Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission
Abstract
:1. Introduction
2. Development of a Brighter Bioluminescence System
2.1. Engineering on Luciferase and Luciferin
2.2. BRET-Based Approaches
3. Development of Red-Shifted Bioluminescence System
3.1. Engineering on Luciferase and Luciferin
3.2. BRET-Based Approaches
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Y.; Shi, P.; Song, W.; Bi, S. Chemiluminescence and Bioluminescence Imaging for Biosensing and Therapy: In Vitro and In Vivo Perspectives. Theranostics 2019, 9, 4047–4065. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.K.; Noguchi, T. Cellular Bioluminescence Imaging. Cold Spring Harb. Protoc. 2012, 2012. [Google Scholar] [CrossRef]
- Pozzo, T.; Akter, F.; Nomura, Y.; Louie, A.Y.; Yokobayashi, Y. Firefly Luciferase Mutant with Enhanced Activity and Thermostability. Acs Omega 2018, 3, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Law, G.H.E.; Gandelman, O.A.; Tisi, L.C.; Lowe, C.R.; Murray, J.A.H. Mutagenesis of solvent-exposed amino acids in Photinus pyralis luciferase improves thermostability and pH-tolerance. Biochem. J. 2006, 397, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aswendt, M.; Vogel, S.; Schäfer, C.; Jathoul, A.; Pule, M.; Hoehn, M. Quantitative in vivo dual-color bioluminescence imaging in the mouse brain. Neurophotonics 2019, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jathoul, A.; Law, E.; Gandelman, O.; Pule, M.; Tisi, L.; Murray, J. Development of a pH-Tolerant Thermostable Photinus pyralis Luciferase for Brighter In Vivo Imaging. In Bioluminescence—Recent Advances in Oceanic Measurements and Laboratory Applications; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Branchini, B.R.; Magyar, R.A.; Murtiashaw, M.H.; Portier, N.C. The Role of Active Site Residue Arginine 218 in Firefly Luciferase Bioluminescence. Biochemistry 2001, 40, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Southworth, T.L.; Murtiashaw, M.H.; Boije, H.; Fleet, S.E. A Mutagenesis Study of the Putative Luciferin Binding Site Residues of Firefly Luciferase. Biochemistry 2003, 42, 10429–10436. [Google Scholar] [CrossRef]
- Bruce, R.B.; Tara, L.S.; Neelum, F.K.; Martha, H.M.; Sarah, E.F. Rational and random mutagenesis of firefly luciferase to identify an efficient emitter of red bioluminescence. Proc. SPIE 2004, 5329. [Google Scholar] [CrossRef]
- Branchini, B.R.; Southworth, T.L.; Khattak, N.F.; Michelini, E.; Roda, A. Red- and green-emitting firefly luciferase mutants for bioluminescent reporter applications. Anal. Biochem. 2005, 345, 140–148. [Google Scholar] [CrossRef]
- Branchini, B.R.; Ablamsky, D.M.; Davis, A.L.; Southworth, T.L.; Butler, B.; Fan, F.; Jathoul, A.P.; Pule, M.A. Red-emitting luciferases for bioluminescence reporter and imaging applications. Anal. Biochem. 2010, 396, 290–297. [Google Scholar] [CrossRef]
