Bacterial Cellulose—Graphene Based Nanocomposites
Abstract
1. Introduction
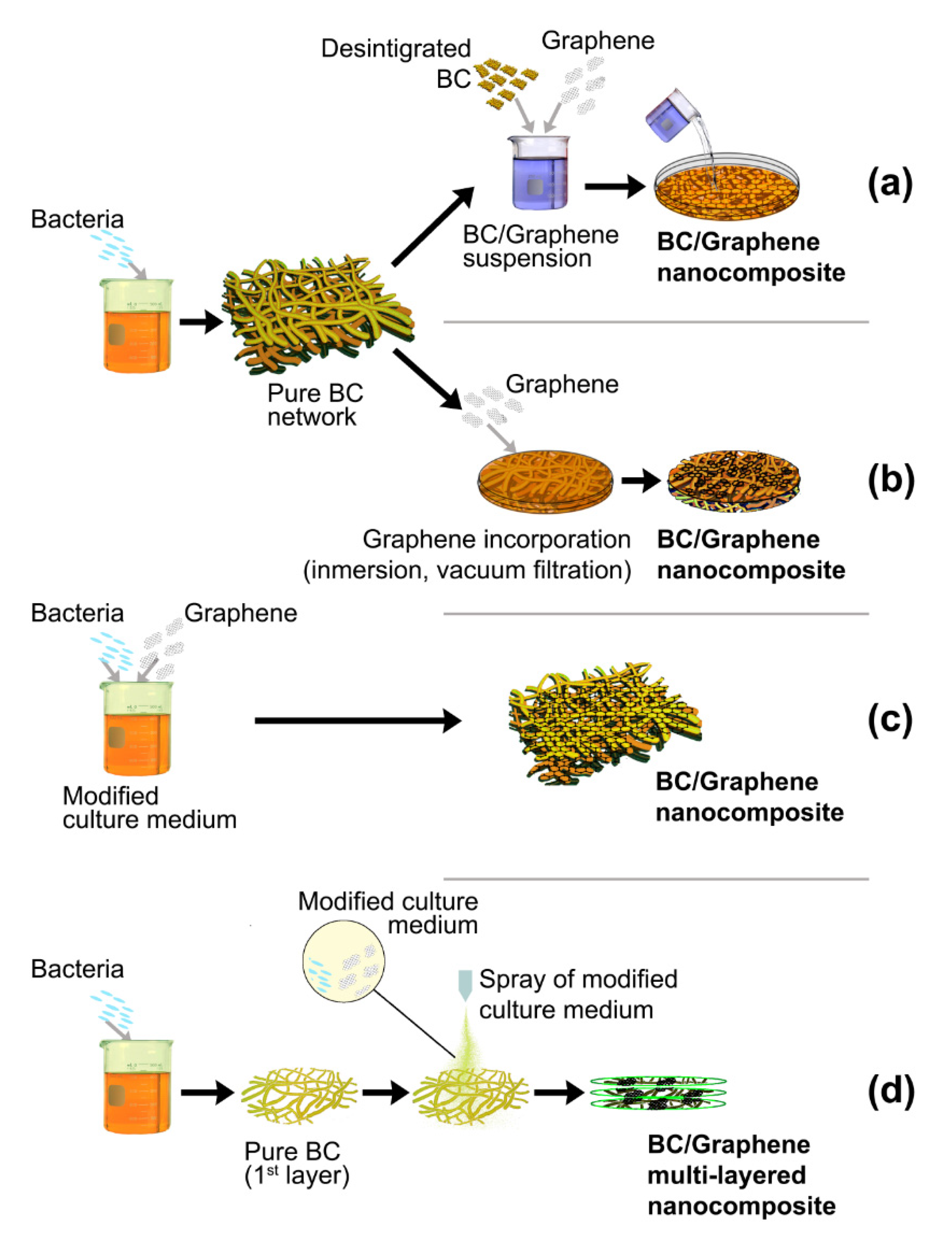
2. Processing Routes
3. Applications of Bacterial Cellulose-Graphene Nanocomposites
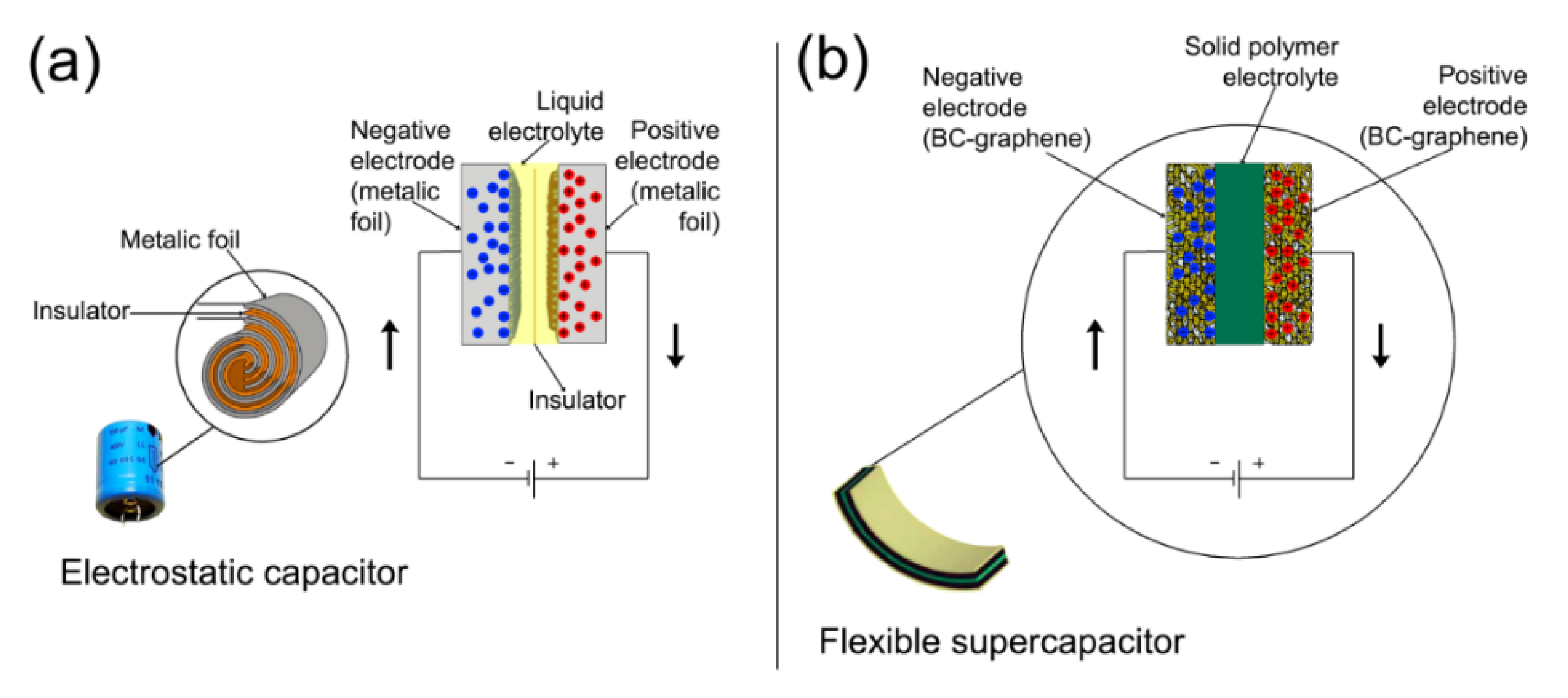
3.1. Electronic and Energy Storage Devices
3.2. Sorbent Nanocomposites for Water Purification
3.3. Bacterial Cellulose-Graphene Nanocomposites for Biomedical Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BC | Bacterial cellulose |
| CNT | Carbon nanotubes |
| DCC | Dicy clohexyl carbodiimide |
| DMF | N,N-dimethyl formamide |
| EDLC | Electrochemical double-layer capacitor |
| GFN | Graphene family nanomaterials |
| GO | Graphene oxide |
| N-GOQD | Nitrogen doped graphene oxide quantum dots |
| PANI | Polyaniline |
| PEDOT | Poly-3,4-ethylenedioxythiophene |
| RGO | Reduced graphene oxide |
| TEMPO-BC | 2,2,6,6-tetramethylpiperidine-1-oxyl radical-oxidized bacterial cellulose |
References
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Cannon, R.E.; Anderson, S.M. Biogenesis of Bacterial Cellulose. Crit. Rev. Microbiol. 1991, 17, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial Cellulose Nanocomposites: An All-Nano Type of Material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Feng, L.; Wu, L.; Qu, X. New Horizons for Diagnostics and Therapeutic Applications of Graphene and Graphene Oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Mayorov, A.S.; Elias, D.C.; Mukhin, I.S.; Morozov, S.V.; Ponomarenko, L.A.; Novoselov, K.S.; Geim, A.K.; Gorbachev, R.V. How Close Can One Approach the Dirac Point in Graphene Experimentally? Nano Lett. 2012, 12, 4629–4634. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Three-Dimensional Graphene Architectures. Nanoscale 2012, 4, 5549. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Yan, M.; Lu, J.; Zhang, M.; Yu, F.; Yu, J.; Song, X.; Yu, S. Electrochemical Biosensor Based on Graphene Oxide–Au Nanoclusters Composites for l-Cysteine Analysis. Biosens. Bioelectron. 2012, 31, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon (N. Y.) 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Highly Conductive Chemically Converted Graphene Prepared from Mildly Oxidized Graphene Oxide. J. Mater. Chem. 2011, 21, 7376. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile Synthesis and Characterization of Graphene Nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene Production via Electrochemical Reduction of Graphene Oxide: Synthesis and Characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z.; Xie, Y.; Xu, H.; Zhu, J.; Zhang, X.; Liu, W.; Yang, G.; Ma, J.; Liu, Y. Photocatalytic Reduction of Graphene Oxide-TiO2 Nanocomposites for Improving Resistive-Switching Memory Behaviors. Small 2018, 14, 1801325. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis Route of Reduced Graphene Oxide via Thermal Reduction of Chemically Exfoliated Graphene Oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Alternative Methods and Nature-Based Reagents for the Reduction of Graphene Oxide: A Review. Carbon (N. Y.) 2015, 94, 224–242. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental Review: Chemical Reduction of Graphene Oxide (GO) to Reduced Graphene Oxide (RGO) by Aqueous Chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
