Levels of Interleukin-6 in Saliva, but Not Plasma, Correlate with Clinical Metrics in Huntington’s Disease Patients and Healthy Control Subjects
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
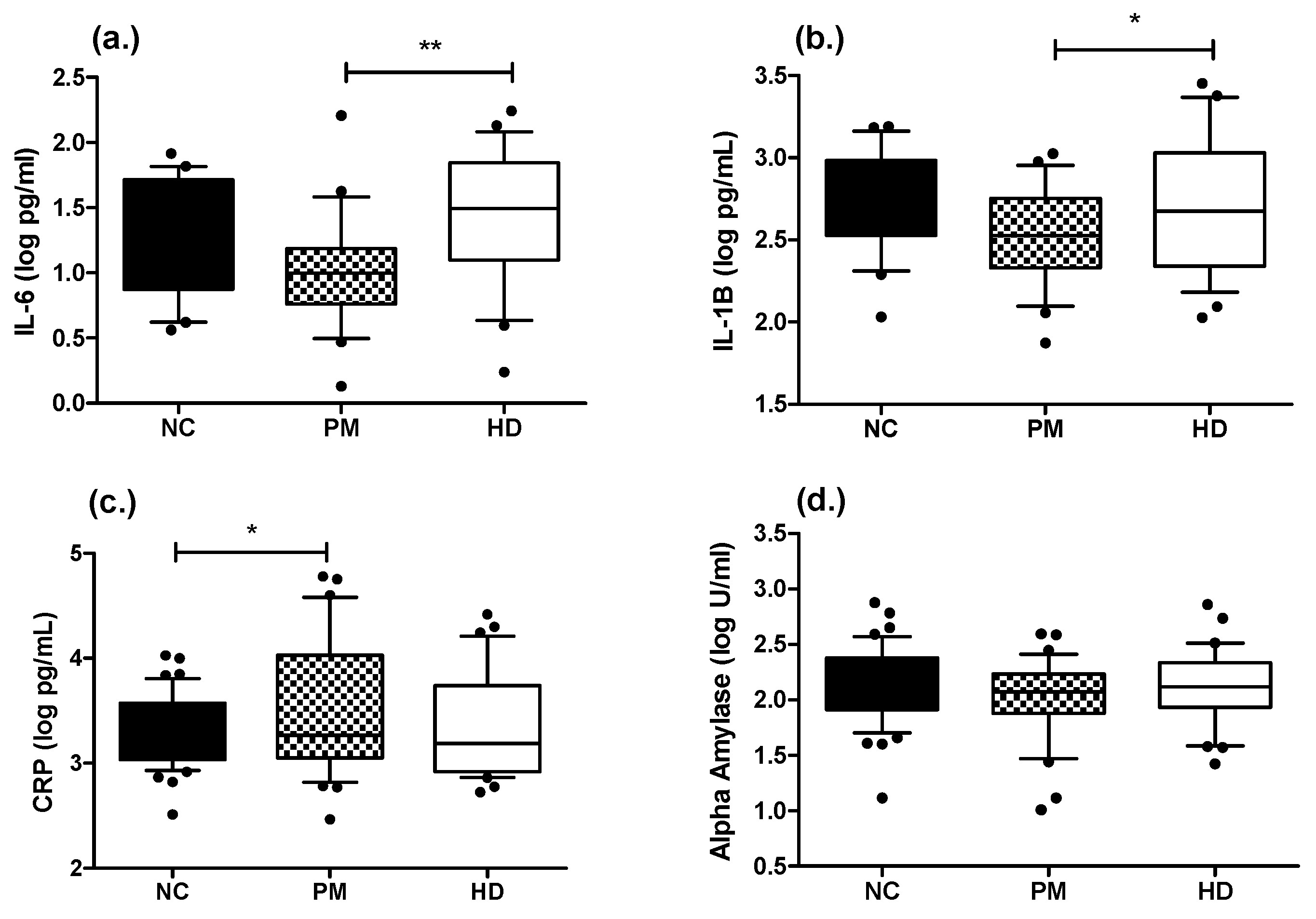
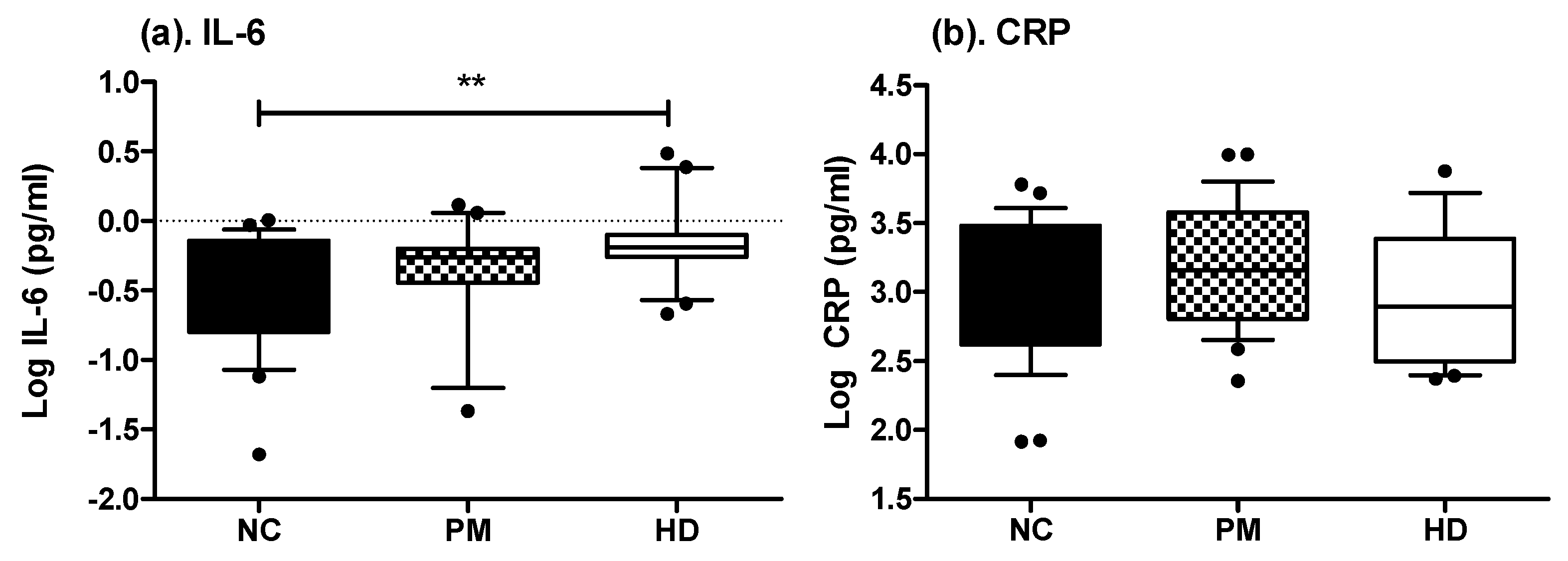
2.2. Measures of Inflammatory-Related Markers in Saliva and Plasma
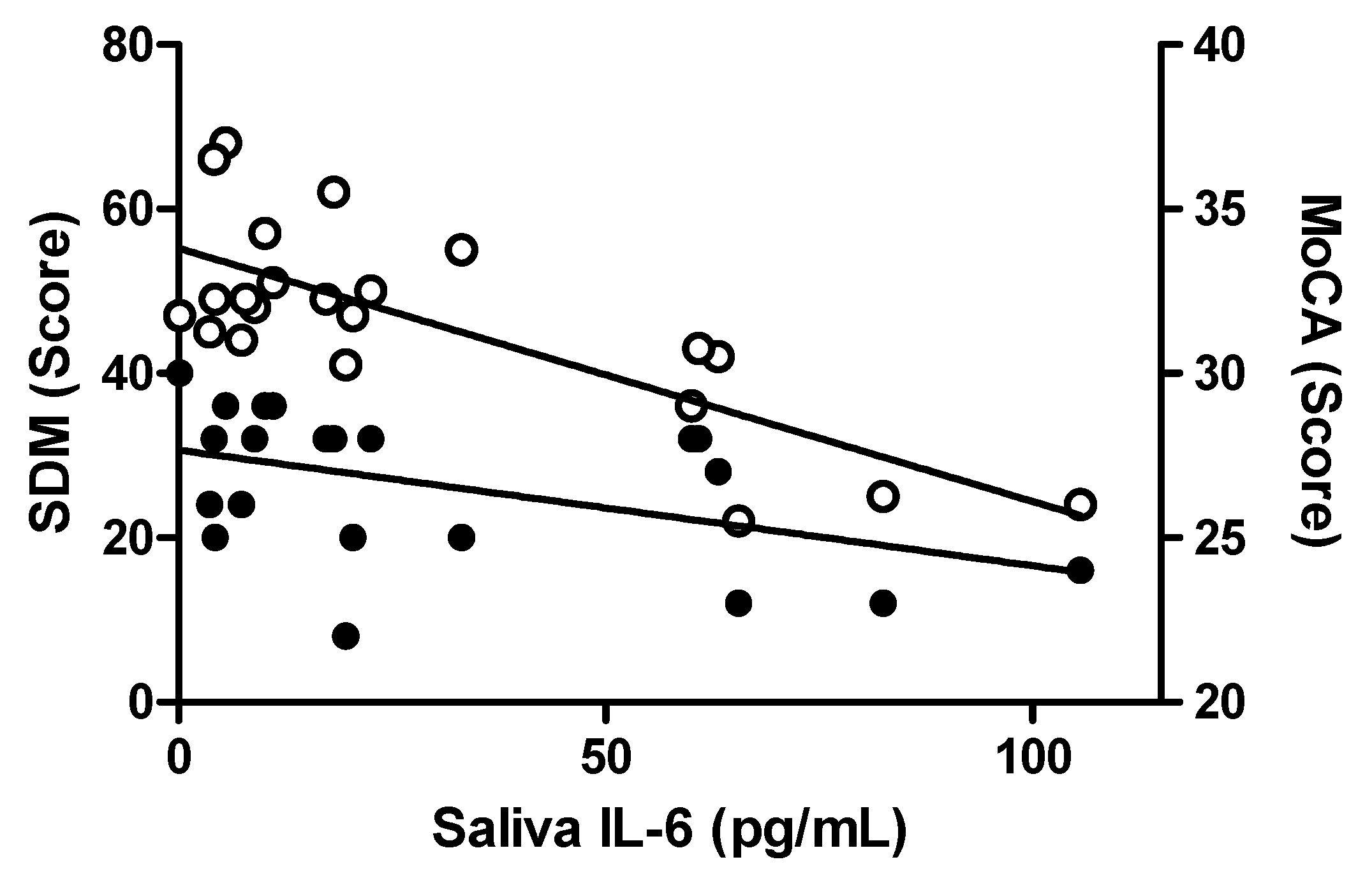
2.3. Associations Between Salivary Inflammatory Markers and Clinical Symptoms
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Clinical Assessments
4.3. Plasma Collection
4.4. Saliva Collection
4.5. ELISA Assays
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HD | Huntington’s disease |
| CRP | C-reactive protein |
| IL-6 | Interleukin-6 |
| IL-1B | Interleukin 1 beta |
| AA | Alpha-amylase |
References
- Huntington Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar]
- Vonsattel, J.P.; Myers, R.H.; Stevens, T.J.; Ferrante, R.J.; Bird, E.D.; Richardson, E.P.J. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985, 44, 559–577. [Google Scholar] [CrossRef]
- DiFiglia, M.; Sapp, E.; Chase, K.; Schwarz, C.; Meloni, A.; Young, C.; Martin, E.; Vonsattel, J.P.; Carraway, R.; Reeves, S.A. Huntingtin is a cytoplasmic protein associated with vesicles human and rat brain neurons. Neuron 1995, 14, 1075–1081. [Google Scholar] [CrossRef]
- Sassone, J.; Colciago, C.; Cislaghi, G.; Silani, V.; Ciammola, A. Huntington’s disease: The current state of research with peripheral tissues. Exp. Neurol. 2009, 219, 385–397. [Google Scholar] [CrossRef]
- Ribchester, R.R.; Thomson, D.; Wood, N.I.; Hinks, T.; Gillingwater, T.H.; Wishart, T.M.; Court, F.A.; Morton, A.J. Progressive abnormalities in skeletal muscle and neuromuscular junctions of transgenic mice expressing the Huntington’s disease mutation. Eur. J. Neurosci. 2004, 20, 3092–3114. [Google Scholar] [CrossRef]
