Identification of CD34+/PGDFRα+ Valve Interstitial Cells (VICs) in Human Aortic Valves: Association of Their Abundance, Morphology and Spatial Organization with Early Calcific Remodeling
Abstract
1. Introduction
2. Results
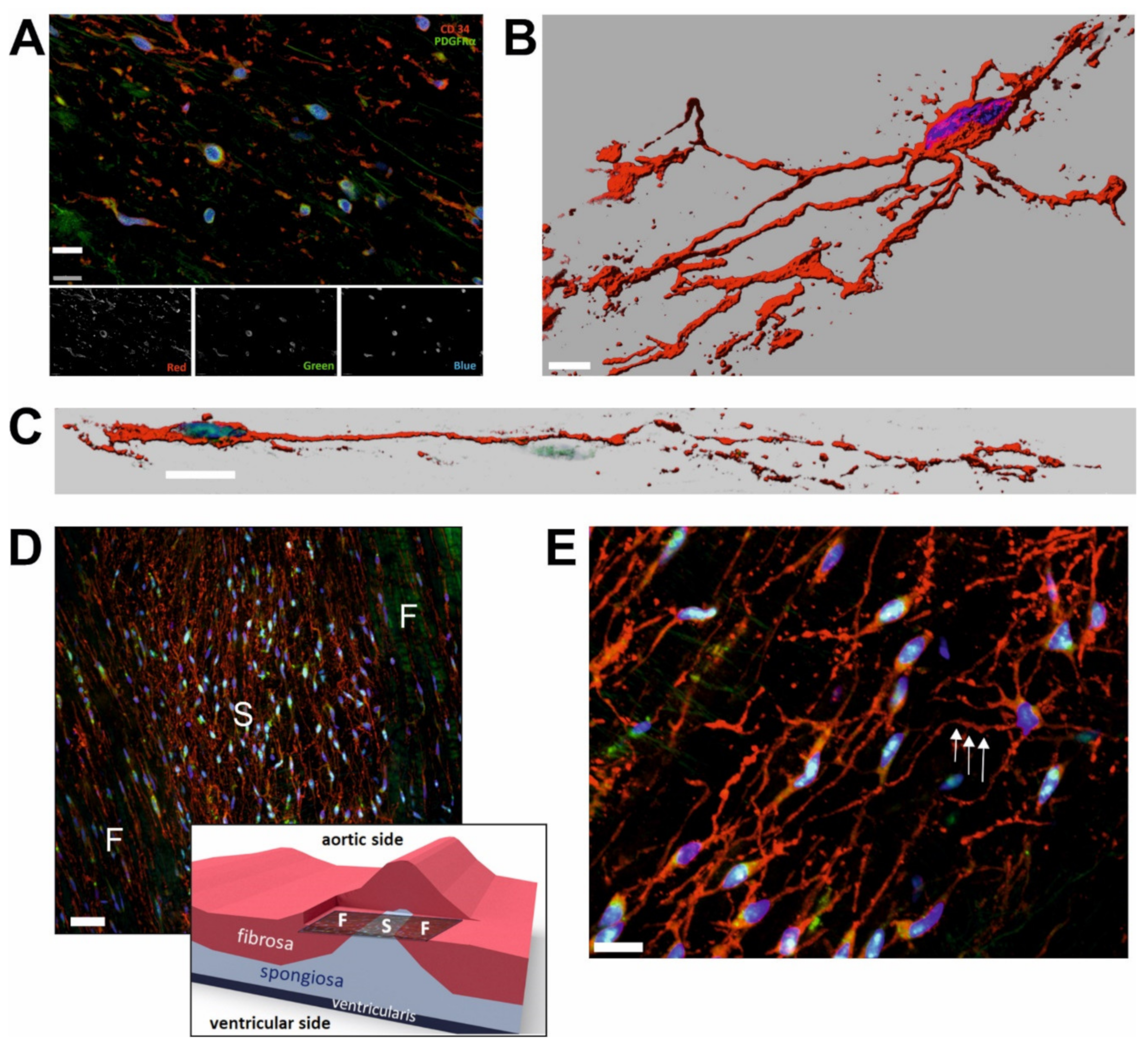
2.1. CD34+/PDGFRα+ Cells Constitute Substantial Subpopulation of VICs
2.2. Morphology and Spatial Organization of CD34+/PDGFRα+ Cells Is Regionally Different
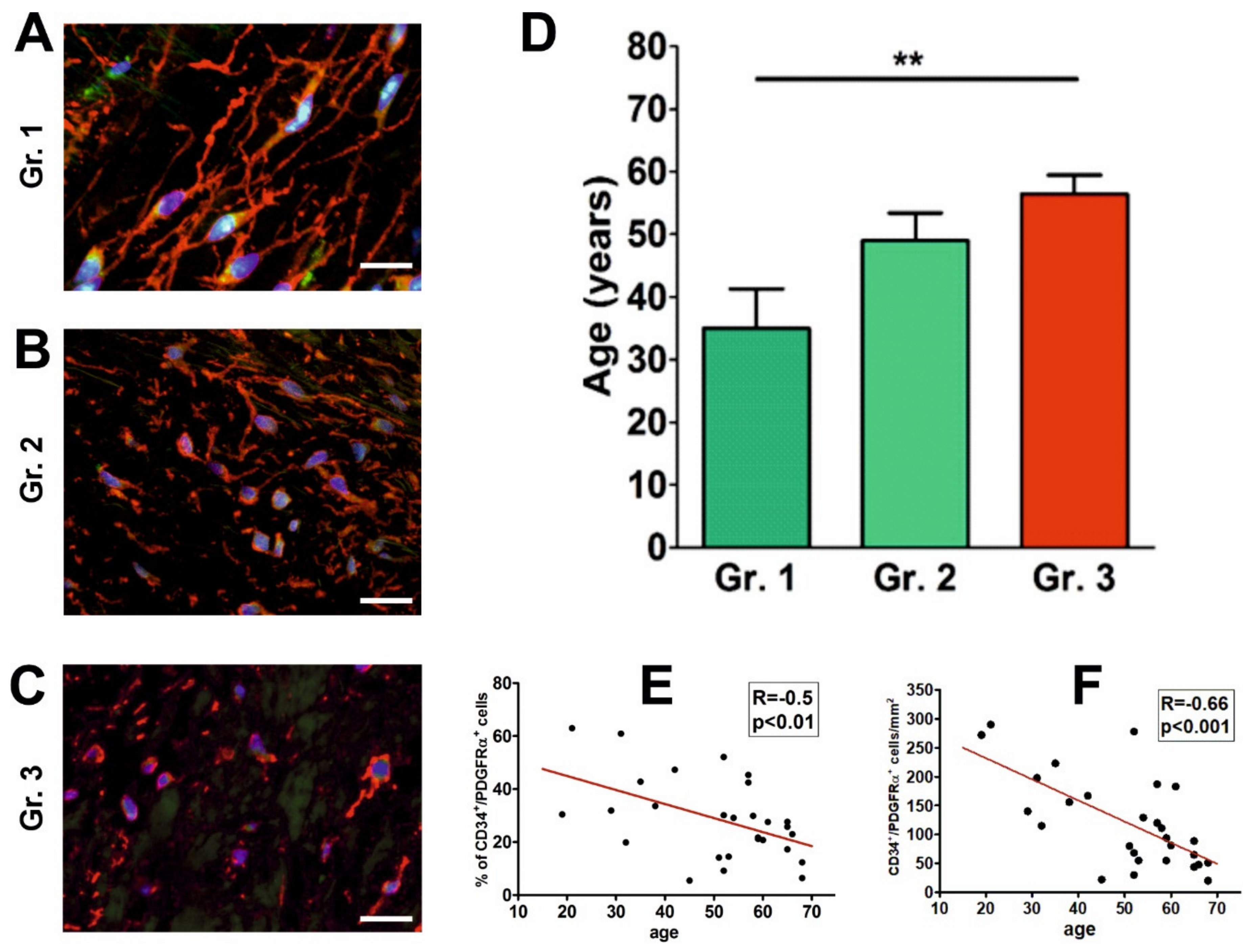
2.3. The Abundance and Morphology of CD34+/PDGFRα+ Cells Are Associated with Age
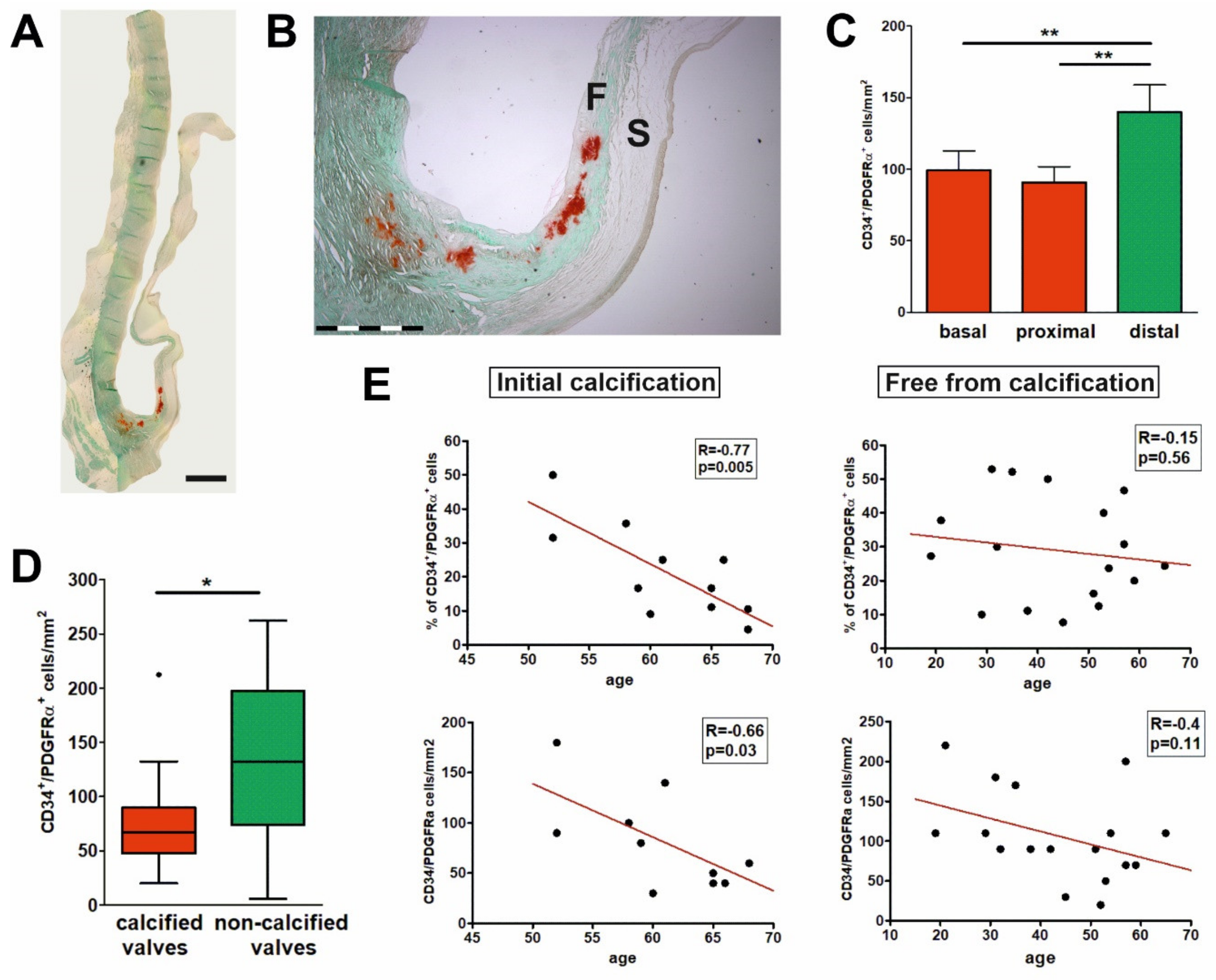
2.4. Early Calcification Is Associated with Decrease in the Number and Proportion of CD34+/PDGFRα+ Cells
3. Discussion
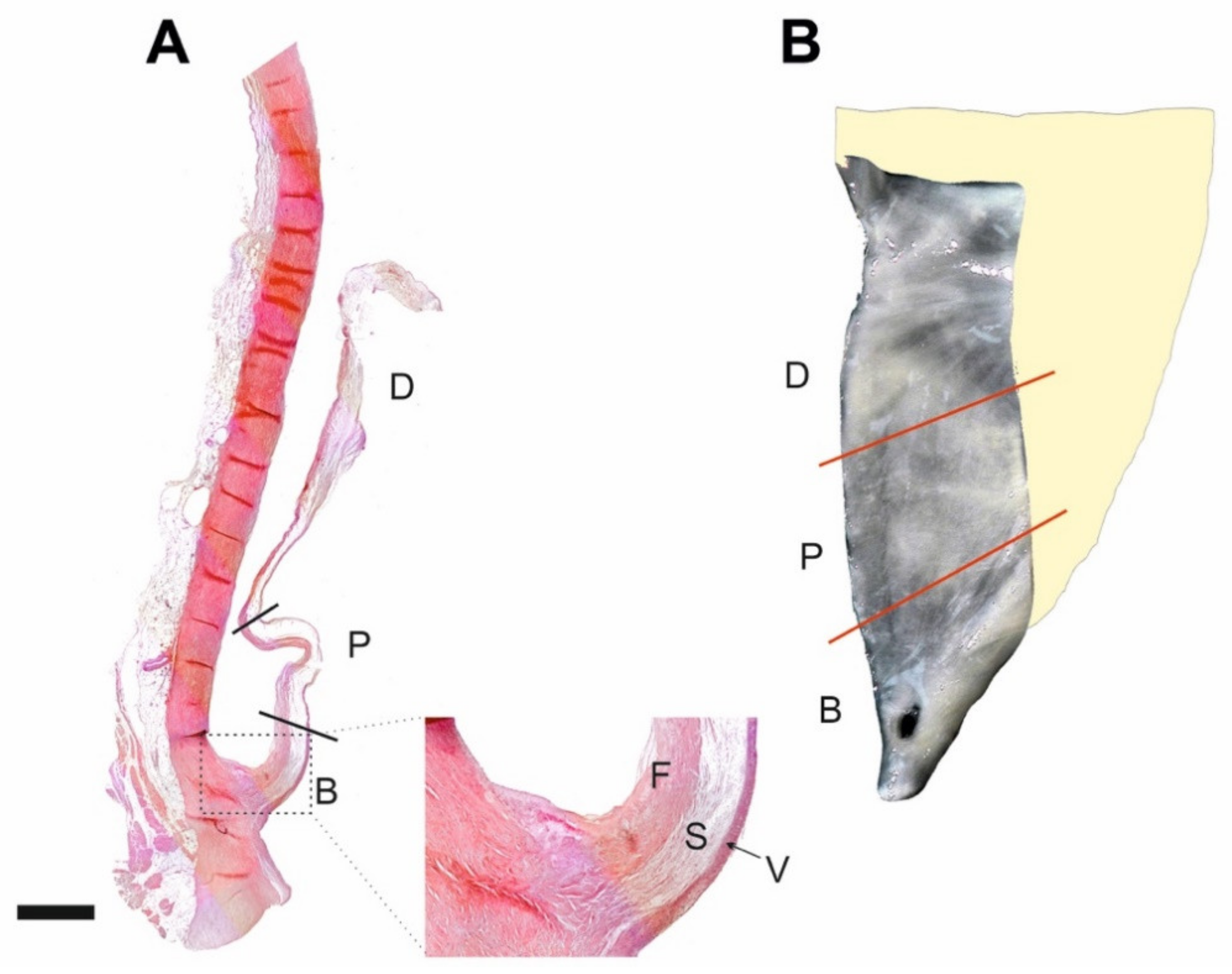
4. Materials and Methods
4.1. Subjects
4.2. Ethical Approval
4.3. Tissue Processing
4.4. Immunofluorescence
4.5. Microscopic Examination
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Brien, K.D. Pathogenesis of calcific aortic valve disease: A disease process comes of age (and a good deal more). Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.R., 3rd; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef]
- Lis, G.J.; Czapla-Masztafiak, J.; Kwiatek, W.M.; Gajda, M.; Jasek, E.; Jasinska, M.; Czubek, U.; Borchert, M.; Appel, K.; Nessler, J.; et al. Distribution of selected elements in calcific human aortic valves studied by microscopy combined with SR-muXRF: Influence of lipids on progression of calcification. Micron 2014, 67, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lis, G.J.; Czubek, U.; Jasek-Gajda, E.; Łoboda, A.; Dulak, J.; Nessler, J.; Kapelak, B.; Sadowski, J.; Litwin, J.A. Influence of osteoclasts and osteoprotegerin on the mode of calcific degeneration of aortic valves. Pol. Arch. Intern. Med. 2016, 126, 149–158. [Google Scholar] [CrossRef][Green Version]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis, histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef]