- Guhados, G.; Wan, W.; Hutter, J.L. Measurement of the Elastic Modulus of Single Bacterial Cellulose Fibers Using Atomic Force Microscopy. Langmuir 2005, 21, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial Cellulose—A Masterpiece of Nature’s Arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Tang, W.; Jia, S.; Jia, Y.; Yang, H. The Influence of Fermentation Conditions and Post-Treatment Methods on Porosity of Bacterial Cellulose Membrane. World J. Microbiol. Biotechnol. 2010, 26, 125–131. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Chen, C.; Chen, X.; Huang, Y.; Sun, D. In Situ Controllable Fabrication of Porous Bacterial Cellulose. Mater. Lett. 2019, 249, 104–107. [Google Scholar] [CrossRef]
- Hu, Y.H.; Wang, H.; Hu, B. Thinnest Two-Dimensional Nanomaterial-Graphene for Solar Energy. ChemSusChem 2010, 3, 782–796. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and Extrinsic Performance Limits of Graphene Devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Lopez, D.; Grande, C.; Gomez, C.M. Reversible Stress Softening and Stress Recovery of Cellulose Networks. Soft Matter 2009, 5, 4185. [Google Scholar] [CrossRef]
- Thompson, M.A.; Onyeziri, M.C.; Fuqua, C. Function and Regulation of Agrobacterium Tumefaciens Cell Surface Structures That Promote Attachment. In Agrobacterium Biology. Current Topics in Microbiology and Immunology; Gelvin, S., Ed.; Springer: Cham, Switzerland, 2018; pp. 143–184. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In Situ and Ex Situ Modifications of Bacterial Cellulose for Applications in Tissue Engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Tanskul, S.; Amornthatree, K.; Jaturonlak, N. A New Cellulose-Producing Bacterium, Rhodococcus Sp. MI 2: Screening and Optimization of Culture Conditions. Carbohydr. Polym. 2013, 92, 421–428. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of Bacterial Cellulose Using Different Carbon Sources and Culture Media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical Structure Variations of Bacterial Cellulose Produced by Different Komagataeibacter Xylinus Strains and Carbon Sources in Static and Agitated Conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Chang, Y.; Zhou, L.; Xiao, Z.; Liang, J.; Kong, D.; Li, Z.; Zhang, X.; Li, X.; Zhi, L. Embedding Reduced Graphene Oxide in Bacterial Cellulose-Derived Carbon Nanofibril Networks for Supercapacitors. ChemElectroChem 2017, 4, 2448–2452. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, X.; Deng, W.; Zheng, Z.; Liu, Z. Freestanding Bacterial Cellulose-Graphene Oxide Composite Membranes with High Mechanical Strength for Selective Ion Permeation. Sci. Rep. 2016, 6, 33185. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Tang, J.; Tang, W. Three-Dimensional, Chemically Bonded Polypyrrole/Bacterial Cellulose/Graphene Composites for High-Performance Supercapacitors. Chem. Mater. 2015, 27, 7034–7041. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Niu, H.; Zhao, M.; Huang, Y. Flexible and Freestanding Electrode Based on Polypyrrole/Graphene/Bacterial Cellulose Paper for Supercapacitor. Compos. Sci. Technol. 2016, 137, 87–93. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Zhang, R. Anti-Bacterial Performances and Biocompatibility of Bacterial Cellulose/Graphene Oxide Composites. RSC Adv. 2015, 5, 4795–4803. [Google Scholar] [CrossRef]
- Shao, W.; Wang, S.; Liu, H.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Preparation of Bacterial Cellulose/Graphene Nanosheets Composite Films with Enhanced Mechanical Performances. Carbohydr. Polym. 2016, 138, 166–171. [Google Scholar] [CrossRef]