- Wang, R.; Ross, C.A.; Cai, H.; Cong, W.N.; Daimon, C.M.; Carlson, O.D.; Egan, J.M.; Siddiqui, S.; Maudsley, S.; Martin, B. Metabolic and hormonal signatures in pre-manifest and manifest Huntington’s disease patients. Front. Physiol. 2014, 5, 231. [Google Scholar] [CrossRef]
- Ellrichmann, G.; Reick, C.; Saft, C.; Linker, R.A. The role of the immune system in Huntington’s disease. Clin. Dev. Immunol. 2013, 2013, 541259. [Google Scholar] [CrossRef]
- Rocha, N.P.; Ribeiro, F.M.; Furr-Stimming, E.; Teixeira, A.L. Neuroimmunology of Huntington’s Disease: Revisiting Evidence from Human Studies. Mediat. Inflamm. 2016, 2016, 8653132. [Google Scholar] [CrossRef]
- Silvestroni, A.; Faull, R.L.; Strand, A.D.; Moller, T. Distinct neuroinflammatory profile in post-mortem human Huntington’s disease. Neuroreport 2009, 20, 1098–1103. [Google Scholar] [CrossRef]
- Dalrymple, A.; Wild, E.J.; Joubert, R.; Sathasivam, K.; Bjorkqvist, M.; Petersen, A.; Jackson, G.S.; Isaacs, J.D.; Kristiansen, M.; Bates, G.P.; et al. Proteomic profiling of plasma in Huntington’s disease reveals neuroinflammatory activation and biomarker candidates. J. Proteome Res. 2007, 6, 2833–2840. [Google Scholar] [CrossRef]
- Bjorkqvist, M.; Wild, E.J.; Thiele, J.; Silvestroni, A.; Andre, R.; Lahiri, N.; Raibon, E.; Lee, R.V.; Benn, C.L.; Soulet, D.; et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J. Exp. Med. 2008, 205, 1869–1877. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Morris, C.D.; Jacques, V.; Gottesfeld, J.M.; Rusche, J.R.; Thomas, E.A. The Effects of Pharmacological Inhibition of Histone Deacetylase 3 (HDAC3) in Huntington’s Disease Mice. PLoS ONE 2016, 11, e0152498. [Google Scholar] [CrossRef]
- Chang, K.H.; Wu, Y.R.; Chen, Y.C.; Chen, C.M. Plasma inflammatory biomarkers for Huntington’s disease patients and mouse model. Brain Behav. Immun. 2015, 44, 121–127. [Google Scholar] [CrossRef]