- Misfeld, M.; Sievers, H.H. Heart valve macro- and microstructure. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1421–1436. [Google Scholar] [CrossRef]
- Lerman, D.A.; Prasad, S.; Alotti, N. Calcific Aortic Valve Disease: Molecular Mechanisms and Therapeutic Approaches. Eur. Cardiol. 2015, 10, 108–112. [Google Scholar] [CrossRef]
- Leopold, J.A. Cellular mechanisms of aortic valve calcification. Circ. Cardiovasc. Interv. 2012, 5, 605–614. [Google Scholar] [CrossRef]
- Niaz, T.; Hagler, D.J. Is there a genetic basis to the different morphological subtypes of bicuspid aortic valve? Ann. Transl. Med. 2018, 6, S117. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.O.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Curici, A.; Wang, E.; Zhang, H.; Hu, S.; Gherghiceanu, M. Telocytes and putative stem cells in ageing human heart. J. Cell Mol. Med. 2015, 19, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.P.; Costabel, U. Cell number in human heart in atrophy, hypertrophy, and under the influence of cytostatics. Recent Adv. Stud. Cardiac Struct. Metab. 1975, 6, 343–355. [Google Scholar] [PubMed]
- Weber, A.; Barth, M.; Selig, J.I.; Raschke, S.; Dakaras, K.; Hof, A.; Hesse, J.; Schrader, J.; Lichtenberg, A.; Akhyari, P. Enzymes of the purinergic signaling system exhibit diverse effects on the degeneration of valvular interstitial cells in a 3-D microenvironment. FASEB J. 2018, 32, 4356–4369. [Google Scholar] [CrossRef]
- Li, C.; Xu, S.; Gotlieb, A.I. The progression of calcific aortic valve disease through injury, cell dysfunction, and disruptive biologic and physical force feedback loops. Cardiovasc. Pathol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Liu, A.C.; Joag, V.R.; Gotlieb, A.I. The Emerging Role of Valve Interstitial Cell Phenotypes in Regulating Heart Valve Pathobiology. Am. J. Pathol. 2007, 171, 1407–1418. [Google Scholar] [CrossRef]