- Shen, Y.-D.; Xiao, Z.-C.; Miao, L.-X.; Kong, D.-B.; Zheng, X.-Y.; Chang, Y.-H.; Zhi, L.-J. Pyrolyzed Bacterial Cellulose/Graphene Oxide Sandwich Interlayer for Lithium–Sulfur Batteries. Rare Met. 2017, 36, 418–424. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Zhu, E.; Tang, J.; Liu, X.; Tang, W. Facile Synthesis of Bacterial Cellulose Fibres Covalently Intercalated with Graphene Oxide by One-Step Cross-Linking for Robust Supercapacitors. J. Mater. Chem. C 2015, 3, 1011–1017. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Yang, Z.; Raman, S.R.; Si, H.; Wan, Y. A Novel Three-Dimensional Graphene/Bacterial Cellulose Nanocomposite Prepared by in Situ Biosynthesis. RSC Adv. 2014, 4, 14369–14372. [Google Scholar] [CrossRef]
- Sanchis, M.J.; Carsí, M.; Gómez, C.M.; Culebras, M.; Gonzales, K.N.; Torres, F.G. Monitoring Molecular Dynamics of Bacterial Cellulose Composites Reinforced with Graphene Oxide by Carboxymethyl Cellulose Addition. Carbohydr. Polym. 2017, 157, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, W.; He, Y.; Duan, T. In-Situ Biopreparation of Biocompatible Bacterial Cellulose/Graphene Oxide Composites Pellets. Appl. Surf. Sci. 2015, 338, 22–26. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, Z.; Kuddannaya, S.; Wu, D.; Zhang, Y.; Wang, Z. Biocompatible, Free-Standing Film Composed of Bacterial Cellulose Nanofibers–Graphene Composite. ACS Appl. Mater. Interfaces 2016, 8, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Schlufter, K.; Schmauder, H.-P.; Dorn, S.; Heinze, T. Efficient Homogeneous Chemical Modification of Bacterial Cellulose in the Ionic Liquid 1-N-Butyl-3-Methylimidazolium Chloride. Macromol. Rapid Commun. 2006, 27, 1670–1676. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, F.; Li, C.; Xiong, G.; Zhu, Y.; Luo, H. Facile and Scalable Production of Three-Dimensional Spherical Carbonized Bacterial Cellulose/Graphene Nanocomposites with a Honeycomb-like Surface Pattern as Potential Superior Absorbents. J. Mater. Chem. A 2015, 3, 24389–24396. [Google Scholar] [CrossRef]
- Luo, H.; Dong, J.; Zhang, Y.; Li, G.; Guo, R.; Zuo, G.; Ye, M.; Wang, Z.; Yang, Z.; Wan, Y. Constructing 3D Bacterial Cellulose/Graphene/Polyaniline Nanocomposites by Novel Layer-by-Layer in Situ Culture toward Mechanically Robust and Highly Flexible Freestanding Electrodes for Supercapacitors. Chem. Eng. J. 2018, 334, 1148–1158. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Xiong, L.; Zhu, Y.; Yang, Z.; Wan, Y. Fabrication of Flexible, Ultra-Strong and Highly Conductive Bacterial Cellulose-Based Paper by Engineering Dispersion of Graphene Nanosheets. Compos. Part B Eng. 2019, 162, 484–490. [Google Scholar] [CrossRef]
- Dhar, P.; Pratto, B.; Gonçalves Cruz, A.J.; Bankar, S. Valorization of Sugarcane Straw to Produce Highly Conductive Bacterial Cellulose/Graphene Nanocomposite Films through in Situ Fermentation: Kinetic Analysis and Property Evaluation. J. Clean. Prod. 2019, 238, 117859. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, T.; Zhang, Q.; Chen, X.; Zhu, C.; Xu, Y.; Yang, J.; Liu, J.; Sun, D. Biointerface by Cell Growth on Graphene Oxide Doped Bacterial Cellulose/Poly(3,4-Ethylenedioxythiophene) Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 10183–10192. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Yang, Z.; Ao, H.; Xiong, L.; Luo, H. Simultaneously Depositing Polyaniline onto Bacterial Cellulose Nanofibers and Graphene Nanosheets toward Electrically Conductive Nanocomposites. Curr. Appl. Phys. 2018, 18, 933–940. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Niu, H.; Wang, F.; Liu, L.; Huang, Y. Freestanding Conductive Film Based on Polypyrrole/Bacterial Cellulose/Graphene Paper for Flexible Supercapacitor: Large Areal Mass Exhibits Excellent Areal Capacitance. Electrochim. Acta 2016, 222, 429–437. [Google Scholar] [CrossRef]