- Bouwens, J.A.; van Duijn, E.; Cobbaert, C.M.; Roos, R.A.; van der Mast, R.C.; Giltay, E.J. Plasma Cytokine Levels in Relation to Neuropsychiatric Symptoms and Cognitive Dysfunction in Huntington’s disease. J. Huntingt. Dis. 2016, 5, 369–377. [Google Scholar] [CrossRef]
- Sánchez-López, F.; Tasset, I.; Agüera, E.; Feijóo, M.; Fernández-Bolaños, R.; Sánchez, F.M.; Ruiz, M.C.; Cruz, A.H.; Gascón, F.; Túnez, I. Oxidative stress and inflammation biomarkers in the blood of patients with Huntington’s disease. Neurol. Res. 2012, 34, 721–724. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Byrne, L.M.; McColgan, P.; Robertson, N.; Tabrizi, S.J.; Zetterberg, H.; Wild, E.J. Cerebrospinal Fluid Inflammatory Biomarkers Reflect Clinical Severity in Huntington’s Disease. PLoS ONE 2016, 11, e0163479. [Google Scholar] [CrossRef]
- Rathnayake, N.; Akerman, S.; Klinge, B.; Lundegren, N.; Jansson, H.; Tryselius, Y.; Sorsa, T.; Gustafsson, A. Salivary biomarkers for detection of systemic diseases. PLoS ONE 2013, 8, e61356. [Google Scholar] [CrossRef]
- La Fratta, I.; Tatangelo, R.; Campagna, G.; Rizzuto, A.; Franceschelli, S.; Ferrone, A.; Patruno, A.; Speranza, L.; De Lutiis, M.A.; Felaco, M.; et al. The plasmatic and salivary levels of IL-1β, IL-18 and IL-6 are associated to emotional difference during stress in young male. Sci. Rep. 2018, 8, 3031. [Google Scholar] [CrossRef]
- Williamson, S.; Munro, C.; Pickler, R.; Grap, M.J.; Elswick, R.K., Jr. Comparison of biomarkers in blood and saliva in healthy adults. Nurs. Res. Pract. 2012, 2012, 246178. [Google Scholar] [CrossRef]
- Sjögren, E.; Leanderson, P.; Kristenson, M.; Ernerudh, J. Interleukin-6 levels in relation to psychosocial factors: Studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain Behav. Immun. 2006, 20, 270–278. [Google Scholar] [CrossRef]
- Minetto, M.; Rainoldi, A.; Gazzoni, M.; Terzolo, M.; Borrione, P.; Termine, A.; Saba, L.; Dovio, A.; Angeli, A.; Paccotti, P. Differential responses of serum and salivary interleukin-6 to acute strenuous exercise. Eur. J. Appl. Physiol. 2005, 93, 679–686. [Google Scholar] [CrossRef]
