Rocuronium Has a Suppressive Effect on Platelet Function via the P2Y12 Receptor Pathway In Vitro That Is Not Reversed by Sugammadex
Abstract
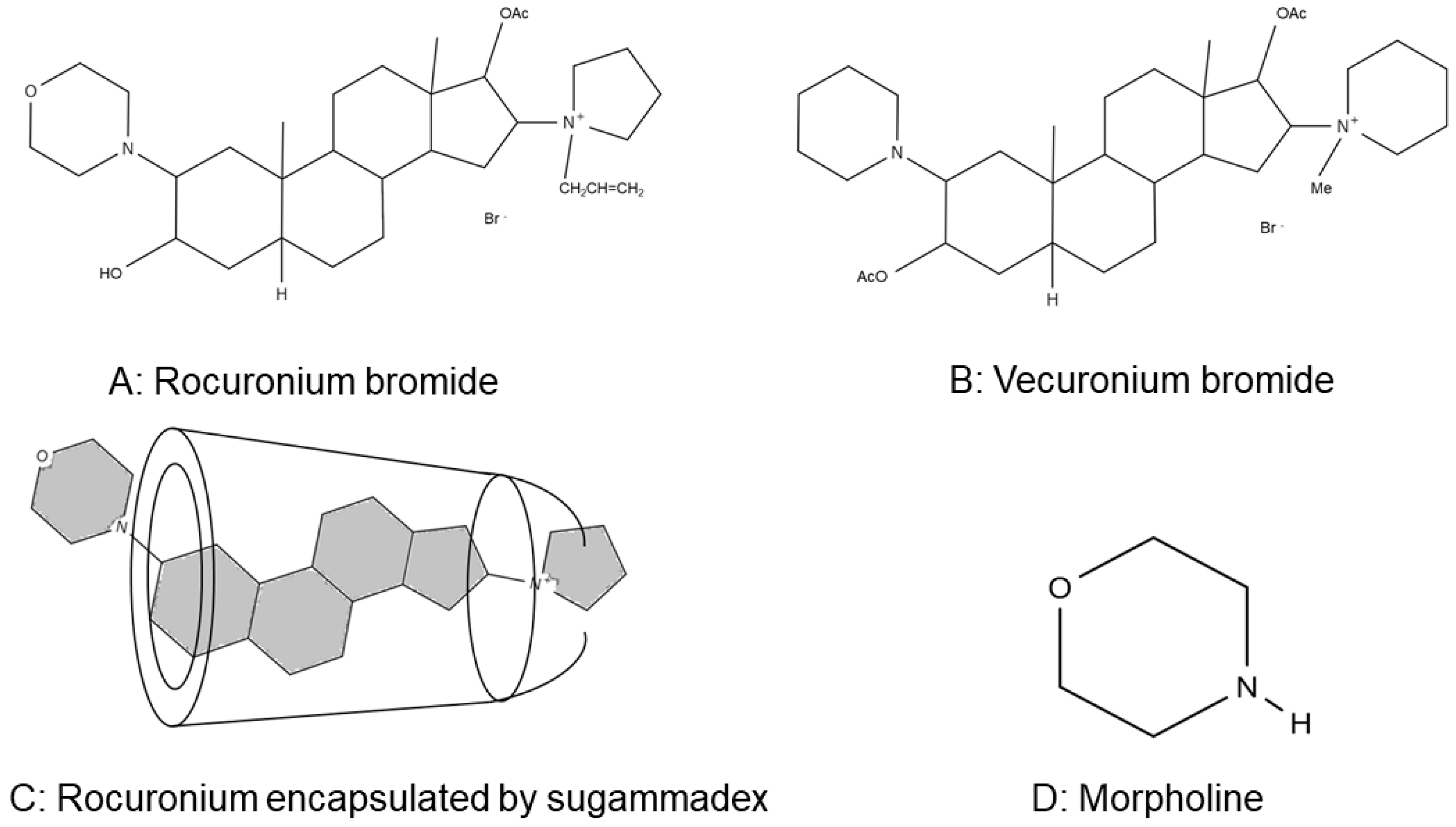
:1. Introduction
2. Results
2.1. Platelet Aggregation
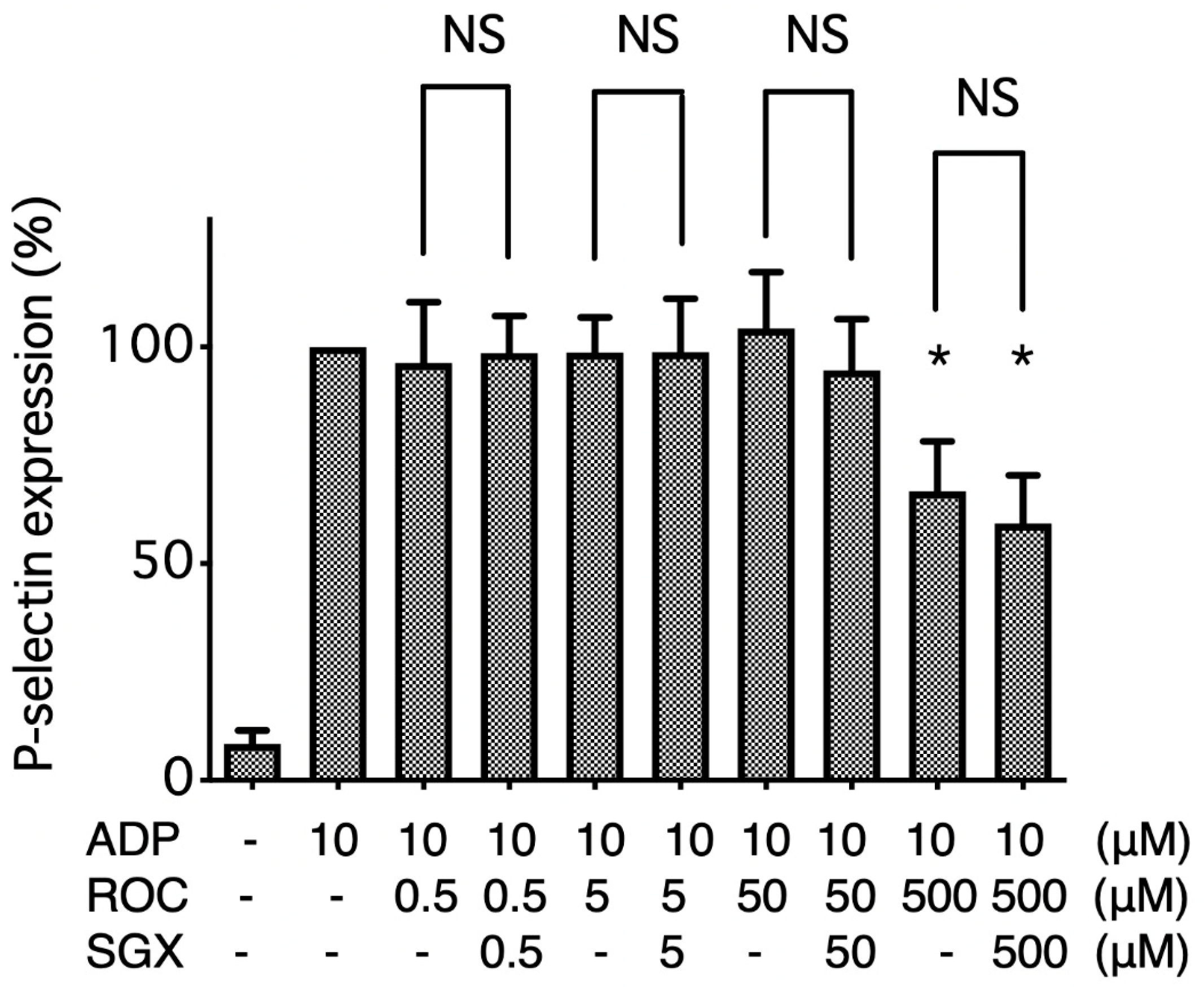
2.2. Flow Cytometry Analysis of P-Selectin Expression on ADP-Stimulated Platelets
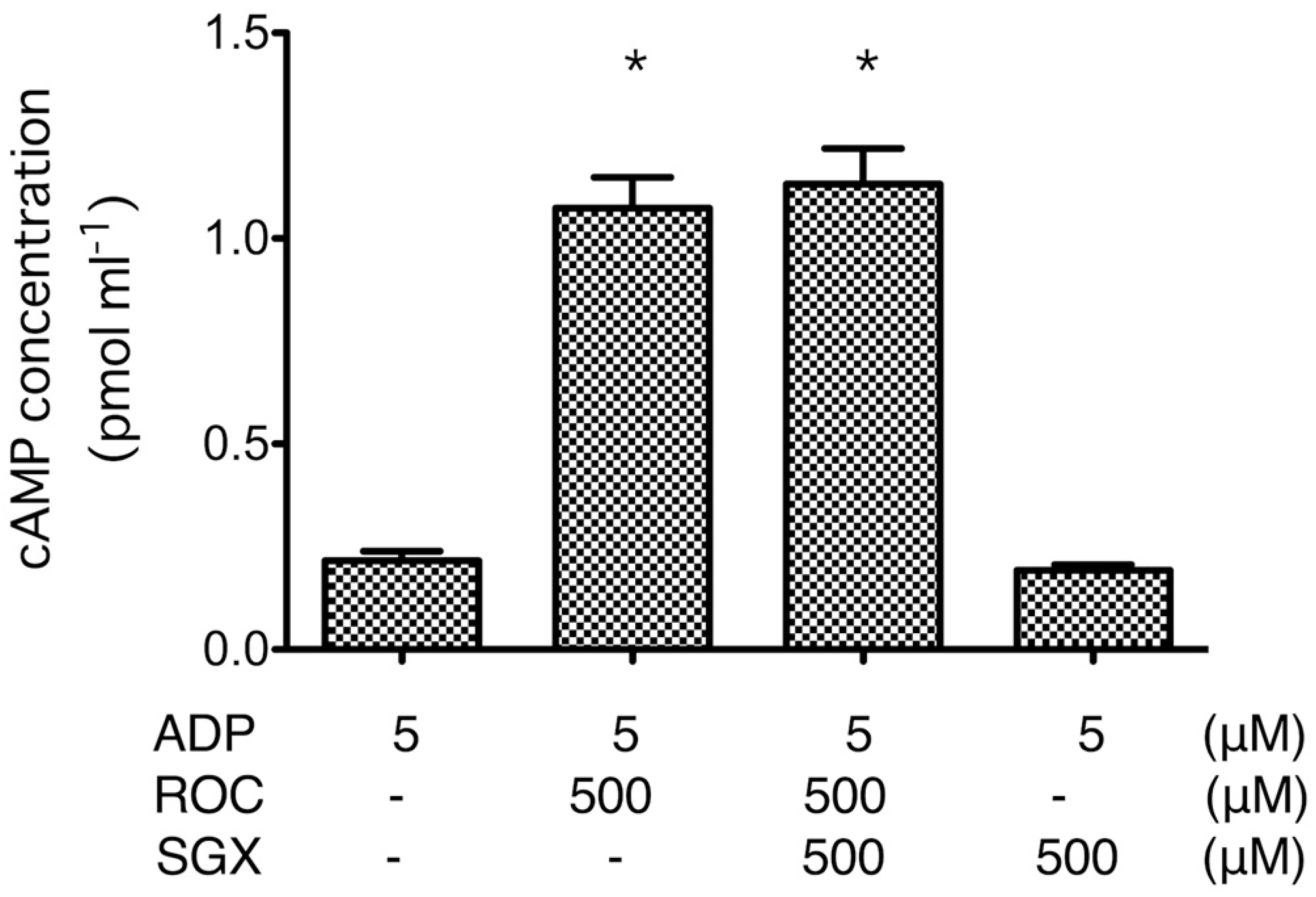
2.3. cAMP Formation
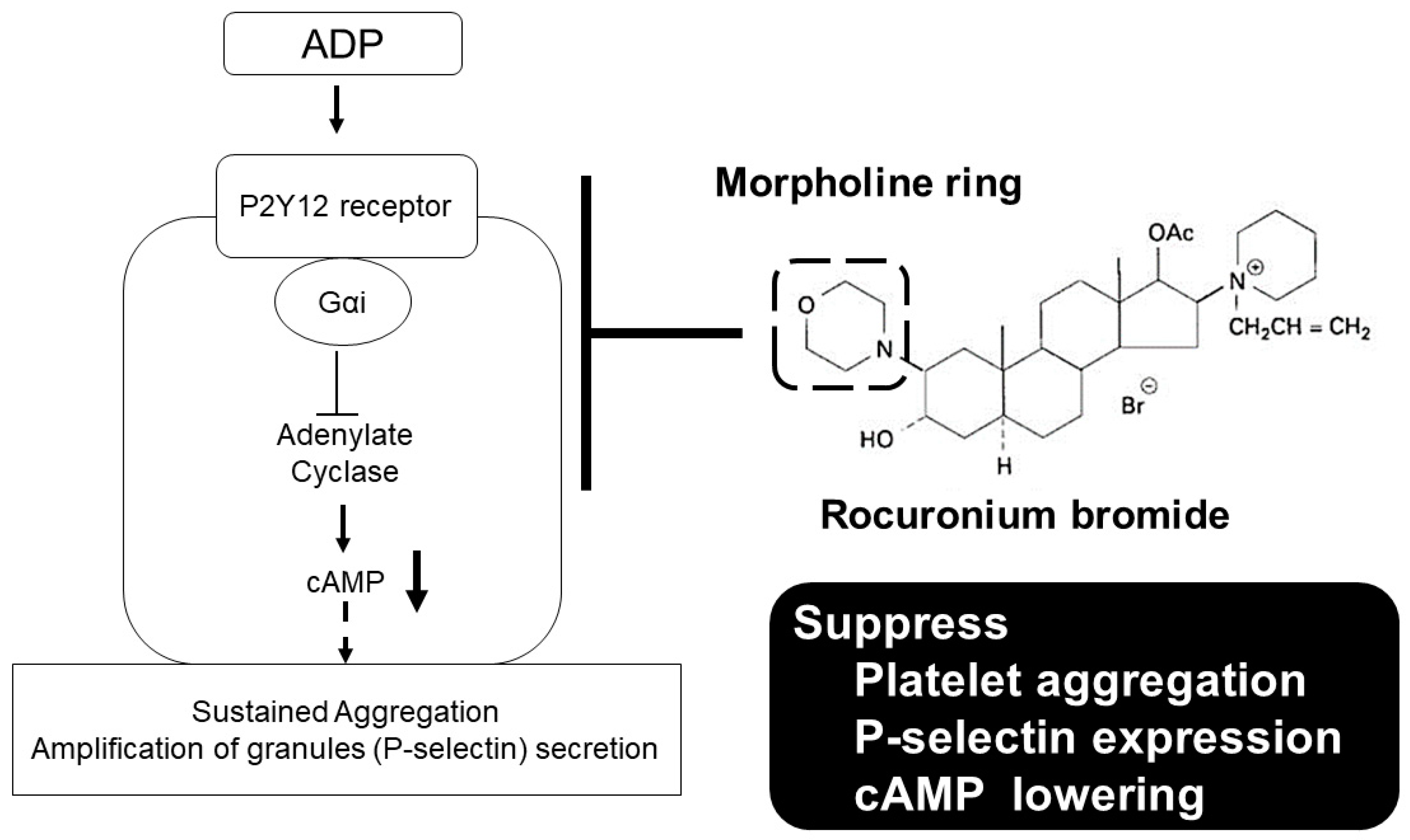
3. Discussion
4. Materials and Methods
4.1. Platelet Preparation
4.2. Chemicals and Drugs
4.3. Measurement of ADP-Induced Platelet Aggregation
4.4. Flow Cytometry Analysis of P-Selectin Expression on ADP-Stimulated Platelets
4.5. cAMP Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| cAMP | Cyclic adenosine monophosphate |
| EDTA | Ethylenediaminetetraacetic acid |
| FACS | Fluorescence-activated cell sorting |
| HEPES | N-2-hydroxyethylpiperazine-N′-2′-ethanesulfonic acid |
| MFI | Mean fluorescent intensity |
| MOR | Morpholine |
| PBS | Phosphate-buffered saline |
| PE | Phycoerythrin |
| PerCP | Peridinin Chlorophyll Protein |
| PPP | Platelet-poor plasma |
| PRP | Platelet-rich plasma |
| ROC | Rocuronium |
| SGX | Sugammadex |
| VEC | Vecuronium |
References
- Hirakata, H.; Hatano, Y.; Ushikubi, F.; Mori, K.; Narumiya, S.; Nakamura, K. The Effect of Inhaled Anesthetics on the Platelet Aggregation and the Ligand-Binding Affinity of the Platelet Thromboxane A2 Receptor. Anesth. Analg. 1995, 81, 114–118. [Google Scholar] [PubMed]
