Regulation of Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Activity of Advanced Cooling Composition (ACC) in UVB-Irradiated Human HaCaT Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
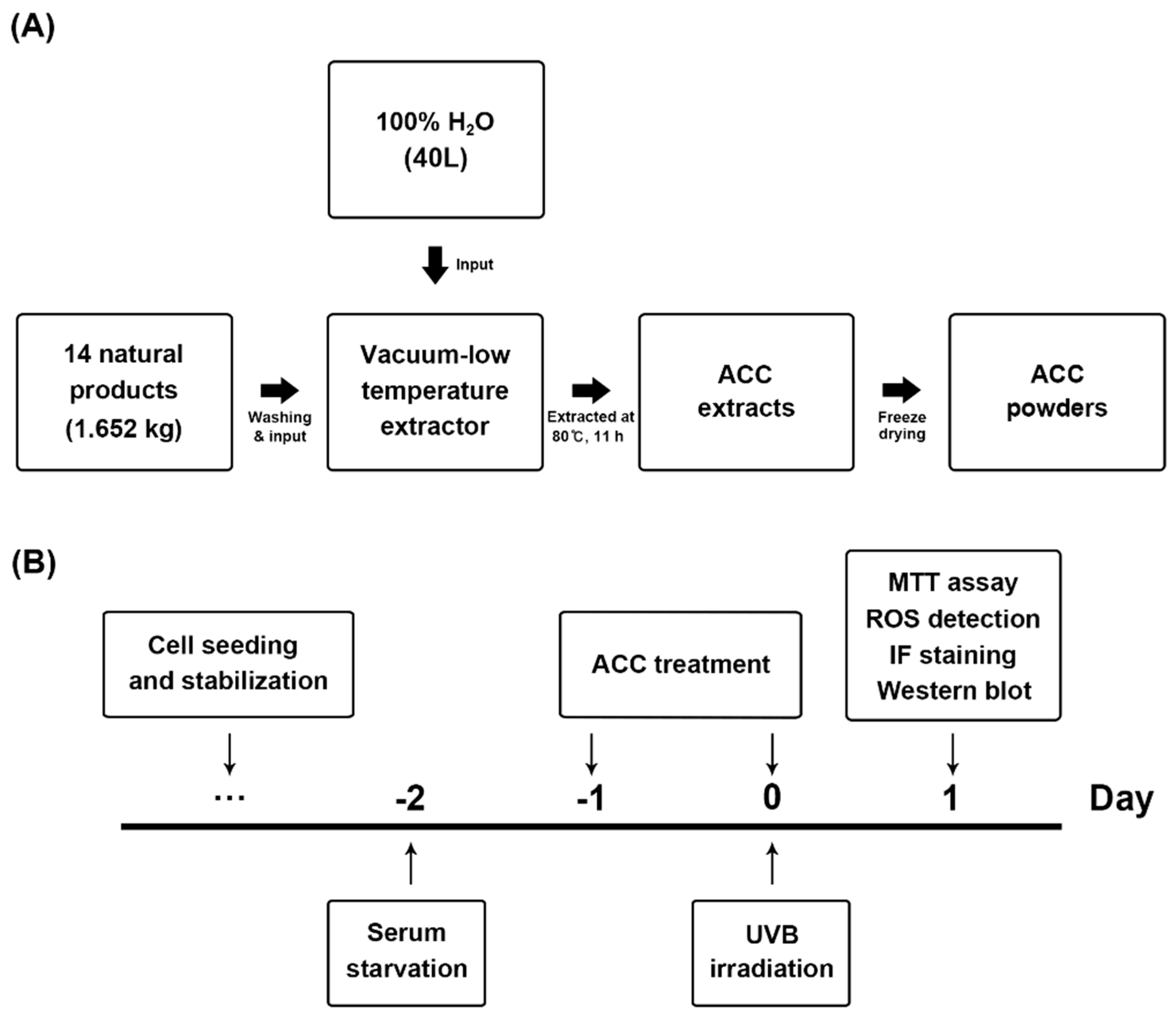
2.1. Preparation of ACC
2.2. Constitutions Analysis with HPLC
2.3. Cell Culture
2.4. UVB Irradiation
2.5. Cell Viability Assay
2.6. DPPH Radical Scavenging Assay
2.7. Intracellular ROS Detection
2.8. Immunofluorescence Staining Procedure
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Identification of Active Compounds from ACC
3.2. Cell Viability after UVB Irradiation in ACC-Treated HaCaT Cells
3.3. Attenuation of UVB-Induced Intracellular ROS in ACC-Treated HaCaT Cells
3.4. Effect of ACC on the Downregulation of the Pro-Inflammatory Cytokines, COX-2, and iNOS in UVB-Irradiated HaCaT Cells
3.5. Regulation of JNK/p38/ERK Signaling Pathways by ACC Pretreatment
3.6. Inhibition Effect of ACC in UVB-Induced Pro-Apoptotic Molecules
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Yeom, M.; Kim, S.-H.; Lee, B.; Han, J.-J.; Chung, G.H.; Choi, H.-D.; Lee, H.; Hahm, D.-H. Oral administration of glucosylceramide ameliorates inflammatory dry-skin condition in chronic oxazolone-induced irritant contact dermatitis in the mouse ear. J. Dermatol. Sci. 2012, 67, 101–110. [Google Scholar] [CrossRef]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Hyun, Y.J.; Cho, S.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Ko, M.H.; Hyun, J.W. An ethanol extract derived from Bonnemaisonia hamifera scavenges ultraviolet B (UVB) radiation-induced reactive oxygen species and attenuates UVB-induced cell damage in human keratinocytes. Mar. Drugs 2012, 10, 2826–2845. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Skin cancer and solar UV radiation. Eur. J. Cancer 1999, 35, 2003–2009. [Google Scholar] [CrossRef]
- Halliday, G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef]
- Rafferty, T.S.; Beckett, G.J.; Walker, C.; Bisset, Y.C.; McKenzie, R.C. Selenium protects primary human keratinocytes from apoptosis induced by exposure to ultraviolet radiation. Clin. Exp. Dermatol. 2003, 28, 294–300. [Google Scholar] [CrossRef]
- Tsang, M.S.; Jiao, D.; Chan, B.C.; Hon, K.-L.; Leung, P.C.; Lau, C.B.; Wong, E.C.; Cheng, L.; Chan, C.K.; Lam, C.W.; et al. Anti-Inflammatory Activities of Pentaherbs Formula, Berberine, Gallic Acid and Chlorogenic Acid in Atopic Dermatitis-Like Skin Inflammation. Molecules 2016, 21, 519. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.Y.; Kim, M.H.; Han, J.M.; Hong, J.; Lee, T.-H.; Kim, S.-H.; Yang, W.M. The anti-inflammatory potential of Cortex Phellodendron in vivo and in vitro: Down-regulation of NO and iNOS through suppression of NF-κB and MAPK activation. Int. Immunopharmacol. 2014, 19, 214–220. [Google Scholar] [CrossRef]
- Dinesh, P.; Rasool, M. Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int. Immunopharmacol. 2017, 44, 26–37. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Liu, W.-B.; Zhou, M.; Dai, Y.-J.; Xu, C.; Tian, H.-Y.; Xu, W.-N. Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish. Immunol. 2016, 55, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, X.; Li, H.; Yuan, J.; Peng, Y.; Dong, L.; Dai, S. Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed. Pharmacother. 2016, 84, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ning, M.; Yang, G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 2012, 17, 4672–4683. [Google Scholar] [CrossRef] [Green Version]
