Biological Responses to Cadmium Stress in Liverwort Conocephalum conicum (Marchantiales)
Abstract
:1. Introduction
2. Results
2.1. Metal Bioaccumulation
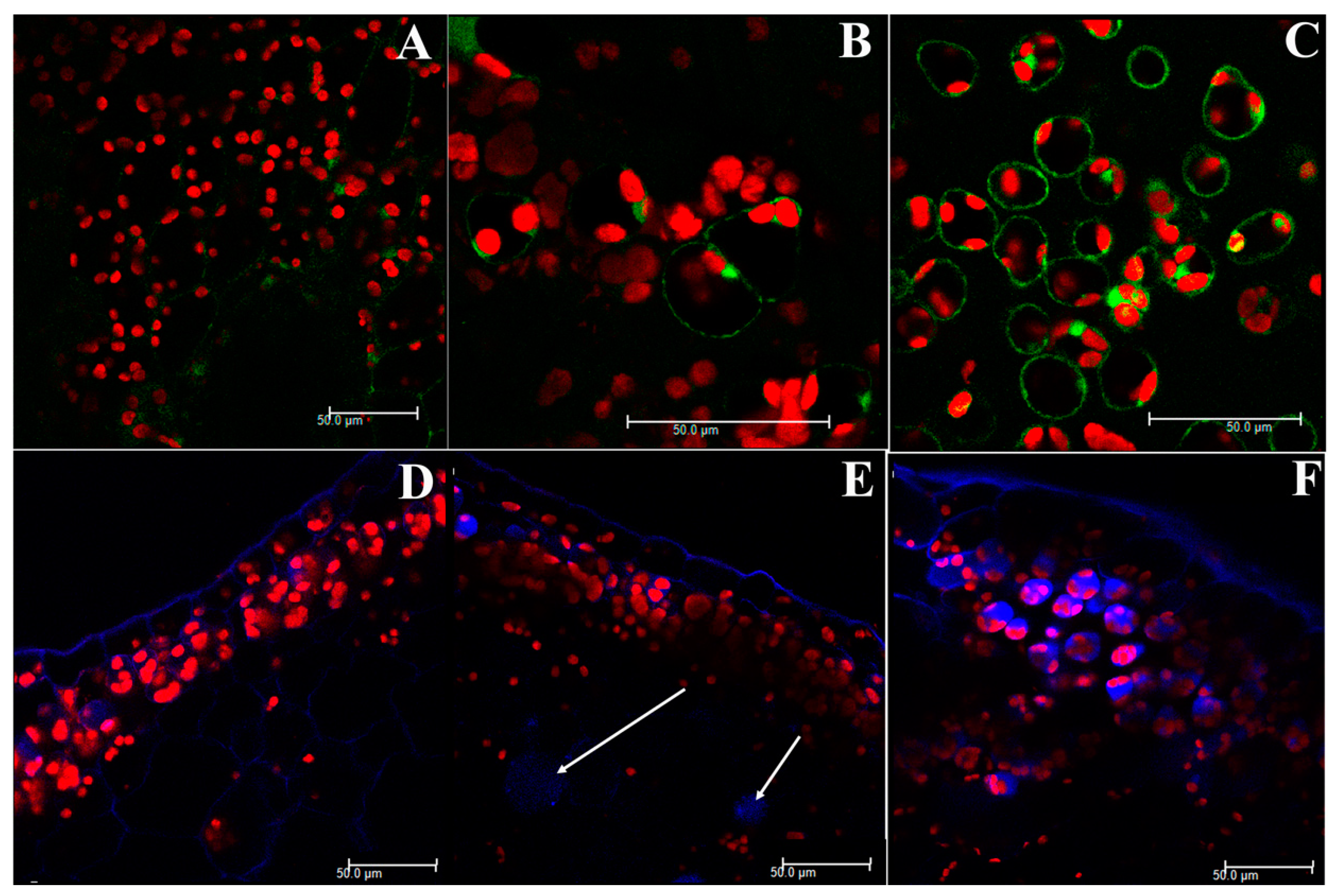
2.2. Confocal Microscopy
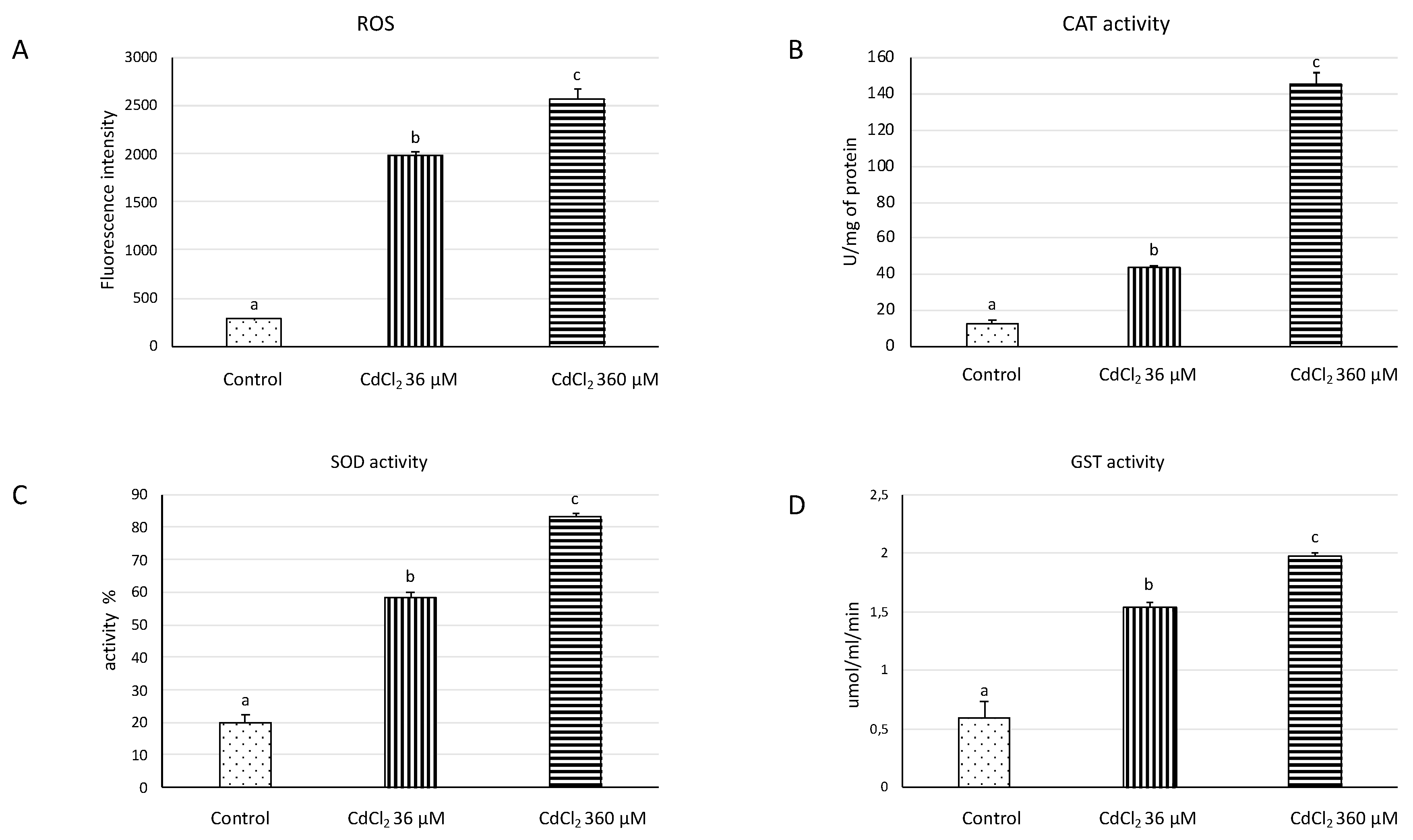
2.3. ROS and Antioxidant Activity Enzyme
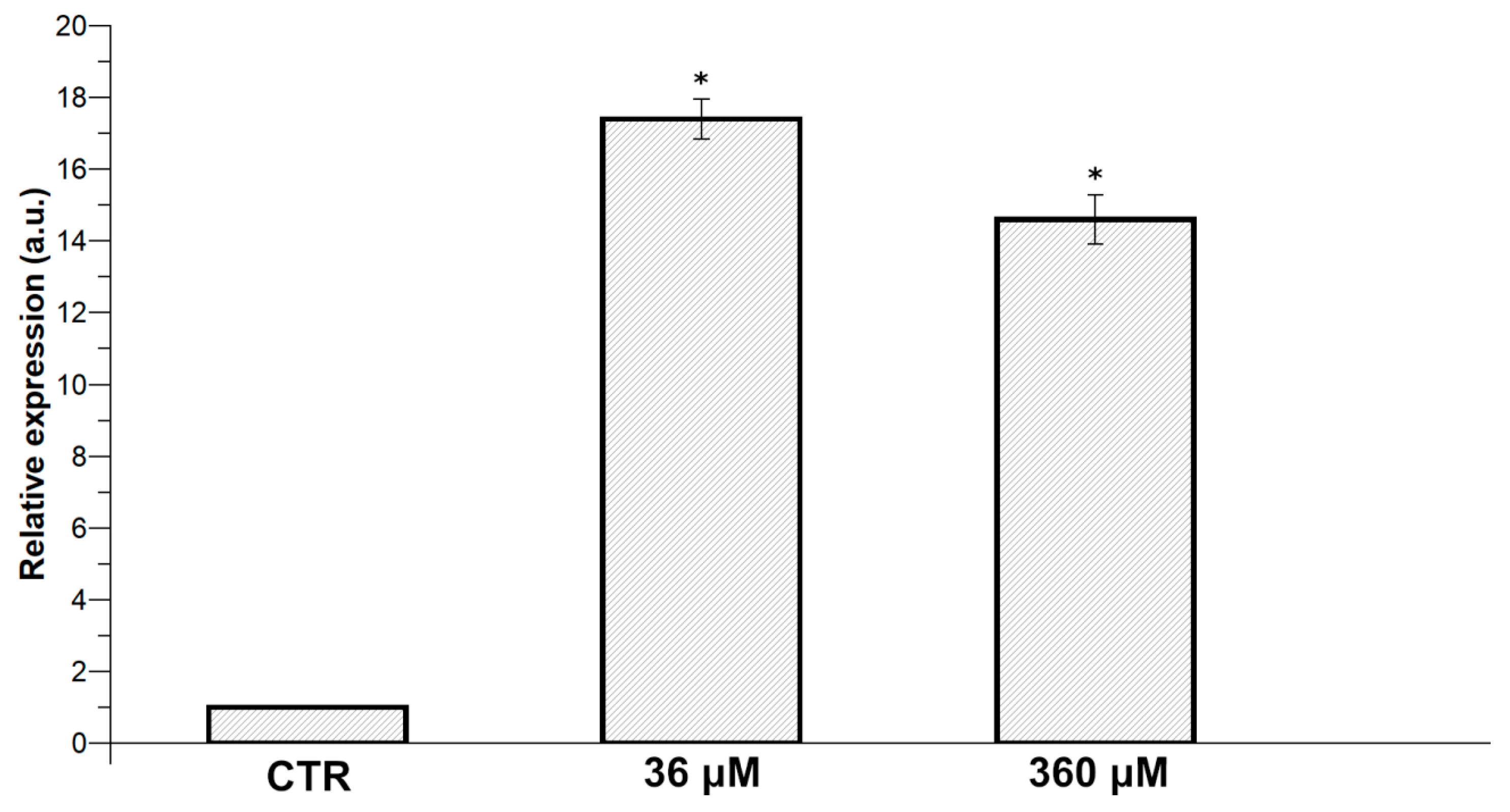
2.4. Expression of Hsp70
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Gametophyte Culture
4.3. Metal Bioaccumulation
4.4. Confocal Microscopy
4.5. Detection of ROS
4.6. Antioxidant Activity Enzyme
4.7. Total RNA Extraction, cDNA Synthesis, and Real-Time qPCR of Hsp70 Expression
4.8. Pearson’s Correlation
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GSH | Glutathione |
| γ–EC | γ–glutamylcysteine |
| GS–bimane | Glutathione–bimane |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GST | Glutathione-S–transferase |
| MCB | Monochlorobimane |
| HSP70 | Heat Shock Proteins 70 |
References
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausió, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the properties of sperm protamine-like II protein after exposure of Mytilus galloprovincialis (Lamarck 1819) to sub-toxic doses of cadmium. Ecotoxicol. Environ. Saf. 2019, 169, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; Brundo, M.V.; Basile, A.; et al. Protamine-like proteins analyses as emerging biotechnique for cadmium impact assessment on male mollusk Mytilus galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M. Seasonal dependence of cadmium molecular effects on Mytilus galloprovincialis (Lamarck, 1819) protamine-like protein properties. Mol. Reprod. Dev. 2019, 86, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Yang, X.; He, Z.; Baligar, V.C. Morphological