- Taylor, P.M.; Batten, P.; Brand, N.J.; Thomas, P.; Yacoub, M.H. The cardiac valve interstitial cell. Int. J. Biochem. Cell Biol. 2003, 35, 113–118. [Google Scholar] [CrossRef]
- Wirrig, E.E.; Yutzey, K.E. Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 737–741. [Google Scholar] [CrossRef]
- Rattazzi, M.; Donato, M.; Bertacco, E.; Millioni, R.; Franchin, C.; Mortarino, C.; Faggin, E.; Nardin, C.; Scarpa, R.; Cinetto, F.; et al. l-Arginine prevents inflammatory and pro-calcific differentiation of interstitial aortic valve cells. Atherosclerosis 2020, 298, 27–35. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J.H. Involvement of Immune Cell Network in Aortic Valve Stenosis: Communication between Valvular Interstitial Cells and Immune Cells. Immune Netw. 2016, 16, 26–32. [Google Scholar] [CrossRef]
- Martin, P.S.; Kloesel, B.; Norris, R.A.; Lindsay, M.; Milan, D.; Body, S.C. Embryonic Development of the Bicuspid Aortic Valve. J. Cardiovasc. Dev. Dis. 2015, 2, 248–272. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, S.; Amarnath, A.; Baily, K.M.; Ferdous, Z. Comparison of calcification potential of valvular interstitial cells isolated from individual aortic valve cusps. Cardiovasc. Pathol. 2016, 25, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Torzewski, M. Fibroblasts and Their Pathological Functions in the Fibrosis of Aortic Valve Sclerosis and Atherosclerosis. Biomolecules 2019, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Combs, M.D.; Yutzey, K.E. Heart valve development: Regulatory networks in development and disease. Circ. Res. 2009, 105, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Radu, B.M.; Banciu, A.; Banciu, D.D.; Radu, M.; Cretoiu, D.; Cretoiu, S.M. Calcium Signaling in Interstitial Cells: Focus on Telocytes. Int. J. Mol. Sci. 2017, 18, 397. [Google Scholar] [CrossRef]
- Kostin, S.; Popescu, L.M. A distinct type of cell in myocardium: Interstitial Cajal-like cells (ICLCs). J. Cell Mol. Med. 2009, 13, 295–308. [Google Scholar] [CrossRef]