- Izawa, S.; Sugaya, N.; Kimura, K.; Ogawa, N.; Yamada, K.C.; Shirotsuki, K.; Mikami, I.; Hirata, K.; Nagano, Y.; Nomura, S. An increase in salivary interleukin-6 level following acute psychosocial stress and its biological correlates in healthy young adults. Biol. Psychol. 2013, 94, 249–254. [Google Scholar] [CrossRef]
- Bjorkqvist, M.; Wild, E.J.; Tabrizi, S.J. Harnessing immune alterations in neurodegenerative diseases. Neuron 2009, 64, 21–24. [Google Scholar] [CrossRef][Green Version]
- Riis, J.L.; Granger, D.A.; DiPietro, J.A.; Bandeen-Roche, K.; Johnson, S.B. Salivary cytokines as a minimally-invasive measure of immune functioning in young children: Correlates of individual differences and sensitivity to laboratory stress. Dev. Psychobiol. 2015, 57, 153–167. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1335–1347. [Google Scholar] [CrossRef]
- Lightfoot, A.P.; Cooper, R.G. The role of myokines in muscle health and disease. Curr. Opin. Rheumatol. 2016, 28, 661–666. [Google Scholar] [CrossRef]
- Waters, C.W.; Varuzhanyan, G.; Talmadge, R.J.; Voss, A.A. Huntington disease skeletal muscle is hyperexcitable owing to chloride and potassium channel dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 9160–9165. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Koshikawa, M.; Harada, M.; Noyama, S.; Kiyono, K.; Motoike, Y.; Nomura, Y.; Nishimura, A.; Izawa, H.; Watanabe, E.; Ozaki, Y. Association between inflammation and skeletal muscle proteolysis, skeletal mass and strength in elderly heart failure patients and their prognostic implications. BMC Cardiovasc. Disord. 2020, 20, 228. [Google Scholar] [CrossRef]
- Belizário, J.E.; Fontes-Oliveira, C.C.; Borges, J.P.; Kashiabara, J.A.; Vannier, E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. Springerplus 2016, 5, 619. [Google Scholar] [CrossRef]
