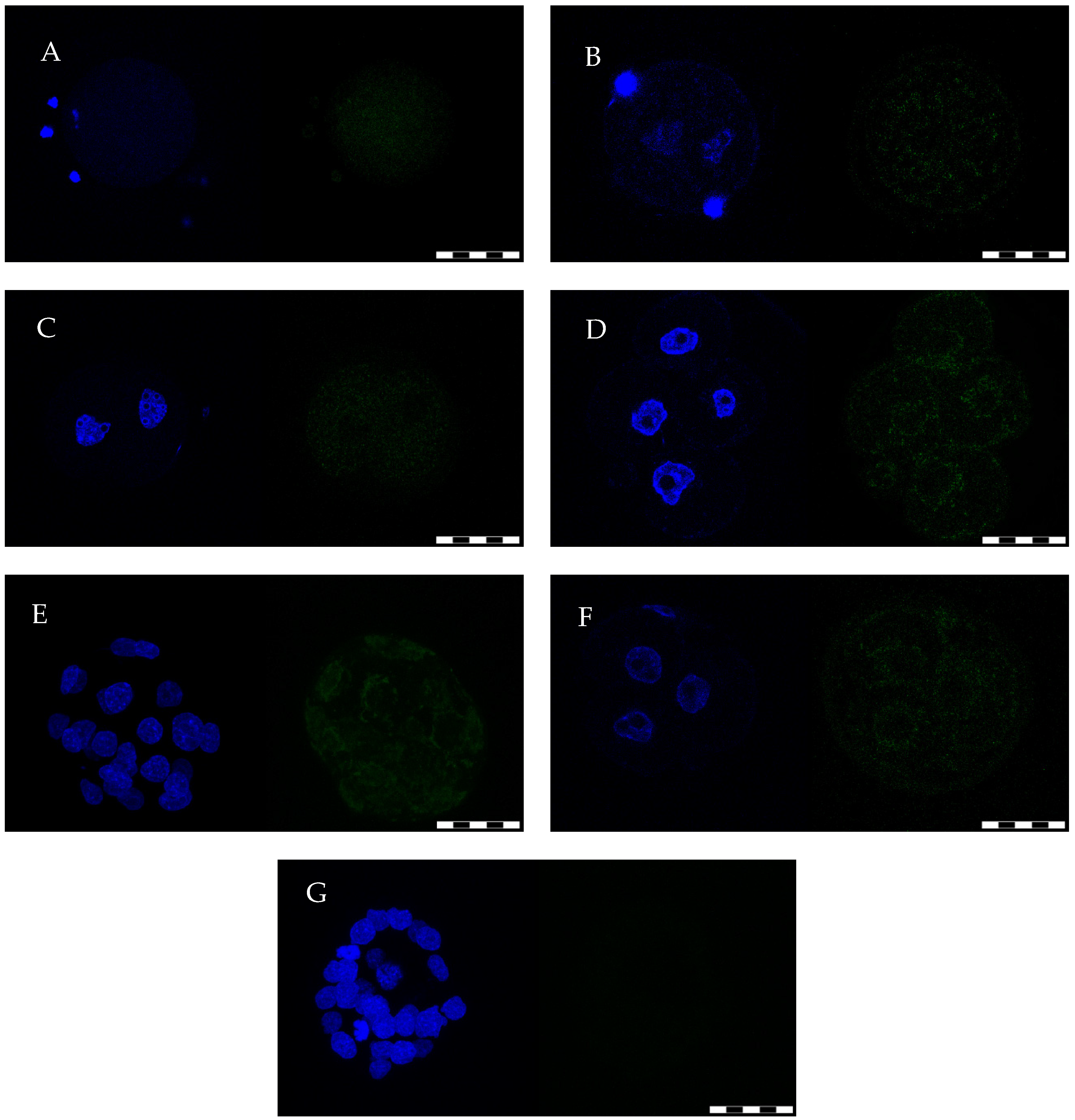
2.1. Oocytes/Preimplantation Embryo Isolation and GPx8 IF Detection
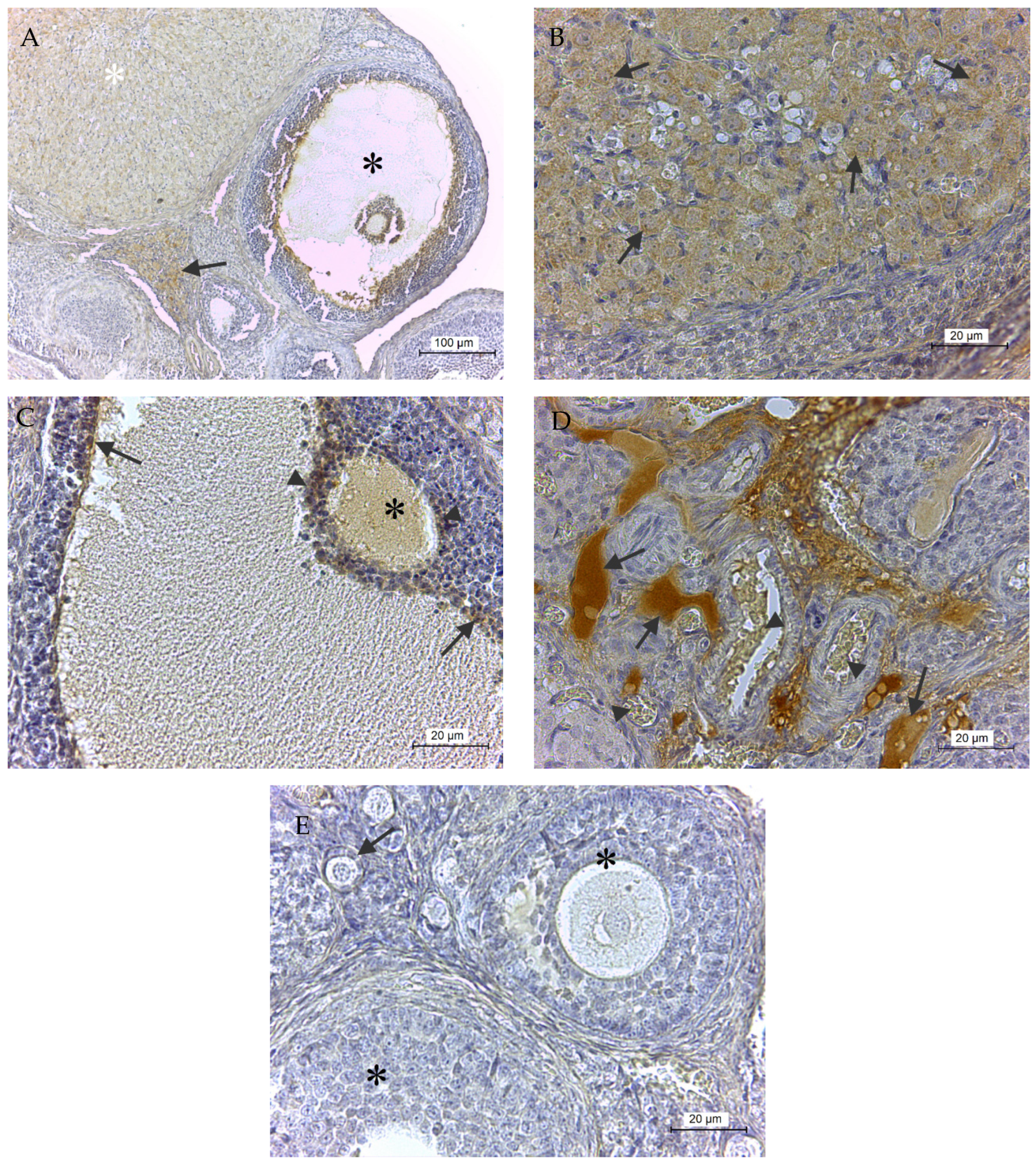
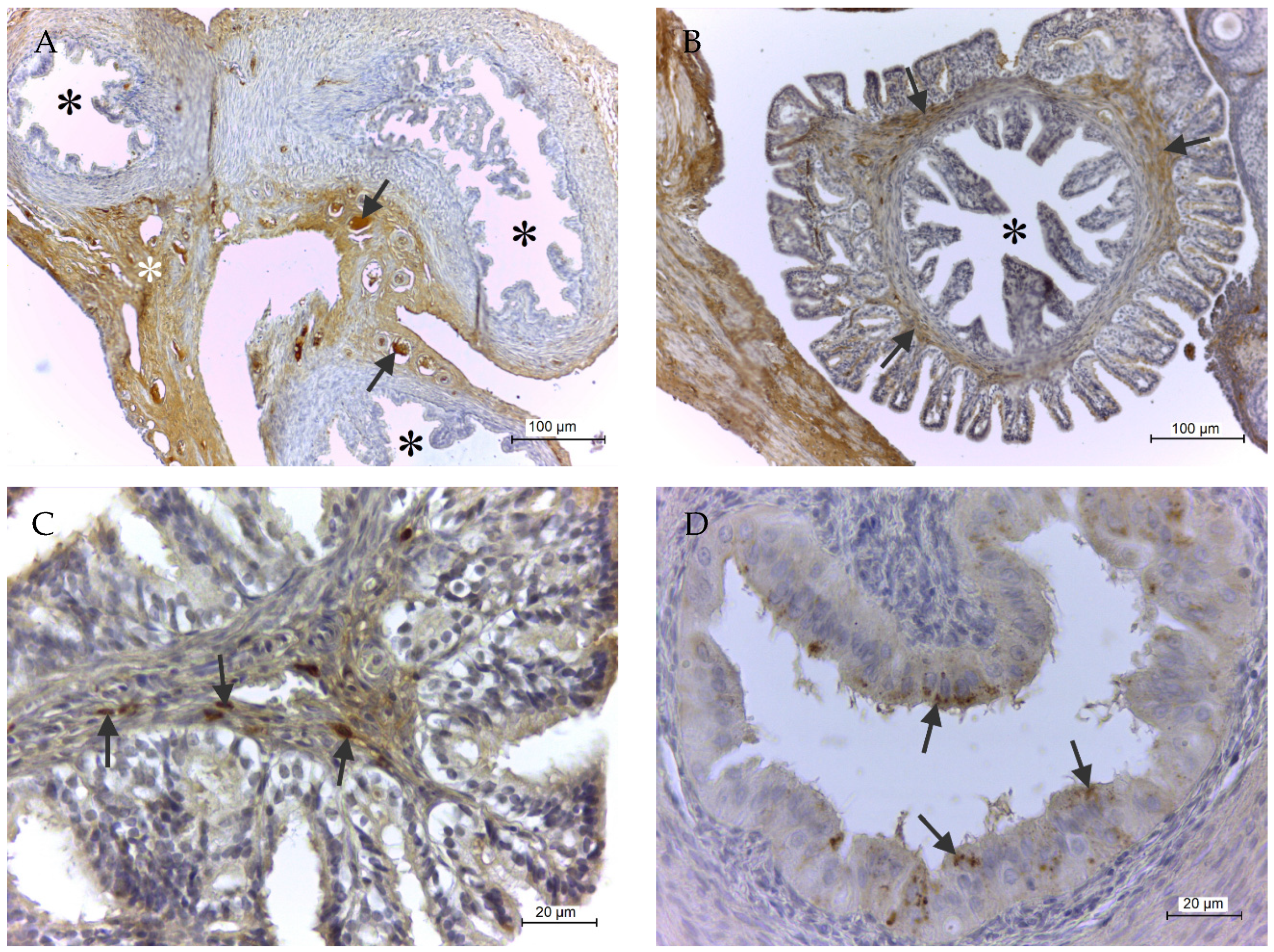
2.2. Immunohistochemical GPx8 Detection
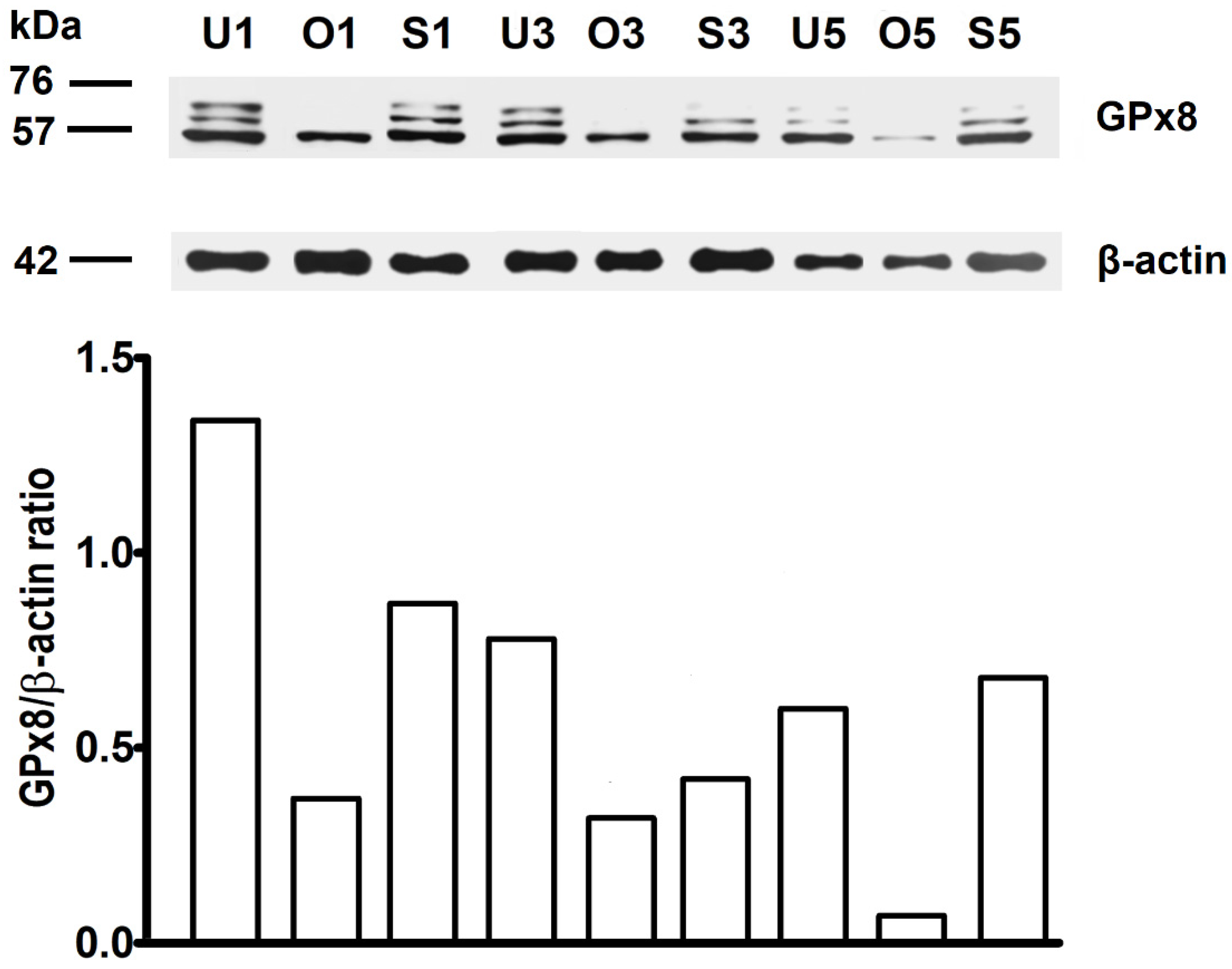
2.3. WB Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Oocytes/Embryo Isolation and GPx8 Immunofluorescence (IF) Detection
4.3. Immunohistochemistry
4.4. Western Blot (WB) Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| CAT | Catalase |
| Cys | Cysteine |
| D1–D5 | Corresponding day of pregnancy |
| ECM | Extracellular matrix |
| Ero1α | endoplasmic reticulum disulfide oxidase 1α |
| FF | Follicular fluid |
| FITC | fluorescein-5-isothiocyanate |
| GnRH | gonadotropin-releasing hormone |
| GPxs | Glutathione peroxidases |
| GPx8 | Glutathione peroxidase 8 |
| GSH | Glutathione |
| H2O2 | hydrogen peroxide |
| IF | Immunofluorescence |
| IgG | Immunoglobulin G |
| OS | Oxidative stress |
| MMPs | Matrix metalloproteinases |
| PBS | Phosphate buffer saline |
| PGF2α | Prostaglandin F2 Alpha |
| PDI | protein disulfide isomerase |
| ROOH | Hydroperoxides |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| Sec | Selenocysteine |
| SODs | Superoxide dismutases |
| TIMPs | Tissue inhibitors of metalloproteinases |
| WB | Western blot |
| ZGA | Zygotic genome activation |
References
- Rowe, P.; Comhaire, F.H.; Hargreave, T. WHO Manual for the Standardized Investigation of the Infertile Couple; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Macaluso, M.; Wright-Schnapp, T.J.; Chandra, A.; Johnson, R.; Satterwhite, C.L.; Pulver, A.; Berman, S.M.; Wang, R.Y.; Farr, S.L.; Pollack, L.A. A public health focus on infertility prevention, detection, and management. Fertil. Steril. 2010, 93, 16.e1–16.e10. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.; Bunting, I.