- Yang, X.-N.; Xue, D.-D.; Li, J.-Y.; Liu, M.; Jia, S.-R.; Chu, L.-Q.; Wahid, F.; Zhang, Y.-M.; Zhong, C. Improvement of Antimicrobial Activity of Graphene Oxide/Bacterial Cellulose Nanocomposites through the Electrostatic Modification. Carbohydr. Polym. 2016, 136, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Kokabi, M.; Mousavi, S.M. Conductive Network Formation in Bacterial Cellulose-Based Nanocomposite Aerogels. Compos. Part B Eng. 2019, 174, 106981. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Wang, J.; Yao, F.; Yang, Z.; Wan, Y. Step-by-Step Self-Assembly of 2D Few-Layer Reduced Graphene Oxide into 3D Architecture of Bacterial Cellulose for a Robust, Ultralight and Recyclable All-Carbon Absorbent. Carbon (N. Y.) 2018, 139, 824–832. [Google Scholar] [CrossRef]
- Pinto, S.C.; Gonçalves, G.; Sandoval, S.; López-Periago, A.M.; Borras, A.; Domingo, C.; Tobias, G.; Duarte, I.; Vicente, R.; Marques, P.A.A.P. Bacterial Cellulose/Graphene Oxide Aerogels with Enhanced Dimensional and Thermal Stability. Carbohydr. Polym. 2020, 230, 115598. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A.; Khan, W.U. A Review of Graphene Oxide, Graphene Buckypaper and Polymer/Graphene Composites: Properties and Fabrication Techniques. J. Plast. Film Sheeting 2016, 32, 336–379. [Google Scholar] [CrossRef]
- Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: A Review. Nanoscale Res. Lett. 2017, 12, 387. [Google Scholar] [CrossRef]
- Wei, T.; Luo, G.; Fan, Z.; Zheng, C.; Yan, J.; Yao, C.; Li, W.; Zhang, C. Preparation of Graphene Nanosheet/Polymer Composites Using in Situ Reduction–Extractive Dispersion. Carbon (N. Y.) 2009, 47, 2296–2299. [Google Scholar] [CrossRef]
- Wang, T.; Liang, G.; Yuan, L.; Gu, A. Unique Hybridized Graphene and Its High Dielectric Constant Composites with Enhanced Frequency Stability, Low Dielectric Loss and Percolation Threshold. Carbon (N. Y.) 2014, 77, 920–932. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Mahanta, N.; Valiyaveettil, S. Flexible Conductive Graphene/Poly(Vinyl Chloride) Composite Thin Films with High Mechanical Strength and Thermal Stability. Carbon (N. Y.) 2011, 49, 198–205. [Google Scholar] [CrossRef]
- Allaoui, A.; Hoa, S.V.; Pugh, M.D. The Electronic Transport Properties and Microstructure of Carbon Nanofiber/Epoxy Composites. Compos. Sci. Technol. 2008, 68, 410–416. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Bernal, M.M.; Hernandez, M.; Verdejo, R.; Lopez-Manchado, M.A. Comparison of Filler Percolation and Mechanical Properties in Graphene and Carbon Nanotubes Filled Epoxy Nanocomposites. Eur. Polym. J. 2013, 49, 1347–1353. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Shenai, P.M.; Kovalev, E.; Baudot, C.; Mathews, N.; Zhao, Y. Characteristics of the Electrical Percolation in Carbon Nanotubes/Polymer Nanocomposites. J. Phys. Chem. C 2011, 115, 21685–21690. [Google Scholar] [CrossRef]
- Luo, H.; Ao, H.; Peng, M.; Yao, F.; Yang, Z.; Wan, Y. Effect of Highly Dispersed Graphene and Graphene Oxide in 3D Nanofibrous Bacterial Cellulose Scaffold on Cell Responses: A Comparative Study. Mater. Chem. Phys. 2019, 235, 121774. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Shen, Y.; Yoshino, K.; Feng, W. A Mechanically Strong, Flexible and Conductive Film Based on Bacterial Cellulose/Graphene Nanocomposite. Carbohydr. Polym. 2012, 87, 644–649. [Google Scholar] [CrossRef]
- Ccorahua, R.; Troncoso, O.P.; Rodriguez, S.; Lopez, D.; Torres, F.G. Hydrazine Treatment Improves Conductivity of Bacterial Cellulose/Graphene Nanocomposites Obtained by a Novel Processing Method. Carbohydr. Polym. 2017, 171, 68–76. [Google Scholar] [CrossRef]