- Nakatsu, T.; Ichiyama, S.; Hiratake, J.; Saldanha, A.; Kobashi, N.; Sakata, K.; Kato, H. Structural basis for the spectral difference in luciferase bioluminescence. Nature 2006, 440, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Harwood, K.R.; Mofford, D.M.; Reddy, G.R.; Miller, S.C. Identification of mutant firefly luciferases that efficiently utilize aminoluciferins. Chem. Biol. 2011, 18, 1649–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mofford, D.M.; Reddy, G.R.; Miller, S.C. Aminoluciferins Extend Firefly Luciferase Bioluminescence into the Near-Infrared and Can Be Preferred Substrates over d-Luciferin. J. Am. Chem. Soc. 2014, 136, 13277–13282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jathoul, A.P.; Grounds, H.; Anderson, J.C.; Pule, M.A. A Dual-Color Far-Red to Near-Infrared Firefly Luciferin Analogue Designed for Multiparametric Bioluminescence Imaging. Angew. Chem. Int. Ed. 2014, 53, 13059–13063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stowe, C.L.; Burley, T.A.; Allan, H.; Vinci, M.; Kramer-Marek, G.; Ciobota, D.M.; Parkinson, G.N.; Southworth, T.L.; Agliardi, G.; Hotblack, A.; et al. Near-infrared dual bioluminescence imaging in mouse models of cancer using infraluciferin. eLife 2019, 8, e45801. [Google Scholar] [CrossRef] [PubMed]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
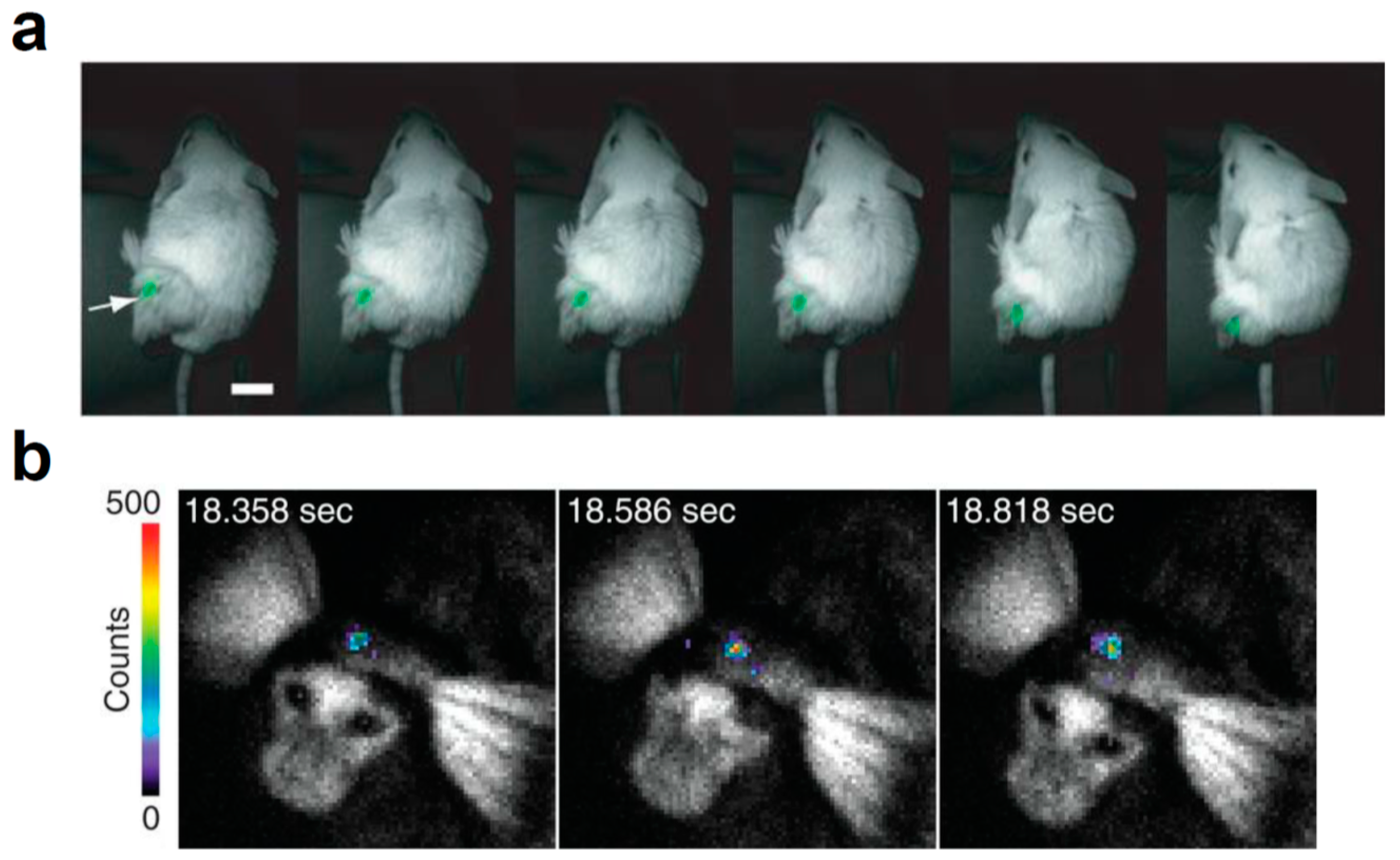
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Zhang, B.S.; Steinhardt, R.C.; Mills, J.H.; Prescher, J.A. Multicomponent Bioluminescence Imaging with a π-Extended Luciferin. J. Am. Chem. Soc. 2020, 142, 14080–14089. [Google Scholar] [CrossRef]
- Hall, M.P.; Woodroofe, C.C.; Wood, M.G.; Que, I.; van’t Root, M.; Ridwan, Y.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Wood, K.V.; et al. Click beetle luciferase mutant and near infrared naphthyl-luciferins for improved bioluminescence imaging. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Loening, A.M.; Fenn, T.D.; Wu, A.M.; Gambhir, S.S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng. Des. Sel. 2006, 19, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Loening, A.M.; Wu, A.M.; Gambhir, S.S. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat. Methods 2007, 4, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef]
- Yeh, H.-W.; Karmach, O.; Ji, A.; Carter, D.; Martins-Green, M.M.; Ai, H. Red-shifted luciferase–luciferin pairs for enhanced bioluminescence imaging. Nat. Methods 2017, 14, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-W.; Xiong, Y.; Wu, T.; Chen, M.; Ji, A.; Li, X.; Ai, H. ATP-Independent Bioluminescent Reporter Variants to Improve in Vivo Imaging. ACS Chem. Biol. 2019, 14, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, H.; Nakajima, Y.; Ohmiya, Y. Luciferase-YFP fusion tag with enhanced emission for single-cell luminescence imaging. Nat. Methods 2007, 4, 637–639. [Google Scholar] [CrossRef]