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ghulmiyyah, J.; Sharma, R.; Halabi, J.; Agarwal, A. Power of Proteomics in Linking Oxidative Stress and Female Infertility. BioMed Res. Int. 2014, 2014, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox Considerations in Female Reproductive Function and Assisted Reproduction: From Molecular Mechanisms to Health Implications. Antioxidants Redox Signal. 2008, 10, 1375–1404. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. React. Oxyg. Species ROS Living Cells 2018. [Google Scholar] [CrossRef] [Green Version]
- Tsunoda, S.; Kimura, N.; Fujii, J. Oxidative stress and redox regulation of gametogenesis, fertilization, andembryonic development. Reprod. Med. Biol. 2013, 13, 71–79. [Google Scholar] [CrossRef]
- Toppo, S.; Flohé, L.; Ursini, F.; Vanin, S.; Maiorino, M. Catalytic mechanisms and specificities of glutathione peroxidases: Variations of a basic scheme. Biochim. Biophys. Acta BBA Gen. Subj. 2009, 1790, 1486–1500. [Google Scholar] [CrossRef]
- Utomo, A.; Jiang, X.; Furuta, S.; Yun, J.; Levin, D.S.; Wang, Y.C.J.; Desai, K.V.; Green, J.E.; Chen, P.L.; Lee, W.H. Identification of a novel putative non-selenocysteine containing phospholipid hydroperoxide glutathioneper- oxidase (NPGPx) essential for alleviating oxidative stress generated from polyunsaturated fatty acids in breast cancer cells. J. Biol. Chem. 2004, 279, 43522–43529. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.D.; Saaranen, M.J.; Karala, A.-R.; Lappi, A.-K.; Wang, L.; Raykhel, I.B.; Alanen, H.I.; Salo, K.E.; Wang, C.-C.; Ruddock, L.W. Two Endoplasmic Reticulum PDI Peroxidases Increase the Efficiency of the Use of Peroxide during Disulfide Bond Formation. J. Mol. Biol. 2011, 406, 503–515. [Google Scholar] [CrossRef]
- Rejraji, H.; Vernet, P. GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. Mol. Reprod. Dev. 2002, 63, 96–103. [Google Scholar] [CrossRef]