- Torres, F.G.; Ccorahua, R.; Arroyo, J.; Troncoso, O.P. Enhanced Conductivity of Bacterial Cellulose Films Reinforced with NH 4 I-Doped Graphene Oxide. Polym. Technol. Mater. 2019, 58, 1585–1595. [Google Scholar]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Biopolymer Electrolyte for Supercapacitor. In Biopolymer Electrolytes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–116. [Google Scholar] [CrossRef]
- Williamson, S.S.; Cassani, P.A.; Lukic, S.; Blunier, B. Energy Storage. In Power Electronics Handbook; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1331–1356. [Google Scholar] [CrossRef]
- Choi, B.G.; Hong, J.; Hong, W.H.; Hammond, P.T.; Park, H. Facilitated Ion Transport in All-Solid-State Flexible Supercapacitors. ACS Nano 2011, 5, 7205–7213. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, L.; Huang, S.; Mei, J.; Xu, J.; Yuan, G. A Flexible Polyaniline/Graphene/Bacterial Cellulose Supercapacitor Electrode. New J. Chem. 2017, 41, 857–864. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Niu, H.; Xing, L.; Liu, L.; Huang, Y. Flexible and Freestanding Supercapacitor Electrodes Based on Nitrogen-Doped Carbon Networks/Graphene/Bacterial Cellulose with Ultrahigh Areal Capacitance. ACS Appl. Mater. Interfaces 2016, 8, 33608–33618. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Zhang, C.; Zhou, Y.; Zhou, X.; Liu, Z.; Yu, G. Enabling Graphene-Oxide-Based Membranes for Large-Scale Energy Storage by Controlling Hydrophilic Microstructures. Chem 2018, 4, 1035–1046. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Wen, F.; Yuan, K.; Tan, J.; Zhang, Z.; Wang, H. Tubular Structured Bacterial Cellulose-Based Nitrite Sensor: Preparation and Environmental Application. J. Solid State Electrochem. 2017, 21, 3649–3657. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, H.; Mensah, A.; Feng, Q.; Lu, K.; Huang, J.; Li, D.; Cai, Y.; Lucia, L.; Wei, Q. In Situ 3D Bacterial Cellulose/Nitrogen-Doped Graphene Oxide Quantum Dot-Based Membrane Fluorescent Probes for Aggregation-Induced Detection of Iron Ions. Cellulose 2019, 26, 6073–6086. [Google Scholar] [CrossRef]
- Hosseini, H.; Kokabi, M.; Mousavi, S.M. BC/RGO Conductive Nanocomposite Aerogel as a Strain Sensor. Polymer (Guildf.) 2018, 137, 82–96. [Google Scholar] [CrossRef]
- Kim, S.-S.; Jeon, J.-H.; Kim, H.-I.; Kee, C.D.; Oh, I.-K. High-Fidelity Bioelectronic Muscular Actuator Based on Graphene-Mediated and TEMPO-Oxidized Bacterial Cellulose. Adv. Funct. Mater. 2015, 25, 3560–3570. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhaskarwar, A.N. A Review on Sorbent Devices for Oil-Spill Control. Environ. Pollut. 2018, 243, 1758–1771. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.-O.; Jung, E.; Park, S.; Park, H.S. Surface Modification and Partial Reduction of Three-Dimensional Macroporous Graphene Oxide Scaffolds for Greatly Improved Adsorption Capacity. RSC Adv. 2014, 4, 899–902. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yadav, S.; Heinlein, T.; Konjik, V.; Breitzke, H.; Buntkowsky, G.; Schneider, J.J.; Zhang, K. Ultra-Light Nanocomposite Aerogels of Bacterial Cellulose and Reduced Graphene Oxide for Specific Absorption and Separation of Organic Liquids. RSC Adv. 2014, 4, 21553. [Google Scholar] [CrossRef]