- Xu, Y.; Piston, D.W.; Johnson, C.H. A bioluminescence resonance energy transfer (BRET) system: Application to interacting circadian clock proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, L.; Parent, S.; Caron, M.; Legault, M.; Joly, E.; Angers, S.; Bouvier, M.; Brown, M.; Houle, B.; Ménard, L. The BRET2/ARRESTIN assay in stable recombinant cells: A platform to screen for compounds that interact with g protein-coupled receptors (GPCRS). J. Recept. Signal Transduct. 2002, 22, 533–541. [Google Scholar] [CrossRef]
- De, A.; Ray, P.; Loening, A.M.; Gambhir, S.S. BRET3: A red-shifted bioluminescence resonance energy transfer (BRET)-based integrated platform for imaging protein-protein interactions from single live cells and living animals. FASEB J. 2009, 23, 2702–2709. [Google Scholar] [CrossRef]
- Dragulescu-Andrasi, A.; Chan, C.T.; De, A.; Massoud, T.F.; Gambhir, S.S. Bioluminescence resonance energy transfer (BRET) imaging of protein–protein interactions within deep tissues of living subjects. Proc. Natl. Acad. Sci. USA 2011, 108, 12060–12065. [Google Scholar] [CrossRef] [Green Version]
- Dimri, S.; Basu, S.; De, A. Use of BRET to Study Protein–Protein Interactions In Vitro and In Vivo. In The Nuclear Receptor Superfamily: Methods and Protocols; McEwan, I.J., Ed.; Springer: New York, NY, USA, 2016; pp. 57–78. [Google Scholar]
- Rumyantsev, K.A.; Turoverov, K.K.; Verkhusha, V.V. Near-infrared bioluminescent proteins for two-color multimodal imaging. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Nishihara, R.; Paulmurugan, R.; Nakajima, T.; Yamamoto, E.; Natarajan, A.; Afjei, R.; Hiruta, Y.; Iwasawa, N.; Nishiyama, S.; Citterio, D.; et al. Highly bright and stable NIR-BRET with blue-shifted coelenterazine derivatives for deep-tissue imaging of molecular events in vivo. Theranostics 2019, 9, 2646–2661. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Chang, Y.F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, A.; Nakano, M.; Saito, K.; Haruno, R.; Watanabe, T.M.; Ohyanagi, T.; Jin, T.; Okada, Y.; Nagai, T. Expanded palette of Nano-lanterns for real-time multicolor luminescence imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 4352–4356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Grailhe, R. Nanoluciferase signal brightness using furimazine substrates opens bioluminescence resonance energy transfer to widefield microscopy. Cytom. Part A 2016, 89, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Goyet, E.; Bouquier, N.; Ollendorff, V.; Perroy, J. Fast and high resolution single-cell BRET imaging. Sci. Rep. 2016, 6, 28231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyre, N.S.; Aloia, A.L.; Joyce, M.A.; Chulanetra, M.; Tyrrell, D.L.; Beard, M.R. Sensitive luminescent reporter viruses reveal appreciable release of hepatitis C virus NS5A protein into the extracellular environment. Virology 2017, 507, 20–31. [Google Scholar] [CrossRef]