- Toppo, S.; Vanin, S.; Bosello, V.; Tosatto, S.C.E. Evolutionaryandstructural insights into the multifaceted glutathioneperoxidase (Gpx) superfamily. Antioxid. Redox Signal. 2008, 10, 1501–1513. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C.C. The roles of glutathione peroxidases during embryo development. Front. Mol. Neurosci. 2011, 4. [Google Scholar] [CrossRef] [Green Version]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Bosello-Travain, V.; Forman, H.J.; Roveri, A.; Toppo, S.; Ursini, F.; Venerando, R.; Warnecke, C.; Zaccarin, M.; Maiorino, M. Glutathione peroxidase 8 is transcriptionally regulated by HIFα and modulates growth factor signaling in HeLa cells. Free Radic. Biol. Med. 2015, 81, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Travain, V.B.; Miotto, G.; Vučković, A.-M.; Cozza, G.; Roveri, A.; Toppo, S.; Ursini, F.; Venerando, R.; Zaccarin, M.; Maiorino, M. Lack of glutathione peroxidase-8 in the ER impacts on lipid composition of HeLa cells microsomal membranes. Free Radic. Biol. Med. 2020, 147, 80–89. [Google Scholar] [CrossRef]
- Yoboue, E.D.; Rimessi, A.; Anelli, T.; Pinton, P.; Sitia, R. Regulation of Calcium Fluxes by GPX8, a Type-II Transmembrane Peroxidase Enriched at the Mitochondria-Associated Endoplasmic Reticulum Membrane. Antioxid. Redox Signal. 2017, 27, 583–595. [Google Scholar] [CrossRef]
- Morikawa, K.; Gouttenoire, J.; Hernandez, C.; Thi, V.L.D.D.; Tran, H.T.; Lange, C.M.; Dill, M.T.; Heim, M.H.; Donzé, O.; Penin, F.; et al. Quantitative proteomics identifies the membrane-associated peroxidase GPx8 as a cellular substrate of the hepatitis C virus NS3-4A protease. Hepatology 2013, 59, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.P.; Weissman, J.S. Oxidative protein folding in eukaryotes. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Iemura, S.-I.; Kamiya, Y.; Ron, D.; Kato, K.; Natsume, T.; Nagata, K. Ero1-α and PDIs constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases. J. Cell Biol. 2013, 202, 861–874. [Google Scholar] [CrossRef]