- Mensah, A.; Lv, P.; Narh, C.; Huang, J.; Wang, D.; Wei, Q. Sequestration of Pb(II) Ions from Aqueous Systems with Novel Green Bacterial Cellulose Graphene Oxide Composite. Materials 2019, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Heydari, A.; Sillanpää, M. Superparamagnetic Fe3O4@EDTA Nanoparticles as an Efficient Adsorbent for Simultaneous Removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) from Water and Soil Environmental Samples. Microchem. J. 2017, 131, 51–56. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane Materials for Water Purification: Design, Development and Application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Zia-ur-Rehman, M.; Al-Wabel, M.I. A Critical Review of Mechanisms Involved in the Adsorption of Organic and Inorganic Contaminants through Biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial Cellulose/Attapulgite Magnetic Composites as an Efficient Adsorbent for Heavy Metal Ions and Dye Treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.; Yang, L.; Cui, J.; Hao, Q.; Sun, D. Handy Purifier Based on Bacterial Cellulose and Ca-Montmorillonite Composites for Efficient Removal of Dyes and Antibiotics. Carbohydr. Polym. 2019, 222, 115017. [Google Scholar] [CrossRef]
- Wang, B.; Qi, G.; Huang, C.; Yang, X.-Y.; Zhang, H.-R.; Luo, J.; Chen, X.-F.; Xiong, L.; Chen, X.-D. Preparation of Bacterial Cellulose/Inorganic Gel of Bentonite Composite by In Situ Modification. Indian J. Microbiol. 2016, 56, 72–79. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken Experimental Design for Chromium(VI) Ions Removal by Bacterial Cellulose-Magnetite Composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional Adsorbent Based on Metal-Organic Framework Modified Bacterial Cellulose/Chitosan Composite Aerogel for High Efficient Removal of Heavy Metal Ion and Organic Pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Shi, L.; Zheng, X.; Wang, J. Adsorption of Sr(II) from Water by Mercerized Bacterial Cellulose Membrane Modified with EDTA. J. Hazard. Mater. 2019, 364, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium Oxide-Bacterial Cellulose Bioadsorbent for the Removal of Lead Ions from Aqueous Solution. Int. J. Biol. Macromol. 2019, 129, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A Review on Heavy Metal Ions Adsorption from Water by Graphene Oxide and Its Composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Nupearachchi, C.N.; Mahatantila, K.; Vithanage, M. Application of Graphene for Decontamination of Water; Implications for Sorptive Removal. Groundw. Sustain. Dev. 2017, 5, 206–215. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial Cellulose in Biomedical Applications: A Review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial Cellulose Scaffolds and Cellulose Nanowhiskers for Tissue Engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and Structural Characterization of Surface Modified Microporous Bacterial Cellulose Scaffolds: A Potential Material for Skin Regeneration Applications in Vitro and in Vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The Growing Merits and Dwindling Limitations of Bacterial Cellulose-Based Tissue Engineering Scaffolds. Curr. Opin. Chem. Eng. 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial Cellulose-Based Scaffold Materials for Bone Tissue Engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, K.; Feng, J.; Liu, J.; Huang, R.; Chen, Z.; Yang, J.; Dai, Z.; Chen, Y.; Wang, N.; et al. Construction of Small-Diameter Vascular Graft by Shape-Memory and Self-Rolling Bacterial Cellulose Membrane. Adv. Healthc. Mater. 2017, 6, 1601343. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial Cellulose: A Versatile Biopolymer for Wound Dressing Applications. Microb. Biotechnol. 2019, 1, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of Bacterial Cellulose in Food, Cosmetics and Drug Delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, Y.S. The Role of Bacterial Cellulose in Artificial Blood Vessels. Mol. Cell. Toxicol. 2017, 13, 257–261. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial Activity of Graphene-Based Materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced Review of Graphene-Based Nanomaterials in Drug Delivery Systems: Synthesis, Modification, Toxicity and Application. Mater. Sci. Eng. C 2017, 77, 1363–1375. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. Stimuli Responsive Drug Delivery Systems Based on Nano-Graphene for Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef]