- Suzuki, K.; Kimura, T.; Shinoda, H.; Bai, G.; Daniels, M.J.; Arai, Y.; Nakano, M.; Nagai, T. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat. Commun. 2016, 7, 13718. [Google Scholar] [CrossRef]
- Schaub, F.X.; Reza, M.S.; Flaveny, C.A.; Li, W.; Musicant, A.M.; Hoxha, S.; Guo, M.; Cleveland, J.L.; Amelio, A.L. Fluorophore-NanoLuc BRET Reporters Enable Sensitive In Vivo Optical Imaging and Flow Cytometry for Monitoring Tumorigenesis. Cancer Res. 2015, 75, 5023–5033. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Oh, Y.; Sens, A.; Ataie, N.; Dana, H.; Macklin, J.J.; Laviv, T.; Welf, E.S.; Dean, K.M.; Zhang, F.; et al. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat. Biotechnol. 2016, 34, 760–767. [Google Scholar] [CrossRef]
- Su, Y.; Walker, J.R.; Park, Y.; Smith, T.P.; Liu, L.X.; Hall, M.P.; Labanieh, L.; Hurst, R.; Wang, D.C.; Encell, L.P.; et al. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat. Methods 2020, 17, 852–860. [Google Scholar] [CrossRef]
- Arai, R.; Nakagawa, H.; Kitayama, A.; Ueda, H.; Nagamune, T. Detection of protein-protein interaction by bioluminescence resonance energy transfer from firefly luciferase to red fluorescent protein. J. Biosci. Bioeng. 2002, 94, 362–364. [Google Scholar] [CrossRef]
- Iglesias, P.; Costoya, J.A. A novel BRET-based genetically encoded biosensor for functional imaging of hypoxia. Biosens. Bioelectron. 2009, 24, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Rosenberg, J.C.; Ablamsky, D.M.; Taylor, K.P.; Southworth, T.L.; Linder, S.J. Sequential bioluminescence resonance energy transfer–fluorescence resonance energy transfer-based ratiometric protease assays with fusion proteins of firefly luciferase and red fluorescent protein. Anal. Biochem. 2011, 414, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Veda, H.; Kitayama, A.; Nagamune, T. Rapid homogeneous immunoassay of peptides based on bioluminescence resonance energy transfer from firefly luciferase. J. Biosci. Bioeng. 2002, 93, 537–542. [Google Scholar] [CrossRef]
- Branchini, B.R.; Ablamsky, D.M.; Rosenberg, J.C. Chemically Modified Firefly Luciferase Is an Efficient Source of Near-Infrared Light. Bioconjug. Chem. 2010, 21, 2023–2030. [Google Scholar] [CrossRef]
- Hiblot, J.; Yu, Q.; Sabbadini, M.D.B.; Reymond, L.; Xue, L.; Schena, A.; Sallin, O.; Hill, N.; Griss, R.; Johnsson, K. Luciferases with Tunable Emission Wavelengths. Angew. Chem. Int. Ed. 2017, 56, 14556–14560. [Google Scholar] [CrossRef]
- So, M.-K.; Xu, C.; Loening, A.M.; Gambhir, S.S.; Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 2006, 24, 339–343. [Google Scholar] [CrossRef]
- Zhang, Y.; So, M.-k.; Loening, A.M.; Yao, H.; Gambhir, S.S.; Rao, J. HaloTag Protein-Mediated Site-Specific Conjugation of Bioluminescent Proteins to Quantum Dots. Angew. Chem. Int. Ed. 2006, 45, 4936–4940. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Xiao, F.; Xia, Z.; Rao, J. Quantum Dot/Bioluminescence Resonance Energy Transfer Based Highly Sensitive Detection of Proteases. Angew. Chem. Int. Ed. 2007, 46, 4346–4349. [Google Scholar] [CrossRef]