- Ngan, L.T.; Moon, J.-K.; Shibamoto, T.; Ahn, Y.-J. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J. Agric. Food Chem. 2012, 60, 9062–9073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Nakano, Y.; Rahman, M.A.; Yatsuzuka, R.; Kamei, C. Effects of a Dictamnus dasycarpus T. extract on allergic models in mice. Biosci. Biotechnol. Biochem. 2008, 72, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; Di Paola, R.; Suzuki, H.; Paterniti, I.; Ahmad, A.; Mariotto, S.; Cuzzocrea, S. Costunolide and Dehydrocostuslactone, two natural sesquiterpene lactones, ameliorate the inflammatory process associated to experimental pleurisy in mice. Eur. J. Pharmacol. 2014, 730, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bachelier, A.; Mayer, R.; Klein, C.D. Sesquiterpene lactones are potent and irreversible inhibitors of the antibacterial target enzyme MurA. Bioorg. Med. Chem. Lett. 2006, 16, 5605–5609. [Google Scholar] [CrossRef]
- Ryu, J.-H.; An, J.-H.; Woo, Y.-K.; Cho, H.-J. The anti-inflammation effects of A.C.C. extracts on the LPS-induced Raw 264.7 cell. J. Korea Acad. Industr. Coop. Soc. 2017, 18, 503–511. [Google Scholar]
- Kalivendi, S.V.; Kotamraju, S.; Cunningham, S.; Shang, T.; Hillard, C.J.; Kalyanaraman, B. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: Role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem. J. 2003, 371, 151–164. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickoloff, B.J.; Turka, L.A. Keratinocytes: Key immunocytes of the integument. Am. J. Pathol. 1993, 143, 325–331. [Google Scholar]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ma, H.; Fan, J.; Li, Y.; Wang, J.; Ni, H.; Xia, G.; Chen, S. Crude polysaccharide from an anti-UVB cell clone of Bupleurum scorzonerifolium protect HaCaT cells against UVB-induced oxidative stress. Cytotechnology 2011, 63, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soter, N.A. Acute effects of ultraviolet radiation on the skin. Semin. Dermatol. 1990, 9, 11–15. [Google Scholar] [PubMed]
- Afaq, F.; Mukhtar, H. Effects of solar radiation on cutaneous detoxification pathways. J. Photochem. Photobiol. B 2001, 63, 61–69. [Google Scholar] [CrossRef]
- Paz, M.L.; González Maglio, D.H.; Weill, F.S.; Bustamante, J.; Leoni, J. Mitochondrial dysfunction and cellular stress progression after ultraviolet B irradiation in human keratinocytes. Photodermatol. Photoimmunol. Photomed. 2008, 24, 115–122. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [Green Version]