- Ramming, T.; Hansen, H.G.; Nagata, K.; Ellgaard, L.; Appenzeller-Herzog, C. GPx8 peroxidase prevents leakage of H2O2 from the endoplasmic reticulum. Free Radic. Biol. Med. 2014, 70, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Tatsumi, T. Degradation of maternal factors during preimplantation embryonic development. J. Reprod. Dev. 2018, 64, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J. Zygotic Genome Activation in Vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F. Dual Function of the Selenoprotein PHGPx During Sperm Maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [Green Version]
- Olson, G.E.; Whitin, J.C.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Austin, L.M.; Deal, J.; Cohen, H.J.; Burk, R.F. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am. J. Physiol. Physiol. 2010, 298, F1244–F1253. [Google Scholar] [CrossRef] [Green Version]
- Burk, R.F.; Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Yin, D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. Physiol. Liver Physiol. 2011, 301, G32–G38. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J. Gpx5 protects the family jewels. J. Clin. Investig. 2009, 119, 1849–1851. [Google Scholar] [CrossRef]
- De Oliveira, P.V.S.; Garcia-Rosa, S.; Sachetto, A.T.A.; Moretti, A.I.S.; Debbas, V.; De Bessa, T.C.; Silva, N.T.; Pereira, A.D.C.; Martins-De-Souza, D.; Santoro, M.L.; et al. Protein disulfide isomerase plasma levels in healthy humans reveal proteomic signatures involved in contrasting endothelial phenotypes. Redox Boil. 2019, 22, 101142. [Google Scholar] [CrossRef]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [Green Version]
- Bagulho, A.; Vilas-Boas, F.; Pena, A.; Peneda, C.; Santos, F.C.; Jerónimo, A.; De Almeida, R.F.M.; Real, C. The extracellular matrix modulates H2O2 degradation and redox signaling in endothelial cells. Redox Boil. 2015, 6, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J Cell Sci. 2002, 115, 2817–2828. [Google Scholar] [PubMed]