- Zielonka, D.; Piotrowska, I.; Marcinkowski, J.T.; Mielcarek, M. Skeletal muscle pathology in Huntington’s disease. Front. Physiol. 2014, 5, 380. [Google Scholar] [CrossRef]
- Lodi, R.; Schapira, A.H.; Manners, D.; Styles, P.; Wood, N.W.; Taylor, D.J.; Warner, T.T. Abnormal in vivo skeletal muscle energy metabolism in Huntington’s disease and dentatorubropallidoluysian atrophy. Ann. Neurol. 2000, 48, 72–76. [Google Scholar] [CrossRef]
- Arenas, J.; Campos, Y.; Ribacoba, R.; Martin, M.A.; Rubio, J.C.; Ablanedo, P.; Cabello, A. Complex I defect in muscle from patients with Huntington’s disease. Ann. Neurol. 1998, 43, 397–400. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. Basic Clin. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Andrich, J.; Schmitz, T.; Saft, C.; Postert, T.; Kraus, P.; Epplen, J.T.; Przuntek, H.; Agelink, M.W. Autonomic nervous system function in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 72, 726–731. [Google Scholar] [CrossRef]
- Kobal, J.; Meglic, B.; Mesec, A.; Peterlin, B. Early sympathetic hyperactivity in Huntington’s disease. Eur. J. Neurol. 2004, 11, 842–848. [Google Scholar] [CrossRef]
- Ouellet-Morin, I.; Danese, A.; Williams, B.; Arseneault, L. Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain Behav. Immun. 2011, 25, 640–646. [Google Scholar] [CrossRef]
- Haririan, H.; Bertl, K.; Laky, M.; Rausch, W.D.; Böttcher, M.; Matejka, M.; Andrukhov, O.; Rausch-Fan, X. Salivary and serum chromogranin A and α-amylase in periodontal health and disease. J. Periodontol. 2012, 83, 1314–1321. [Google Scholar] [CrossRef]
- Gutiérrez-Corrales, A.; Campano-Cuevas, E.; Castillo-Dalí, G.; Torres-Lagares, D.; Gutiérrez-Pérez, J.L. Ability of salivary biomarkers in the prognostic of systemic and buccal inflammation. J. Clin. Exp. Dent. 2017, 9, e716–e722. [Google Scholar] [CrossRef]