- Reich, A.; Mędrek, K. Effects of narrow band UVB (311 nm) irradiation on epidermal cells. Int. J. Mol. Sci. 2013, 14, 8456–8466. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S. The roles of keratinocyte-derived cytokines in the epidermis and their possible responses to UVA-irradiation. J. Investig. Dermatol. Symp. Proc. 1999, 4, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.J.; Collier, J.J. The gene encoding cyclooxygenase-2 is regulated by IL-1β and prostaglandins in 832/13 rat insulinoma cells. Cell. Immunol. 2011, 271, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Choi, H.G.; Chung, H.J.; Hong, C.K. Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by ultraviolet B in keratinocyte cell lines. Br. J. Dermatol. 2002, 147, 655–662. [Google Scholar] [CrossRef]
- Frank, S.; Kämpfer, H.; Podda, M.; Kaufmann, R.; Pfeilschifter, J. Identification of copper/zinc superoxide dismutase as a nitric oxide-regulated gene in human (HaCaT) keratinocytes: Implications for keratinocyte proliferation. Biochem. J. 2000, 346, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.S.; Seo, S.J.; Hong, C.K. The effect of amniotic membrane extract on the expression of iNOS mRNA and generation of NO in HaCaT cell by ultraviolet B irradiation. Photodermatol. Photoimmunol. Photomed. 2002, 18, 280–286. [Google Scholar] [CrossRef]
- Lewis, T.S.; Shapiro, P.S.; Ahn, N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 1998, 74, 49–139. [Google Scholar] [PubMed]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- López-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Pérez-Plasencia, C.; Alvarez-Sánchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef]
- Kim, B.-M.; Rhee, J.-S.; Lee, K.-W.; Kim, M.-J.; Shin, K.-H.; Lee, S.-J.; Lee, Y.-M.; Lee, J.-S. UV-B radiation-induced oxidative stress and p38 signaling pathway involvement in the benthic copepod Tigriopus japonicus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 167, 15–23. [Google Scholar] [CrossRef]
- Ashida, M.; Bito, T.; Budiyanto, A.; Ichihashi, M.; Ueda, M. Involvement of EGF receptor activation in the induction of cyclooxygenase-2 in HaCaT keratinocytes after UVB. Exp. Dermatol. 2003, 12, 445–452. [Google Scholar] [CrossRef]
- Kim, A.L.; Labasi, J.M.; Zhu, Y.; Tang, X.; McClure, K.; Gabel, C.A.; Athar, M.; Bickers, D.R. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J. Investig. Dermatol. 2005, 124, 1318–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Tang, Q.; Gonzales, M.S.; Bowden, G.T. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene 2001, 20, 3921–3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impey, S.; Obrietan, K.; Storm, D.R. Making new connections: Role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 1999, 23, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Kolch, W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 2000, 351, 289–305. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.-X.; Zhao, Y.; Brautigan, D.L.; Zhang, Z.-Y. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J. Biol. Chem. 2002, 277, 31818–31825. [Google Scholar] [CrossRef] [Green Version]
- Tantini, B.; Pignatti, C.; Fattori, M.; Fiumana, E.; Facchini, A.; Stefanelli, C.; Caldarera, C.M.; Pegg, A.E.; Flamigni, F. Polyamine depletion inhibits etoposide-induced NF-kappaB activation in transformed mouse fibroblasts. Amino Acids 2004, 27, 207–214. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Mathov, I.; Aguirre, J.I.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Mechanical stimulation prevents osteocyte apoptosis: Requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell Physiol. 2005, 289, C633–C643. [Google Scholar] [CrossRef] [Green Version]