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 2009, 2, 198–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, P.; Malik, A.; Malhotra, S.S.; Gupta, S.K. Role of STAT signaling and autocrine action of chemokines during H2 O 2 induced HTR-8/SVneo trophoblastic cells invasion. J. Cell. Physiol. 2018, 234, 1380–1397. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Barker, G.; Menon, R.; Lappas, M. Increased oxidative stress in human fetal membranes overlying the cervix from term non-labouring and post labour deliveries. Placenta 2012, 33, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Solar, P.; Juica, N.; Orihuela, P.A.; Cardenas, H.; Christodoulides, M.; Vargas, R.; Velásquez, L. Differential expression of extracellular matrix components in the Fallopian tubes throughout the menstrual cycle. Reprod. Biol. Endocrinol. 2012, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Juica, N.E.; Rodas, P.I.; Solar, P.; Borda, P.; Vargas, R.; Muñoz, C.; Paredes, R.; Christodoulides, M.; Velasquez, L. Neisseria gonorrhoeae Challenge Increases Matrix Metalloproteinase-8 Expression in Fallopian Tube Explants. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Noguchi, Y.; Sato, T.; Hirata, M.; Hara, T.; Ohama, K.; Ito, A. Identification and Characterization of Extracellular Matrix Metalloproteinase Inducer in Human Endometrium during the Menstrual Cyclein Vivoandin Vitro. J. Clin. Endocrinol. Metab. 2003, 88, 6063–6072. [Google Scholar] [CrossRef] [Green Version]
- Nazari, A.; Dirandeh, E.; Ansari-Pirsaraei, Z.; Deldar, H. Antioxidant levels, copper and zinc concentrations were associated with postpartum luteal activity, pregnancy loss and pregnancy status in Holstein dairy cows. Theriogenology 2019, 133, 97–103. [Google Scholar] [CrossRef]