and Physiological Responses of Plants to Cadmium Toxicity: A Review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- He, S.; He, Z.; Yang, X.; Stoffella, P.J.; Baligar, V.C. Chapter Four—Soil Biogeochemistry, Plant Physiology, and Phytoremediation of Cadmium-Contaminated Soils. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 134, pp. 135–225. [Google Scholar]
- Irfan, M.; Hayat, S.; Ahmad, A.; Alyemeni, M.N. Soil cadmium enrichment: Allocation and plant physiological manifestations. Saudi J. Biol. Sci. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Proctor, J. The influence of cadmium, copper, lead, and zinc on the distribution and evolution of metallophytes in the British Isles. Plant Syst. Evol. 1990, 173, 91–108. [Google Scholar] [CrossRef]
- Assche, F.V.; Clijsters, H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Basile, A.; Alba di Nuzzo, R.; Capasso, C.; Sorbo, S.; Capasso, A.; Carginale, V. Effect of cadmium on gene expression in the liverwort Lunularia cruciata. Gene 2005, 356, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Loppi, S.; Piscopo, M.; Paoli, L.; Vannini, A.; Monaci, F.; Sorbo, S.; Lentini, M.; Esposito, S. The biological response chain to pollution: A case study from the “Italian Triangle of Death” assessed with the liverwort Lunularia cruciata. Environ. Sci. Pollut. Res. 2017, 24, 26185–26193. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Sorbo, S.; Loppi, S.; Funaro, F.; Del Prete, D.; Basile, A. Biological effects from environmental pollution by toxic metals in the “land of fires” (Italy) assessed using the biomonitor species Lunularia cruciata L. (Dum). Environ. Pollut. 2020, 265, 115000. [Google Scholar] [CrossRef]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Sanità di Toppi, L. A Cd/Fe/Zn-Responsive Phytochelatin Synthase is Constitutively Present in the Ancient Liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef] [Green Version]
- Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borsò, M.; Bruno, L.; Sorbo, S.; et al. The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Fan, M.; Hu, R.; Zhao, J.; Wu, Y. Mosses Are Better than Leaves of Vascular Plants in Monitoring Atmospheric Heavy Metal Pollution in Urban Areas. Int. J. Environ. Res. Public Health 2018, 15, 1105. [Google Scholar] [CrossRef] [Green Version]
- Basile, A.; Sorbo, S.; Aprile, G.; Conte, B.; Castaldo Cobianchi, R. Comparison of the heavy metal bioaccumulation capacity of an epiphytic moss and an epiphytic lichen. Environ. Pollut. 2008, 151, 401–407. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Cardi, M.; Esposito, S. Ultrastructural changes and Heat Shock Proteins 70 induced by atmospheric pollution are similar to the effects observed under in vitro heavy metals stress in Conocephalum conicum (Marchantiales-Bryophyta). Environ. Pollut. 2013, 182, 209–216. [Google Scholar] [CrossRef]
- Carginale, V.; Sorbo, S.; Capasso, C.; Trinchella, F.; Cafiero, G.; Basile, A. Accumulation, localisation, and toxic effects of cadmium in the liverwort Lunularia cruciata. Protoplasma 2004, 223, 53–61. [Google Scholar] [CrossRef]
- Dal Corso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy Metal Pollutions: State of the Art and Innovation in Phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piscopo, M.; Ricciardiello, M.; Palumbo, G.; Troisi, J. Selectivity of metal bioaccumulation and its relationship with glutathione S-transferase levels in gonadal and gill tissues of Mytilus