- Kostin, S. Myocardial telocytes: A specific new cellular entity. J. Cell Mol. Med. 2010, 14, 1917–1921. [Google Scholar] [CrossRef]
- Kostin, S. Cardiac telocytes in normal and diseased hearts. Semin. Cell Dev. Biol. 2016, 55, 22–30. [Google Scholar] [CrossRef]
- Aleksandrovych, V.; Pasternak, A.; Basta, P.; Sajewicz, M.; Walocha, J.A.; Gil, K. Telocytes: Facts, speculations and myths (Review article). Folia Med. Cracov. 2017, 57, 5–22. [Google Scholar]
- Suciu, L.; Nicolescu, M.I.; Popescu, L.M. Cardiac telocytes: Serial dynamic images in cell culture. J. Cell Mol. Med. 2010, 14, 2687–2692. [Google Scholar] [CrossRef]
- Marini, M.; Ibba-Manneschi, L.; Manetti, M. Cardiac Telocyte-Derived Exosomes and Their Possible Implications in Cardiovascular Pathophysiology. Adv. Exp. Med. Biol. 2017, 998, 237–254. [Google Scholar] [PubMed]
- Bani, D.; Formigli, L.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J. Cell Mol. Med. 2010, 14, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S.; Bani, D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J. Cell Mol. Med. 2010, 14, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wei, L.; Zhong, C.; Fu, S.; Bei, Y.; Huică, R.I.; Wang, F.; Xiao, J. Cardiac telocytes are double positive for CD34/PDGFR-α. J. Cell Mol. Med. 2015, 19, 2036–2042. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, W.; Wu, S.M.; Xiao, J.; Kong, X. Telocytes in human heart valves. J. Cell Mol. Med. 2014, 18, 759–765. [Google Scholar] [CrossRef]
- Chang, Y.; Li, C.; Lu, Z.; Li, H.; Guo, Z. Multiple immunophenotypes of cardiac telocytes. Exp. Cell Res. 2015, 338, 239–244. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; Díaz-Flores, L., Jr.; Gonzalez Goméz, M.; Sáez, F.J.; Madrid, J.F. Behaviour of telocytes during physiopathological activation. Semin. Cell Dev. Biol. 2016, 55, 50–61. [Google Scholar] [CrossRef]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts. 2014, 5, 353–369. [Google Scholar] [CrossRef]