- Hirakata, H.; Ushikubi, F.; Toda, H.; Nakamura, K.; Sai, S.; Urabe, N.; Hatano, Y.; Narumiya, S.; Mori, K. Sevoflurane inhibits human platelet aggregation and thromboxane A 2 formation, possibly by suppression of cyclooxygenase activity. Anesthesiology 1996, 85, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, H.; Nakamura, K.; Sai, S.; Okuda, H.; Hatano, Y.; Urabe, N.; Mori, K. Platelet aggregation is impaired during anaesthesia with sevoflurane but not with isoflurane. Can. J. Anaesth. 1997, 44, 1157–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakata, H.; Nakamura, K.; Yokubol, B.; Toda, H.; Hatano, Y.; Urabe, N.; Mori, K. Propofol Has Both Enhancing and Suppressing Eflects on Human Platelet Aggregation. Anesthesiology 1999, 91, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hirakata, H.; Sato, M.; Nakamura, K.; Hatano, Y.; Nakamura, T.; Fukuda, K. Ketamine suppresses platelet aggregation possibly by suppressed inositol triphosphate formation and subsequent suppression of cytosolic calcium increase. Anesthesiology 2002, 96, 1147–1152. [Google Scholar] [CrossRef]
- Kawamoto, S.; Hirakata, H.; Sugita, N.; Fukuda, K. Bidirectional effects of dexmedetomidine on human platelet functions in vitro. Eur. J. Pharmacol. 2015, 766, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamoto, S.; Fukuda, K. Dexmedetomidine increases human platelet-derived microparticles via the α2-adrenoceptor. J. Jpn. Soc. Intensive Care Med. 2018, 25, 457–459. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.M. Rocuronium: The newest aminosteroid neuromuscular blocking drug. Br. J. Anaesth. 1996, 76, 481–483. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Welliver, M.; McDonough, J.; Kalynych, N.; Redfern, R. Discovery, development, and clinical application of sugammadex sodium, a selective relaxant binding agent. Drug Des. Dev. Ther. 2008, 2, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Kahner, B.N.; Shankar, H.; Murugappan, S.; Prasad, G.L.; Kunapuli, S.P. Nucleotide receptor signaling in platelets. J. Thromb. Haemost. 2006, 4, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Gachet, C. P2 receptors, platelet function and pharmacological implications. Thromb. Haemost. 2008, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Keularts, I.M.L.W.; van Gorp, R.M.A.; Feijge, M.A.H.; Vuist, W.M.J.; Heemskerk, J.W.M. α 2A-Adrenergic Receptor Stimulation Potentiates Calcium Release in Platelets by Modulating cAMP Levels. J. Biol. Chem. 2000, 275, 1763–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabinger, I.; Thaler, J.; Ay, C. Biomarkers for prediction of venous thromboembolism in cancer. Blood 2013, 122, 2011–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merten, M.; Thiagarajan, P. P-Selectin Expression on Platelets Determines Size and Stability of Platelet Aggregates. Circulation 2000, 102, 1931–1936. [Google Scholar] [CrossRef]
- Christersson, C.; Johnell, M.; Siegbahn, A. Tissue factor and IL8 production by P-selectin-dependent platelet-monocyte aggregates in whole blood involves phosphorylation of Lyn and is inhibited by IL10. J. Thromb. Haemost. 2008, 6, 986–994. [Google Scholar] [CrossRef]