- Corey-Bloom, J.; Haque, A.S.; Park, S.; Nathan, A.S.; Baker, R.W.; Thomas, E.A. Salivary levels of total huntingtin are elevated in Huntington’s disease patients. Sci. Rep. 2018, 8, 7371. [Google Scholar] [CrossRef]
- Ebersole, J.L. Humoral immune responses in gingival crevice fluid: Local and systemic implications. Periodontol. 2000 2003, 31, 135–166. [Google Scholar] [CrossRef] [PubMed]
- Michalke, B.; Rossbach, B.; Göen, T.; Schäferhenrich, A.; Scherer, G. Saliva as a matrix for human biomonitoring in occupational and environmental medicine. Int. Arch. Occup. Environ. Health 2015, 88, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.A.; Fortunato, C.K.; Beltzer, E.K.; Virag, M.; Bright, M.A.; Out, D. Focus on methodology: Salivary bioscience and research on adolescence: An integrated perspective. J. Adolesc. 2012, 35, 1081–1095. [Google Scholar] [CrossRef] [PubMed]

| NC | PM | HD | p-Value | |
|---|---|---|---|---|
| n | 52 | 36 | 37 | |
| Gender, F:M | 27:25 | 19:17 | 25:12 | 0.352 |
| Age, yrs | 54.42 (23−78) | 43.44 (19−71) ** | 56.95 (30−76) | <0.0001 |
| Weight, lbs | 171.94 (106−306) | 172.90 (99−290) | 149.83 (90−263) *^ | 0.024 |
| Education, yrs | 15.40 (12−22) | 16.24 (12−22) | 14.22 (5−24) | 0.438 |
| CAG repeat | NA | 42.33 (38−51) | 42.93 (38−49) | 0.283 |
| DBS | NA | 248.39 (112−368) | 388.14 (273−578) ^^ | <0.0001 |
| AOO, yrs | NA | NA | 48.83 (22−68) | NA |
| PAO, yrs | NA | 48.91 (24−75) | 50.16 (27−70) | 0.222 |
| Saliva IL-6 (pg/mL) | Saliva IL-1B (pg/mL) | Saliva CRP (pg/mL) | Saliva AA (U/mL) | Plasma IL-6 (pg/mL) | Plasma CRP (pg/mL) | ||
|---|---|---|---|---|---|---|---|
| Saliva IL-6 (pg/mL) | Spearman’s rho | — | |||||
| p-value | — | ||||||
| Saliva IL-1B (pg/mL) | Spearman’s rho | 0.527 *** | — | ||||
| p-value | <0.0001 | — | |||||
| Saliva CRP (pg/mL) | Spearman’s rho | 0.377** | 0.06 | — | |||
| p-value | 0.008 | 0.633 | — | ||||
| Saliva AA (U/mL) | Spearman’s rho | 0.393 ** | 0.266* | 0.099 | — | ||
| p-value | 0.006 | 0.027 | 0.378 | — | |||