- Riley, J.C.M.; Behrman, H.R. In Vivo Generation of Hydrogen Peroxide in the Rat Corpus Luteum during Luteolysis. Endocrinology 1991, 128, 1749–1753. [Google Scholar] [CrossRef]
- Behrman, H.R.; Preston, S.L. Luteolytic Actions of Peroxide in Rat Ovarian Cells. Endocrinology 1989, 124, 2895–2900. [Google Scholar] [CrossRef]
- Sugino, N. Roles of reactive oxygen species in the corpus luteum. Anim. Sci. J. 2006, 77, 556–565. [Google Scholar] [CrossRef]
- Dharmarajan, A.M.; Bruce, N.W.; Waddell, B.J. Quantitative changes in steroidogenic organelles in the corpus luteum of the pregnant rat in relation to progestin secretion on day 16 and in the morning and afternoon of day 22. Am. J. Anat. 1991, 190, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, R.; Sklan, D.; Wolfenson, D.; Shaham-Albalancy, A.; Hanukoglu, I. Antioxidant capacity is correlated with steroidogenic status of the corpus luteum during the bovine estrous cycle. Biochim. Biophys. Acta BBA Gen. Subj. 1998, 1380, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elizur, S.E.; Lebovitz, O.; Orvieto, R.; Dor, J.; Zan-Bar, T. Reactive oxygen species in follicular fluid may serve as biochemical markers to determine ovarian aging and follicular metabolic age. Gynecol. Endocrinol. 2014, 30, 705–707. [Google Scholar] [CrossRef]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.M.; Aitken, J.B.; Russell, D.L.; Lane, M.; Rodgers, R.; Harris, H.H. X-Ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics 2015, 7, 71–82. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Updat. 2005, 12, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Huang, V.W.; Zhao, W.; Lee, K.-F.; Lee, C.Y.; Lam, K.K.; Ko, J.K.; Yeung, W.S.B.; Ho, P.-C.; Chiu, P.C.N. Cell membrane proteins from oviductal epithelial cell line protect human spermatozoa from oxidative damage. Fertil. Steril. 2013, 99, 1444–1452. [Google Scholar] [CrossRef]