- Akhavan, O. Graphene Scaffolds in Progressive Nanotechnology/Stem Cell-Based Tissue Engineering of the Nervous System. J. Mater. Chem. B 2016, 4, 3169–3190. [Google Scholar] [CrossRef]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L. Fabrication of Scaffolds in Tissue Engineering: A Review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Jennifer, M.; Maciej, W. Nanoparticle Technology as a Double-Edged Sword: Cytotoxic, Genotoxic and Epigenetic Effects on Living Cells. J. Biomater. Nanobiotechnol. 2013, 04, 53–63. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization Is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Su, G.; Li, L.; Gilbertson, B.O.; Yu, L.H.; Zhang, Q.; Sun, Y.-P.; Yan, B. Size-Dependent Cell Uptake of Protein-Coated Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2012, 4, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Chen, W.-Y. Water/Oil Repellency and Work of Adhesion of Liquid Droplets on Graphene Oxide and Graphene Surfaces. Surf. Coat. Technol. 2011, 205, 4554–4561. [Google Scholar] [CrossRef]
- Wang, A.; Pu, K.; Dong, B.; Liu, Y.; Zhang, L.; Zhang, Z.; Duan, W.; Zhu, Y. Role of Surface Charge and Oxidative Stress in Cytotoxicity and Genotoxicity of Graphene Oxide towards Human Lung Fibroblast Cells. J. Appl. Toxicol. 2013, 33, 1156–1164. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In Vitro Toxicity Evaluation of Graphene Oxide on A549 Cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Tae, G. A Stimuli-Sensitive Injectable Graphene Oxide Composite Hydrogel. Chem. Commun. 2012, 48, 5820. [Google Scholar] [CrossRef]
- Liu, C.; Yu, W.; Chen, Z.; Zhang, J.; Zhang, N. Enhanced Gene Transfection Efficiency in CD13-Positive Vascular Endothelial Cells with Targeted Poly(Lactic Acid)–Poly(Ethylene Glycol) Nanoparticles through Caveolae-Mediated Endocytosis. J. Control. Release 2011, 151, 162–175. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van den Bossche, J.; Kostarelos, K. Purified Graphene Oxide Dispersions Lack In Vitro Cytotoxicity and In Vivo Pathogenicity. Adv. Healthc. Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef]
- Ramani, D.; Sastry, T.P. Bacterial Cellulose-Reinforced Hydroxyapatite Functionalized Graphene Oxide: A Potential Osteoinductive Composite. Cellulose 2014, 21, 3585–3595. [Google Scholar] [CrossRef]
- Si, H.; Luo, H.; Xiong, G.; Yang, Z.; Raman, S.R.; Guo, R.; Wan, Y. One-Step In Situ Biosynthesis of Graphene Oxide-Bacterial Cellulose Nanocomposite Hydrogels. Macromol. Rapid Commun. 2014, 35, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Low, H.R.; Loi, X.Y.; Merel, L.; Mohd Cairul Iqbal, M.A. Fabrication and Evaluation of Bacterial Nanocellulose/Poly(Acrylic Acid)/Graphene Oxide Composite Hydrogel: Characterizations and Biocompatibility Studies for Wound Dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Shaabani, A. Encapsulation of Graphene Quantum Dot-Crosslinked Chitosan by Carboxymethylcellulose Hydrogel Beads as a PH-Responsive Bio-Nanocomposite for the Oral Delivery Agent. Int. J. Biol. Macromol. 2019, 123, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Mianehrow, H.; Moghadam, M.H.M.; Sharif, F.; Mazinani, S. Graphene-Oxide Stabilization in Electrolyte Solutions Using Hydroxyethyl Cellulose for Drug Delivery Application. Int. J. Pharm. 2015, 484, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ao, H.; Li, G.; Li, W.; Xiong, G.; Zhu, Y.; Wan, Y. Bacterial Cellulose/Graphene Oxide Nanocomposite as a Novel Drug Delivery System. Curr. Appl. Phys. 2017, 17, 249–254. [Google Scholar] [CrossRef]