| Plasma IL-6 (pg/mL) | Spearman’s rho | 0.381 ** | 0.109 | 0.254 * | 0.029 | — | |
| p-value | 0.0049 | 0.405 | 0.047 | 0.815 | — | ||
| Plasma CRP (ng/mL) | Spearman’s rho | 0.165 | 0.115 | 0.345 ** | −0.134 | 0.135 | — |
| p-value | 0.181 | 0.358 | 0.006 | 0.266 | 0.274 | — |
| Unadjusted Correlations and p-Values | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saliva IL6 (pg/mL) | Saliva IL1B (pg/mL) | Saliva CRP (pg/mL) | Saliva AA (U/mL) | Plasma IL6 (pg/mL) | Plasma CRP (ng/mL) | |||||||
| rho | p-Value | rho | p-Value | rho | p-Value | rho | p-value | rho | p-value | rho | p-Value | |
| MMSE | −0.219 | 0.154 | −0.194 | 0.191 | −0.177 | 0.243 | 0.106 | 0.479 | −0.215 | 0.183 | 0.041 | 0.786 |
| MoCA | −0.317 | 0.036 | −0.317 | 0.03 | −0.02 | 0.897 | −0.004 | 0.98 | −0.117 | 0.472 | 0.027 | 0.86 |
| SDM | −0.415 | 0.006 | −0.226 | 0.135 | 0.007 | 0.965 | 0.048 | 0.758 | −0.177 | 0.337 | 0.341 | 0.027 |
| TFC | −0.477 | 0.001 | −0.348 | 0.016 | 0.069 | 0.652 | −0.19 | 0.201 | −0.289 | 0.07 | 0.224 | 0.134 |
| HADS | −0.047 | 0.767 | 0.062 | 0.686 | 0.07 | 0.654 | −0.136 | 0.373 | 0.027 | 0.87 | −0.02 | 0.902 |
| TMS | 0.51 | < 0.000 | 0.38 | 0.008 | −0.081 | 0.598 | 0.143 | 0.339 | 0.258 | 0.108 | −0.166 | 0.27 |
| Chorea | 0.549 | < 0.000 | 0.333 | 0.022 | −0.038 | 0.803 | 0.221 | 0.135 | 0.152 | 0.35 | −0.203 | 0.181 |
| Age-Adjusted Correlations and p-Values | ||||||||||||
| Saliva IL6 (pg/mL) | Saliva IL1B (pg/mL) | Saliva CRP (pg/mL) | Saliva AA (U/mL) | Plasma IL6 (pg/mL) | Plasma CRP (ng/mL) | |||||||
| rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | p-Value | |
| MMSE | −0.124 | 0.428 | −0.155 | 0.305 | −0.174 | 0.258 | 0.133 | 0.377 | −0.178 | 0.278 | 0.054 | 0.724 |
| MoCA | −0.242 | 0.118 | −0.269 | 0.071 | −0.011 | 0.943 | 0.018 | 0.906 | −0.078 | 0.639 | 0.039 | 0.805 |
| SDM | −0.232 | 0.144 | −0.153 | 0.322 | 0.036 | 0.821 | 0.123 | 0.433 | −0.095 | 0.576 | 0.457 | 0.003 |