- Yokoyama, S.; Ikeda, H.; Haramaki, N.; Yasukawa, H.; Murohara, T.; Imaizumi, T. Platelet P-selectin plays an important role in arterial thrombogenesis by forming large stable platelet-leukocyte aggregates. J. Am. Coll. Cardiol. 2005, 45, 1280–1286. [Google Scholar] [CrossRef] [Green Version]
- Naim, M.J.; Alam, O.; Alam, M.J.; Alam, P.; Shrivastava, N. A review on pharmacological profile of Morpholine derivatives. Int. J. Pharmacol. Pharm. Sci. 2015, 3, 40–51. [Google Scholar]
- Wang, X.M.; Xin, M.H.; Xu, J.; Kang, B.R.; Li, Y.; Lu, S.M.; Zhang, S.Q. Synthesis and antitumor activities evaluation of m-(4-morpholinoquinazolin-2-yl)benzamides in vitro and in vivo. Eur. J. Med. Chem. 2015, 96, 382–395. [Google Scholar] [CrossRef]
- Senwar, K.R.; Sharma, P.; Reddy, T.S.; Jeengar, M.K.; Nayak, V.L.; Naidu, V.G.M.; Kamal, A.; Shankaraiah, N. Spirooxindole-derived morpholine-fused-1,2,3-triazoles: Design, synthesis, cytotoxicity and apoptosis inducing studies. Eur. J. Med. Chem. 2015, 102, 413–424. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Rangelov, M.; Smelcerovic, Z.; Veljkovic, A.; Cherneva, E.; Yancheva, D.; Nikolic, G.M.; Petronijevic, Z.; Kocic, G. Two 6-(propan-2-yl)-4-methyl-morpholine-2,5-diones as new non-purine xanthine oxidase inhibitors and anti-inflammatory agents. Food Chem. Toxicol. 2013, 55, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Khanum, S.A.; Begum, B.A.; Girish, V.; Khanum, N.F. Synthesis and evaluation of benzophenone-n-ethyl morpholine ethers as anti-inflammatory agents. Int. J. Biomed. Sci. 2010, 6, 60–65. [Google Scholar]