- Marshall, C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 1999, 11, 732–736. [Google Scholar] [CrossRef]
- MacKeigan, J.P.; Collins, T.S.; Ting, J.P.-Y. MEK inhibition enhances paclitaxel-induced tumor apoptosis. J. Biol. Chem. 2000, 275, 38953–38956. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Investig. 1998, 101, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Henseleit, U.; Zhang, J.; Wanner, R.; Haase, I.; Kolde, G.; Rosenbach, T. Role of p53 in UVB-induced apoptosis in human HaCaT keratinocytes. J. Investig. Dermatol. 1997, 109, 722–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.-E.; Wani, G.; Patnaik, S.; Zhao, Q.; Arafa, E.-S.; Barakat, B.; Mir, S.N.; Wani, A.A. Naringenin Protects HaCaT Human Keratinocytes Against UVB-induced Apoptosis and Enhances the Removal of Cyclobutane Pyrimidine Dimers from the Genome†. Photochem. Photobiol. 2008, 84, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Sitailo, L.A.; Tibudan, S.S.; Denning, M.F. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J. Biol. Chem. 2002, 277, 19346–19352. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Brooks, C.; Wei, Q.; Feng, L.; Dong, G.; Tao, Y.; Mei, L.; Xie, Z.-J.; Dong, Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc. Natl. Acad. Sci. USA 2007, 104, 11649–11654. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-K.; Jang, B.-C. UVB-induced anti-survival and pro-apoptotic effects on HaCaT human keratinocytes via caspase- and PKC-dependent downregulation of PKB, HIAP-1, Mcl-1, XIAP and ER stress. Int. J. Mol. Med. 2014, 33, 695–702. [Google Scholar] [CrossRef] [Green Version]







| Materials | Proportion (%) |
|---|---|
| Phellodendron bark | 24 |
| Scutellaria baicalensis | 5 |
| Paeonia lactiflora Pall. | 12 |
| Dictamnus dasycarpus Turcz. | 12 |
| Anemarrhena asphodeloides | 6 |
| Alumen | 12 |
| Dryobalanops aromatica Gaertner | 2 |
| Mentha arvensis var. piperascens | 4 |
| Inula helenium | 3 |
| Syringa velutina var. kamibayashi | 1 |
| Corydalis incisa | 6 |
| Eclipta prostrata | 6 |
| Lonicera japonica | 1 |
| Glycyrrhiza uralensis | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Woo, Y.-K.; Cho, H.-J. Regulation of Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Activity of Advanced Cooling Composition (ACC) in UVB-Irradiated Human HaCaT Keratinocytes. Int. J. Mol. Sci. 2020, 21, 6527. https://doi.org/10.3390/ijms21186527
Park J, Woo Y-K, Cho H-J. Regulation of Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Activity of Advanced Cooling Composition (ACC) in UVB-Irradiated Human HaCaT Keratinocytes. International Journal of Molecular Sciences. 2020; 21(18):6527. https://doi.org/10.3390/ijms21186527
Chicago/Turabian StylePark, Jungha, Yong-Kyu Woo, and Hyun-Jeong Cho. 2020. "Regulation of Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Activity of Advanced Cooling Composition (ACC) in UVB-Irradiated Human HaCaT Keratinocytes" International Journal of Molecular Sciences 21, no. 18: 6527. https://doi.org/10.3390/ijms21186527