galloprovincialis exposed to Ni (II), Cu (II) and Cd (II). Rend. Lincei 2016, 27, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Copat, C.; Conti, G.O.; Signorelli, C.; Marmiroli, S.; Sciacca, S.; Vinceti, M.; Ferrante, M. Risk Assessment for Metals and PAHs by Mediterranean Seafood. Food Nutr. Sci. 2013, 4, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Piscopo, M.; Trifuoggi, M.; Scarano, C.; Gori, C.; Giarra, A.; Febbraio, F. Relevance of arginine residues in Cu(II)-induced DNA breakage and Proteinase K resistance of H1 histones. Sci. Rep. 2018, 8, 7414. [Google Scholar] [CrossRef] [Green Version]
- Leharne, S.; McPhee, E.; Kingston, L. The pattern of metal deposition to a woodland ecosystem as revealed by bryophyte analysis. Environ. Monit. Assess. 1990, 15, 131–141. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; del Río, L.A. Imaging of Reactive Oxygen Species and Nitric Oxide In Vivo in Plant Tissues. Methods Enzymol. 2008, 440, 397–409. [Google Scholar]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Zabalza, A.; Corpas, F.J.; Gómez, M.; Río, L.A.D.; Sandalio, L.M. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006, 29, 1532–1544. [Google Scholar] [CrossRef]
- Shapiro, A.D.; Zhang, C. The Role of NDR1 in Avirulence Gene-Directed Signaling and Control of Programmed Cell Death in Arabidopsis. Plant Physiol. 2001, 127, 1089–1101. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2016, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Transcriptome Analysis in Response to Heat Shock and Cadmium in the Aquatic Fungus Blastocladiella Emersonii|Eukaryotic Cell. Available online: https://ec.asm.org/content/6/6/1053.short (accessed on 28 July 2020).