- Matyja, A.; Gil, K.; Pasternak, A.; Sztefko, K.; Gajda, M.; Tomaszewski, K.A.; Matyja, M.; Walocha, J.A.; Kulig, J.; Thor, P. Telocytes: New insight into the pathogenesis of gallstone disease. J. Cell Mol. Med. 2013, 17, 734–742. [Google Scholar] [CrossRef]
- Aleksandrovych, V.; Walocha, J.A.; Gil, K. Telocytes in female reproductive system (human and animal). J. Cell Mol. Med. 2016, 20, 994–1000. [Google Scholar] [CrossRef]
- Hutson, H.N.; Marohl, T.; Anderson, M.; Eliceiri, K.; Campagnola, P.; Masters, K.S. Calcific Aortic Valve Disease Is Associated with Layer-Specific Alterations in Collagen Architecture. PLoS ONE 2016, 11, e0163858. [Google Scholar] [CrossRef] [PubMed]
- Latif, N.; Sarathchandra, P.; Taylor, P.M.; Antoniw, J.; Yacoub, M.H. Localization and pattern of expression of extracellular matrix components in human heart valves. J. Heart Valve Dis. 2005, 14, 218–227. [Google Scholar] [PubMed]
- Vesely, I. Reconstruction of loads in the fibrosa and ventricularis of porcine aortic valves. ASAIO J. 1996, 42, M739–M746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blevins, T.L.; Peterson, S.B.; Lee, E.L.; Bailey, A.M.; Frederick, J.D.; Huynh, T.N.; Gupta, V.; Grande-Allen, K.J. Mitral valve interstitial cells demonstrate regional, adhesional, and synthetic heterogeneity. Cells Tissues Organs 2008, 187, 113–122. [Google Scholar] [CrossRef]
- Simmons, C.A.; Grant, G.R.; Mandachi, E.; Davies, P.F. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ. Res. 2005, 96, 792–799. [Google Scholar] [CrossRef]
- Freeman, R.V.; Otto, C.M. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation 2005, 111, 3316–3326. [Google Scholar] [CrossRef]
- Aikawa, E.; Whittaker, P.; Farber, M.; Mendelson, K.; Padera, R.F.; Aikawa, M.; Schoen, F.J. Human semilunar cardiac valve remodeling by activated cells from fetus to adult. Circulation 2006, 113, 1344–1352. [Google Scholar] [CrossRef]