- Kuettel, S.; Zambon, A.; Kaiser, M.; Brun, R.; Scapozza, L.; Perozzo, R. Synthesis and evaluation of antiparasitic activities of new 4-[5-(4-phenoxyphenyl)-2H-pyrazol-3-yl]morpholine derivatives. J. Med. Chem. 2007, 50, 5833–5839. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Lee, J.Y.; Park, H.D.; Kim, T.H.; Park, M.C.; Choi, G.; Kim, S. Identification of a new morpholine scaffold as a P2Y12 receptor antagonist. Molecules 2016, 21, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Saeki, S.; Takeda, J.; Ozaki, M.; Iwao, Y. Neuromuscular blocking effects, pharmacokinetics and safety of Org 9426 (rocuronium bromide) in Japanese patients. Masui 2006, 55, 419–427. [Google Scholar] [PubMed]
- Bevan, D.R. Rocuronium bromide and organ function. Eur. J. Anaesthesiol. Suppl. 1994, 9, 87–91. [Google Scholar]
- Magorian, T.; Wood, P.; Caldwell, J.; Fisher, D.; Segredo, V.; Szenohradszky, J.; Sharma, M.; Gruenke, L.; Miller, R. The Pharmacokinetics and Neuromuscular Effects of Rocuronium Bromide in Patients with Liver Disease. Anesth. Analg. 1995, 80, 754–759. [Google Scholar]
- Szenohradszky, J.; Fisher, D.M.; Segredo, V.; Caldwell, J.E.; Bragg, P.; Sharma, M.L.; Gruenke, L.D.; Miller, R.D. Pharmacokinetics of Rocuronium Bromide (ORG 9426) in Patients with Normal Renal Function or Patients Undergoing Cadaver Renal Transplantation. Anesthesiology 1992, 77, 899–904. [Google Scholar] [CrossRef]