- Kamkaew, A.; Sun, H.; England, C.G.; Cheng, L.; Liu, Z.; Cai, W. Quantum dot–NanoLuc bioluminescence resonance energy transfer enables tumor imaging and lymph node mapping in vivo. Chem. Commun. 2016, 52, 6997–7000. [Google Scholar] [CrossRef] [Green Version]
- Kazuki, N.; Yoshiro, I.; Yoshihiro, O. Quantum Yield Measurements of Firefly Bioluminescence Reactions Using a Commercial Luminometer. Chem. Lett. 2010, 39, 291–293. [Google Scholar]
- Seliger, H.H.; McElroy, W.D. The colors of firefly bioluminescence: Enzyme configuration and species specificity. Proc. Natl. Acad. Sci. USA 1964, 52, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, K.V.; Lam, Y.A.; McElroy, W.D. Introduction to beetle luciferases and their applications. J. Biolumin. Chemilumin. 1989, 4, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Tisi, L.C.; White, P.J.; Squirrell, D.J.; Murphy, M.J.; Lowe, C.R.; Murray, J.A.H. Development of a thermostable firefly luciferase. Anal. Chim. Acta 2002, 457, 115–123. [Google Scholar] [CrossRef]
- Conti, E.; Franks, N.P.; Brick, P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 1996, 4, 287–298. [Google Scholar] [CrossRef]
- Frydman, J.; Erdjument-Bromage, H.; Tempst, P.; Hartl, F.U. Co-translational domain folding as the structural basis for the rapid de novo folding of firefly luciferase. Nat. Struct. Biol. 1999, 6, 697–705. [Google Scholar] [CrossRef]
- White, P.J.; Squirrell, D.J.; Arnaud, P.; Lowe, C.R.; Murray, J.A.H. Improved thermostability of the North American firefly luciferase: Saturation mutagenesis at position. Biochem. J. 1996, 319, 343–350. [Google Scholar] [CrossRef]
- Eijsink, V.G.H.; Bjørk, A.; Gåseidnes, S.; Sirevåg, R.; Synstad, B.; van der Burg, B.; Vriend, G. Rational engineering of enzyme stability. J. Biotechnol. 2004, 113, 105–120. [Google Scholar] [CrossRef]
- Fujii, H.; Noda, K.; Asami, Y.; Kuroda, A.; Sakata, M.; Tokida, A. Increase in bioluminescence intensity of firefly luciferase using genetic modification. Anal. Biochem. 2007, 366, 131–136. [Google Scholar] [CrossRef]
- Branchini, B.R.; Southworth, T.L.; Murtiashaw, M.H.; Magyar, R.A.; Gonzalez, S.A.; Ruggiero, M.C.; Stroh, J.G. An Alternative Mechanism of Bioluminescence Color Determination in Firefly Luciferase. Biochemistry 2004, 43, 7255–7262. [Google Scholar] [CrossRef]
- Sandalova, T.P.; Ugarova, N.N. Model of the active site of firefly luciferase. Biochem. Biokhimiia 1999, 64, 962–967. [Google Scholar]