- Bierkens, J.; Maes, J.; Plaetse, F.V. Dose-dependent induction of heat shock protein 70 synthesis in Raphidocelis subcapitata following exposure to different classes of environmental pollutants. Environ. Pollut. 1998, 101, 91–97. [Google Scholar] [CrossRef]
- Lewis, S.; Handy, R.D.; Cordi, B.; Billinghurst, Z.; Depledge, M.H. Stress proteins (HSP’s): Methods of Detection and Their Use as an Environmental Biomarker. Ecotoxicology 1999, 8, 351–368. [Google Scholar] [CrossRef]
- Ireland, H.E.; Harding, S.J.; Bonwick, G.A.; Jones, M.; Smith, C.J.; Williams, J.H.H. Evaluation of heat shock protein 70 as a biomarker of environmental stress in Fucus serratus and Lemna minor. Biomarkers 2004, 9, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Singh, B. Phylogenetic analysis of 70 kD heat shock protein sequences suggests a chimeric origin for the eukaryotic cell nucleus. Curr. Biol. 1994, 4, 1104–1114. [Google Scholar] [CrossRef]
- Sadura, I.; Libik-Konieczny, M.; Jurczyk, B.; Gruszka, D.; Janeczko, A. HSP Transcript and Protein Accumulation in Brassinosteroid Barley Mutants Acclimated to Low and High Temperatures. Int. J. Mol. Sci. 2020, 21, 1889. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Yang, X.; Hu, G.; Liu, Q.; Li, W.; Zhang, L.; Song, X. Genome-Wide Investigation of Heat Shock Transcription Factor Family in Wheat (Triticum aestivum L.) and Possible Roles in Anther Development. Int. J. Mol. Sci. 2020, 21, 608. [Google Scholar] [CrossRef] [Green Version]
- Boston, R.S.; Viitanen, P.V.; Vierling, E. Molecular chaperones and protein folding in plants. Plant Mol. Biol. 1996, 32, 191–222. [Google Scholar] [CrossRef]
- Molecular Chaperones in Cellular Protein Folding|Nature. Available online: https://www.nature.com/articles/381571a0 (accessed on 30 July 2020).