- Christie, G.W.; Barratt-Boyes, B.G. Age-dependent changes in the radial stretch of human aortic valve leaflets determined by biaxial testing. Ann. Thorac Surg. 1995, 60, S156–S158. [Google Scholar] [CrossRef]
- Sell, S.; Scully, R.E. Aging changes in the aortic and mitral valves. Histologic and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am. J. Pathol. 1965, 46, 345–365. [Google Scholar] [PubMed]
- Jian, B.; Narula, N.; Li, Q.Y.; Mohler, E.R., 3rd; Levy, R.J. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 2003, 75, 457–465. [Google Scholar] [CrossRef]
- Bonetti, A.; Della Mora, A.; Contin, M.; Gregoraci, G.; Tubaro, F.; Marchini, M.; Ortolani, F. Survival-Related Autophagic Activity Versus Procalcific Death in Cultured Aortic Valve Interstitial Cells Treated With Critical Normophosphatemic-Like Phosphate Concentrations. J. Histochem. Cytochem. 2017, 65, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, F.; Bonetti, A.; Tubaro, F.; Petrelli, L.; Contin, M.; Nori, S.L.; Spina, M.; Marchini, M. Ultrastructural characterization of calcification onset and progression in subdermally implanted aortic valves. Histochemical and spectrometric data. Histol Histopathol. 2007, 22, 261–272. [Google Scholar] [PubMed]
- Richter, M.; Kostin, S. The failing human heart is characterized by decreased numbers of telocytes as result of apoptosis and altered extracellular matrix composition. J. Cell Mol. Med. 2015, 19, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Niculite, C.M.; Regalia, T.M.; Gherghiceanu, M.; Huica, R.; Surcel, M.; Ursaciuc, C.; Leabu, M.; Popescu, L.M. Dynamics of telopodes (telocyte prolongations) in cell culture depends on extracellular matrix protein. Mol. Cell Biochem. 2015, 398, 157–164. [Google Scholar] [CrossRef]
- Cretoiu, D.; Hummel, E.; Zimmermann, H.; Gherghiceanu, M.; Popescu, L.M. Human cardiac telocytes: 3D imaging by FIB-SEM tomography. J. Cell Mol. Med. 2014, 18, 2157–2164. [Google Scholar] [CrossRef]