- Schwinn, M.K.; Machleidt, T.; Zimmerman, K.; Eggers, C.T.; Dixon, A.S.; Hurst, R.; Hall, M.P.; Encell, L.P.; Binkowski, B.F.; Wood, K.V. CRISPR-Mediated Tagging of Endogenous Proteins with a Luminescent Peptide. ACS Chem. Biol. 2018, 13, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Miyasaki, M.; Li, Q.; Kawamura, G.; Ozawa, T. A Detection Method for GLUT4 Exocytosis Based on Spontaneous Split Luciferase Complementation. Anal. Sci. 2019, 35, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Főrster, T. 10th Spiers Memorial Lecture. Transfer mechanisms of electronic excitation. Discuss. Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Clegg, R.M. Fluorescence resonance energy transfer. Curr. Opin. Biotechnol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Drinovec, L.; Kubale, V.; Nøhr Larsen, J.; Vrecl, M. Mathematical models for quantitative assessment of bioluminescence resonance energy transfer: Application to seven transmembrane receptors oligomerization. Front. Endocrinol. (Lausanne) 2012, 3, 104. [Google Scholar] [CrossRef] [Green Version]
- Dacres, H.; Wang, J.; Dumancic, M.M.; Trowell, S.C. Experimental Determination of the Förster Distance for Two Commonly Used Bioluminescent Resonance Energy Transfer Pairs. Anal. Chem. 2010, 82, 432–435. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Mourant, J.R.; Freyer, J.P.; Hielscher, A.H.; Eick, A.A.; Shen, D.; Johnson, T.M. Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics. Appl. Opt. 1998, 37, 3586–3593. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Bringas, M.; Petruk, A.A.; Estrin, D.A.; Capece, L.; Martí, M.A. Tertiary and quaternary structural basis of oxygen affinity in human hemoglobin as revealed by multiscale simulations. Sci. Rep. 2017, 7, 10926. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.J.; Aalders, M.C.G.; Mik, E.G.; Hooper, B.A.; van Gemert, M.J.C.; van Leeuwen, T.G. Oxygen Saturation-Dependent Absorption and Scattering of Blood. Phys. Rev. Lett. 2004, 93, 028102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.-W.; Lee, C.-K.; Tsai, M.-T.; Wang, Y.-M.; Yang, C.C. Measurement of the hemoglobin oxygen saturation level with spectroscopic spectral-domain optical coherence tomography. Opt. Lett. 2008, 33, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Cheun, W.L. The Chemical Structure of Melanin. Pigment Cell Res. 2004, 17, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Sayre, R.M.; Zeise, L.; Chedekel, M.R. New trends in photobiology: Photoprotection by melanin. J. Photochem. Photobiol. B Biol. 1991, 9, 135–160. [Google Scholar] [CrossRef]
- George, Z.; Aikaterini, D.; Ioannis, B.; Dimitrios, G.; Argyrios, T.; Efthimios, K. Melanin absorption spectroscopy: New method for noninvasive skin investigation and melanoma detection. BIOMEDO 2008, 13, 1–8. [Google Scholar]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-μm Wavelength Region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Allen, T.; Beard, P.; Hall, A.; Dhillon, A.; Owen, J. Spectroscopic photoacoustic imaging of lipid-rich plaques in the human aorta in the 740 to 1400 nm wavelength range. BIOMEDO 2012, 17, 061209. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [Green Version]