- El Mouatassim, S.; Guerin, P.; Menezo, Y. Mammalian oviduct and protection against free oxygen radicals: Expression of genes encoding antioxidant enzymes in human and mouse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 89, 1–6. [Google Scholar] [CrossRef]
- Lapointe, J.; Bilodeau, J.-F. Antioxidant Defenses Are Modulated in the Cow Oviduct During the Estrous Cycle1. Biol. Reprod. 2003, 68, 1157–1164. [Google Scholar] [CrossRef]
- Lapointe, J.; Kimmins, S.; MacLaren, L.A.; Bilodeau, J.-F. Estrogen Selectively Up-Regulates the Phospholipid Hydroperoxide Glutathione Peroxidase in the Oviducts. Endocrinology 2005, 146, 2583–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, P.N.; Alvarez, J.G.; Risopatrón, J.; Romero, F.; Sánchez, R. Effect of tubal explants and their secretions on bovine spermatozoa: Modulation of ROS production and DNA damage. Reprod. Fertil. Dev. 2012, 24, 871. [Google Scholar] [CrossRef] [PubMed]
- Maillo, V.; Sánchez-Calabuig, M.J.; Lopera-Vasquez, R.; Hamdi, M.; Gutiérrez-Adán, A.; Lonergan, P.; Rizos, D. Oviductal response to gametes and early embryos in mammals. Reproduction 2016, 152, R127–R141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelleher, A.M.; DeMayo, F.J.; Spencer, T.E. Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev. 2019, 40, 1424–1445. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N. The rat as an animal model for fetoplacental development: A reappraisal of the post-implantation period. Reprod. Biol. 2012, 12, 97–118. [Google Scholar] [CrossRef]
- Mori, M.; Bogdan, A.; Balassa, T.; Csabai, T.; Szekeres-Bartho, J. The decidua—The maternal bed embracing the embryo—Maintains the pregnancy. Semin. Immunopathol. 2016, 38, 635–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.-H.; Chen, P.-C.; Chen, C.-H.; Hsu, C.-F.; Huang, R.-L.; Ding, D.-C.; Chu, T.