- Nomura, A.; Seya, K.; Yu, Z.; Daitoku, K.; Motomura, S.; Murakami, M.; Fukuda, I.; Furukawa, K. CD34-negative mesenchymal stem-like cells may act as the cellular origin of human aortic valve calcification. Biochem. Biophys. Res. Commun. 2013, 440, 780–785. [Google Scholar] [CrossRef]
- Hjortnaes, J.; Shapero, K.; Goettsch, C.; Hutcheson, J.D.; Keegan, J.; Kluin, J.; Mayer, J.E.; Bischoff, J.; Aikawa, E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis 2015, 242, 251–260. [Google Scholar] [CrossRef]
- Schoen, F.J. Evolving concepts of cardiac valve dynamics: The continuum of development, functional structure, pathobiology, and tissue engineering. Circulation 2008, 118, 1864–1880. [Google Scholar] [CrossRef]
- Paruchuri, S.; Yang, J.H.; Aikawa, E.; Melero-Martin, J.M.; Khan, Z.A.; Loukogeorgakis, S.; Schoen, F.J.; Bischoff, J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ. Res. 2006, 99, 861–869. [Google Scholar] [CrossRef]
- Biasin, V.; Crnkovic, S.; Sahu-Osen, A.; Birnhuber, A.; El Agha, E.; Sinn, K.; Klepetko, W.; Olschewski, A.; Bellusci, S.; Marsh, L.M.; et al. PDGFRα and αSMA mark two distinct mesenchymal cell populations involved in parenchymal and vascular remodeling in pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L684–L697. [Google Scholar] [CrossRef]
- Santini, M.P.; Malide, D.; Hoffman, G.; Pandey, G.; D’Escamard, V.; Nomura-Kitabayashi, A.; Rovira, I.; Kataoka, H.; Ochando, J.; Harvey, R.P.; et al. Tissue-Resident PDGFRα+ Progenitor Cells Contribute to Fibrosis versus Healing in a Context- and Spatiotemporally Dependent Manner. Cell Rep. 2020, 30, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cretoiu, D.; Yan, G.; Cretoiu, S.M.; Popescu, L.M.; Fang, H.; Wang, X. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J. Cell Mol. Med. 2014, 18, 1035–1059. [Google Scholar] [CrossRef]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Bennett, M.R.; Shanahan, C.M.; Weissberg, P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000, 87, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, G.J.; Dubrowski, A.; Lis, M.; Solewski, B.; Witkowska, K.; Aleksandrovych, V.; Jasek-Gajda, E.; Hołda, M.K.; Gil, K.; Litwin, J.A. Identification of CD34+/PGDFRα+ Valve Interstitial Cells (VICs) in Human Aortic Valves: Association of Their Abundance, Morphology and Spatial Organization with Early Calcific Remodeling. Int. J. Mol. Sci. 2020, 21, 6330. https://doi.org/10.3390/ijms21176330
Lis GJ, Dubrowski A, Lis M, Solewski B, Witkowska K, Aleksandrovych V, Jasek-Gajda E, Hołda MK, Gil K, Litwin JA. Identification of CD34+/PGDFRα+ Valve Interstitial Cells (VICs) in Human Aortic Valves: Association of Their Abundance, Morphology and Spatial Organization with Early Calcific Remodeling. International Journal of Molecular Sciences. 2020; 21(17):6330. https://doi.org/10.3390/ijms21176330
Chicago/Turabian StyleLis, Grzegorz J., Andrzej Dubrowski, Maciej Lis, Bernard Solewski, Karolina Witkowska, Veronika Aleksandrovych, Ewa Jasek-Gajda, Mateusz K. Hołda, Krzysztof Gil, and Jan A. Litwin. 2020. "Identification of CD34+/PGDFRα+ Valve Interstitial Cells (VICs) in Human Aortic Valves: Association of Their Abundance, Morphology and Spatial Organization with Early Calcific Remodeling" International Journal of Molecular Sciences 21, no. 17: 6330. https://doi.org/10.3390/ijms21176330
APA StyleLis, G. J., Dubrowski, A., Lis, M., Solewski, B., Witkowska, K., Aleksandrovych, V., Jasek-Gajda, E., Hołda, M. K., Gil, K., & Litwin, J. A. (2020). Identification of CD34+/PGDFRα+ Valve Interstitial Cells (VICs) in Human Aortic Valves: Association of Their Abundance, Morphology and Spatial Organization with Early Calcific Remodeling. International Journal of Molecular Sciences, 21(17), 6330. https://doi.org/10.3390/ijms21176330