- Tamás, M.J.; Fauvet, B.; Christen, P.; Goloubinoff, P. Misfolding and aggregation of nascent proteins: A novel mode of toxic cadmium action in vivo. Curr. Genet. 2018, 64, 177–181. [Google Scholar] [CrossRef]
- Sergio, E.; Cobianchi, R.C.; Sorbo, S.; Conte, B.; Basile, A. Ultrastructural alterations and HSP 70 induction in Elodea ca-nadensisMichx. exposed to heavy metals. Caryologia 2007, 60, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Basile, A.; Sorbo, S.; Cardi, M.; Lentini, M.; Castiglia, D.; Cianciullo, P.; Conte, B.; Loppi, S.; Esposito, S. Effects of heavy metals on ultrastructure and Hsp70 induction in Lemna minor L. exposed to water along the Sarno River, Italy. Ecotoxicol. Environ. Saf. 2015, 114, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Conte, B.; Golia, B.; Montanari, S.; Cobianchi, R.C.; Esposito, S. Antioxidant activity in extracts from Leptodictyum riparium (Bryophyta), stressed by heavy metals, heat shock, and salinity. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2011, 145, 77–80. [Google Scholar] [CrossRef]
- Esposito, S.; Sorbo, S.; Conte, B.; Basile, A. Effects of Heavy Metals on Ultrastructure and HSP70S Induction in the Aquatic Moss Leptodictyum Riparium Hedw. Int. J. Phytoremediat. 2012, 14, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Lentini, M.; Conte, B.; Esposito, S. Water pollution causes ultrastructural and functional damages in Pellia neesiana (Gottsche) Limpr. J. Trace Elem. Med. Biol. 2017, 43, 80–86. [Google Scholar] [CrossRef]
- di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Tukaj, S.; Bisewska, J.; Roeske, K.; Tukaj, Z. Time- and Dose-Dependent Induction of HSP70 in Lemna minor Exposed to Different Environmental Stressors. Bull. Environ. Contam. Toxicol. 2011, 87, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Harmens, H.; Norris, D.; Cooper, D.; Hall, J.; Kubin, E.; Piispanen, J.; Poikolainen, J.; Karhu, J.; Metla Kubin, E.; Piispanen, J.; et al. Spatial trends in nitrogen concentrations in mosses across Europe in 2005/2006. In Report on Nitrogen in European Mosses. Work Package 4. The UNECE International Cooperative Programme on Vegetation; Centre for Ecology & Hydrology: Saint Ives, Wales, UK, 2008. [Google Scholar]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausió, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus galloprovincialis (Lamarck, 1819) spermatozoa: hsp70 expression and protamine-like protein property studies. Environ. Sci. Pollut. Res. 2018, 25, 12957–12966. [Google Scholar] [CrossRef]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular effects of copper on the reproductive system of mytilus galloprovincialis. Mol. Reprod. Dev. 2019, 86, 1357–1368. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Cd | Fe | Pb | Hg | Sb | As | Cu | Zn | |
|---|---|---|---|---|---|---|---|---|
| No treatment | n.d. | 83.3 ± 2.4 | 6.2 ± 0.3 | n.d. | n.d. | n.d. | 19.2 ± 2.3 | 32.7 ± 2.8 |
| 36 µM Cd | 162.53 ± 3.9 | 89.1 ± 3.9 | 5.8 ± 0.6 | n.d. | n.d. | n.d. | 23.5 ± 1.8 | 36.4 ± 2.5 |
| 360 µM Cd | 2371 ± 19 | 78.8 ± 5.4 | 6.6 ± 0.5 | n.d. | n.d. | n.d. | 18.7 ± 2.3 | 34.2 ± 3.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maresca, V.; Lettieri, G.; Sorbo, S.; Piscopo, M.; Basile, A. Biological Responses to Cadmium Stress in Liverwort Conocephalum conicum (Marchantiales). Int. J. Mol. Sci. 2020, 21, 6485. https://doi.org/10.3390/ijms21186485
Maresca V, Lettieri G, Sorbo S, Piscopo M, Basile A. Biological Responses to Cadmium Stress in Liverwort Conocephalum conicum (Marchantiales). International Journal of Molecular Sciences. 2020; 21(18):6485. https://doi.org/10.3390/ijms21186485
Chicago/Turabian StyleMaresca, Viviana, Gennaro Lettieri, Sergio Sorbo, Marina Piscopo, and Adriana Basile. 2020. "Biological Responses to Cadmium Stress in Liverwort Conocephalum conicum (Marchantiales)" International Journal of Molecular Sciences 21, no. 18: 6485. https://doi.org/10.3390/ijms21186485
APA StyleMaresca, V., Lettieri, G., Sorbo, S., Piscopo, M., & Basile, A. (2020). Biological Responses to Cadmium Stress in Liverwort Conocephalum conicum (Marchantiales). International Journal of Molecular Sciences, 21(18), 6485. https://doi.org/10.3390/ijms21186485