| TFC | −0.323 | 0.034 | −0.306 | 0.038 | 0.111 | 0.474 | −0.174 | 0.247 | −0.237 | 0.146 | 0.303 | 0.043 |
| HADS | −0.173 | 0.281 | 0.017 | 0.911 | 0.063 | 0.692 | −0.163 | 0.289 | 0.075 | 0.66 | −0.031 | 0.847 |
| TMS | 0.35 | 0.011 | 0.356 | 0.015 | −0.141 | 0.363 | 0.117 | 0.439 | 0.197 | 0.23 | −0.258 | 0.088 |
| Chorea | 0.411 | 0.006 | 0.29 | 0.051 | −0.079 | 0.611 | 0.218 | 0.146 | 0.058 | 0.725 | −0.293 | 0.053 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corey-Bloom, J.; Fischer, R.S.; Kim, A.; Snell, C.; Parkin, G.M.; Granger, D.A.; Granger, S.W.; Thomas, E.A. Levels of Interleukin-6 in Saliva, but Not Plasma, Correlate with Clinical Metrics in Huntington’s Disease Patients and Healthy Control Subjects. Int. J. Mol. Sci. 2020, 21, 6363. https://doi.org/10.3390/ijms21176363
Corey-Bloom J, Fischer RS, Kim A, Snell C, Parkin GM, Granger DA, Granger SW, Thomas EA. Levels of Interleukin-6 in Saliva, but Not Plasma, Correlate with Clinical Metrics in Huntington’s Disease Patients and Healthy Control Subjects. International Journal of Molecular Sciences. 2020; 21(17):6363. https://doi.org/10.3390/ijms21176363
Chicago/Turabian StyleCorey-Bloom, Jody, Ryan S. Fischer, Aeri Kim, Chase Snell, Georgia M. Parkin, Douglas A. Granger, Steven W. Granger, and Elizabeth A. Thomas. 2020. "Levels of Interleukin-6 in Saliva, but Not Plasma, Correlate with Clinical Metrics in Huntington’s Disease Patients and Healthy Control Subjects" International Journal of Molecular Sciences 21, no. 17: 6363. https://doi.org/10.3390/ijms21176363
APA StyleCorey-Bloom, J., Fischer, R. S., Kim, A., Snell, C., Parkin, G. M., Granger, D. A., Granger, S. W., & Thomas, E. A. (2020). Levels of Interleukin-6 in Saliva, but Not Plasma, Correlate with Clinical Metrics in Huntington’s Disease Patients and Healthy Control Subjects. International Journal of Molecular Sciences, 21(17), 6363. https://doi.org/10.3390/ijms21176363