- Matteo, R.S.; Ornstein, E.; Schwartz, A.E.; Ostapkovich, N.; Stone, J.G. Pharmacokinetics and Pharmacodynamics of Rocuronium (Org 9426) in Elderly Surgical Patients. Anesth. Analg. 1993, 77, 1193–1197. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Clarke, R.C.; Sadleir, P.H.M.; Platt, P.R. The role of sugammadex in the development and modification of an allergic response to rocuronium: Evidence from a cutaneous model. Anaesthesia 2012, 67, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Limbird, L.E. Receptors linked to inhibition of adenylate cyclase: Additional signaling mechanisms. FASEB J. 1988, 2, 2686–2695. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, Y.; Kawamoto, S.; Fukuda, K. Rocuronium Has a Suppressive Effect on Platelet Function via the P2Y12 Receptor Pathway In Vitro That Is Not Reversed by Sugammadex. Int. J. Mol. Sci. 2020, 21, 6399. https://doi.org/10.3390/ijms21176399
Murata Y, Kawamoto S, Fukuda K. Rocuronium Has a Suppressive Effect on Platelet Function via the P2Y12 Receptor Pathway In Vitro That Is Not Reversed by Sugammadex. International Journal of Molecular Sciences. 2020; 21(17):6399. https://doi.org/10.3390/ijms21176399
Chicago/Turabian StyleMurata, Yutaka, Shuji Kawamoto, and Kazuhiko Fukuda. 2020. "Rocuronium Has a Suppressive Effect on Platelet Function via the P2Y12 Receptor Pathway In Vitro That Is Not Reversed by Sugammadex" International Journal of Molecular Sciences 21, no. 17: 6399. https://doi.org/10.3390/ijms21176399
APA StyleMurata, Y., Kawamoto, S., & Fukuda, K. (2020). Rocuronium Has a Suppressive Effect on Platelet Function via the P2Y12 Receptor Pathway In Vitro That Is Not Reversed by Sugammadex. International Journal of Molecular Sciences, 21(17), 6399. https://doi.org/10.3390/ijms21176399