| Properties | Value | Observation | References |
|---|---|---|---|
| Bacterial cellulose network | |||
| Diameter | 35–90 nm | Individual nanofibres | [22] |
| Length | 580–960 µm | Individual nanofibres | [23] |
| Young’s modulus | 78 GPa | Individual nanofibres | [22] |
| Porosity | 65–75% | BC native network hydrogel | [24] |
| Surface area | 20 m2/g | BC native network hydrogel | [25] |
| Water holding capacity | 201% | BC native network hydrogel | [25] |
| Graphene | |||
| Surface area | 2600 m2/g | Theoretical prediction | [26] |
| Mobility | 15,000 cm2 V−1 s−1 | Room temperature | [26] |
| Electric conductivity | 106–107 S m−1 | Isolated single particle conductivity | [27] |
| Thermal conductivity | 5.8 × 103 Wm−1K−1 | - | [26] |
| Young’s modulus | 1 TPa | - | [26] |
| Nanocomposite | Graphene Content (%) | Conductivity (S∙m−1) | References |
|---|---|---|---|
| BC-Graphene | 18.4–26.8 | 70–80 | [49] |
| BC-RGO | 2.5–10 | 0.001–0.01 | [69] |
| BC-RGO | 30 | 12 | [70] |
| BC-Graphene-PANI | - | 170 | [52] |
| BC-RGO-NH4I | 30 | 0.013 | [71] |
| BC-Graphene System | Conductivity (S m−1) | Specific Capacitance (F g−1) | Cycling Stability (%) | Reference |
|---|---|---|---|---|
| BC/GO | 171 | 160 | 90.3, after 2000 cycles | [41] |
| BC/GE/PANI | 1660 | 645 | 82.2%, after 1000 cycles | [48] |
| Polypyrrole/Bacterial Cellulose/Graphene | 1320 | 556 | 95.2, after 5000 cycles | [36] |
| BC/RGO | - | 216 | 86, after 10,000 cycles | [34] |
| Nitrogen-Doped Carbon Networks/Graphene/Bacterial Cellulose | - | 263–318 | ~100, after 20,000 cycles | [77] |
| Type of Matrix | Type of Graphene | Biocompatibility Test | Viability (Cells) | Potential Application Assessed | Reference |
|---|---|---|---|---|---|
| BC hydrogel | Graphene oxide (GO) | Mouse fibroblast cell line (L929), CCK-8 assay | 0.55–1.1 (O.D. = 450 nm †) | Drug delivery system | [129] |
| BC hydrogel pellets | Graphene oxide (GO) | Mouse peritoneal macrophages-RAW264.7, MTT assay | ~75 × 104 cells (72 h) | Drug delivery system | [44] |
| BC film | Reduced graphene oxide (RGO) | Human marrow mesenchymal stem cells (hMSCs) | ~5.5 × 104 cells (72 h) | - | [45] |
| BC/Hydroxyapatite porous structure | Graphene oxide (GO) | MG-63 and NIH 3T3 cells, MTT | 110–120% (MG-63 cells, 24 h) 80–110% (NIH 3T3 cells, 24 h) | Tissue engineering | [124] |
| BC/PEDOT film | Graphene oxide (GO) | PC12 neural cells, MTT | 95% (24 h) | Regenerative medicine | [51] |
| BC hydrogel | Graphene oxide (GO) | Human dermal fibroblast | 80% (24 h) | Wound dressing | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troncoso, O.P.; Torres, F.G. Bacterial Cellulose—Graphene Based Nanocomposites. Int. J. Mol. Sci. 2020, 21, 6532. https://doi.org/10.3390/ijms21186532
Troncoso OP, Torres FG. Bacterial Cellulose—Graphene Based Nanocomposites. International Journal of Molecular Sciences. 2020; 21(18):6532. https://doi.org/10.3390/ijms21186532
Chicago/Turabian StyleTroncoso, Omar P., and Fernando G. Torres. 2020. "Bacterial Cellulose—Graphene Based Nanocomposites" International Journal of Molecular Sciences 21, no. 18: 6532. https://doi.org/10.3390/ijms21186532
APA StyleTroncoso, O. P., & Torres, F. G. (2020). Bacterial Cellulose—Graphene Based Nanocomposites. International Journal of Molecular Sciences, 21(18), 6532. https://doi.org/10.3390/ijms21186532