-Y. Platelet-Derived Growth Factor in the Ovarian Follicle Attracts the Stromal Cells of the Fallopian Tube Fimbriae. PLoS ONE 2016, 11, e0158266. [Google Scholar] [CrossRef] [Green Version]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Walter, I. Myofibroblasts in the mucosal layer of the uterine tube. Ital. J. Anat. Embryol. Arch. Ital. Anat. Embriol. 1998, 103, 259. [Google Scholar]
- Oberley, T.D.; Oberley, L.W.; Slattery, A.F.; Elwell, J.H. Immunohistochemical localization of glutathione-S-transferase and glutathione peroxidase in adult Syrian hamster tissues and during kidney development. Am. J. Pathol. 1991, 139, 355–369. [Google Scholar]
- American Psychological Association (APA). Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research; APA Council of Representatives, Office of Research Ethics: Washington, DC, USA, 2012. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihalik, J.; Kreheľová, A.; Kovaříková, V.; Solár, P.; Domoráková, I.; Pavliuk-Karachevtseva, A.; Hladová, A.; Rybárová, S.; Hodorová, I. GPx8 Expression in Rat Oocytes, Embryos, and Female Genital Organs During Preimplantation Period of Pregnancy. Int. J. Mol. Sci. 2020, 21, 6313. https://doi.org/10.3390/ijms21176313
Mihalik J, Kreheľová A, Kovaříková V, Solár P, Domoráková I, Pavliuk-Karachevtseva A, Hladová A, Rybárová S, Hodorová I. GPx8 Expression in Rat Oocytes, Embryos, and Female Genital Organs During Preimplantation Period of Pregnancy. International Journal of Molecular Sciences. 2020; 21(17):6313. https://doi.org/10.3390/ijms21176313
Chicago/Turabian StyleMihalik, Jozef, Andrea Kreheľová, Veronika Kovaříková, Peter Solár, Iveta Domoráková, Andriana Pavliuk-Karachevtseva, Alena Hladová, Silvia Rybárová, and Ingrid Hodorová. 2020. "GPx8 Expression in Rat Oocytes, Embryos, and Female Genital Organs During Preimplantation Period of Pregnancy" International Journal of Molecular Sciences 21, no. 17: 6313. https://doi.org/10.3390/ijms21176313