- White, E.H.; Rapaport, E.; Hopkins, T.A.; Seliger, H.H. Chemi- and bioluminescence of firefly luciferin. J. Am. Chem. Soc. 1969, 91, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Murtiashaw, M.H.; Magyar, R.A.; Portier, N.C.; Ruggiero, M.C.; Stroh, J.G. Yellow-Green and Red Firefly Bioluminescence from 5,5-Dimethyloxyluciferin. J. Am. Chem. Soc. 2002, 124, 2112–2113. [Google Scholar] [CrossRef] [PubMed]
- Naumov, P.; Ozawa, Y.; Ohkubo, K.; Fukuzumi, S. Structure and Spectroscopy of Oxyluciferin, the Light Emitter of the Firefly Bioluminescence. J. Am. Chem. Soc. 2009, 131, 11590–11605. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Hasumi, Y.; Ohtsuka, K.; Maki, S.; Niwa, H.; Yamaji, M.; Hashizume, D. Spectroscopic Studies of the Light-Color Modulation Mechanism of Firefly (Beetle) Bioluminescence. J. Am. Chem. Soc. 2009, 131, 2385–2396. [Google Scholar] [CrossRef]
- Navizet, I.; Liu, Y.-J.; Ferré, N.; Xiao, H.-Y.; Fang, W.-H.; Lindh, R. Color-Tuning Mechanism of Firefly Investigated by Multi-Configurational Perturbation Method. J. Am. Chem. Soc. 2010, 132, 706–712. [Google Scholar] [CrossRef]
- Hosseinkhani, S. Molecular enigma of multicolor bioluminescence of firefly luciferase. Cell. Mol. Life Sci. 2011, 68, 1167–1182. [Google Scholar] [CrossRef]
- Miura, C.; Kiyama, M.; Iwano, S.; Ito, K.; Obata, R.; Hirano, T.; Maki, S.; Niwa, H. Synthesis and luminescence properties of biphenyl-type firefly luciferin analogs with a new, near-infrared light-emitting bioluminophore. Tetrahedron 2013, 69, 9726–9734. [Google Scholar] [CrossRef]
- Shcherbakova, D.M.; Verkhusha, V.V. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nat. Methods 2013, 10, 751–754. [Google Scholar] [CrossRef]
- Furuhata, Y.; Sakai, A.; Murakami, T.; Nagasaki, A.; Kato, Y. Bioluminescent imaging of Arabidopsis thaliana using an enhanced Nano-lantern luminescence reporter system. PLoS ONE 2020, 15, e0227477. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.-X.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef]
- Lavis, L.D.; Raines, R.T. Bright Ideas for Chemical Biology. ACS Chem. Biol. 2008, 3, 142–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinić, I.; Eliseeva, S.V.; Petoud, S. Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials. J. Lumin. 2017, 189, 19–43. [Google Scholar] [CrossRef]
- Keppler, A.; Gendreizig, S.; Gronemeyer, T.; Pick, H.; Vogel, H.; Johnsson, K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 2003, 21, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Los, G.V.; Encell, L.P.; McDougall, M.G.; Hartzell, D.D.; Karassina, N.; Zimprich, C.; Wood, M.G.; Learish, R.; Ohana, R.F.; Urh, M.; et al. HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis. ACS Chem. Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Santone, K.S.; Acosta, D.; Bruckner, J.V. Cadmium toxicity in primary cultures of rat hepatocytes. J. Toxicol. Environ. Health 1982, 10, 169–177. [Google Scholar] [CrossRef]
- Rathbun, C.M.; Porterfield, W.B.; Jones, K.A.; Sagoe, M.J.; Reyes, M.R.; Hua, C.T.; Prescher, J.A. Parallel Screening for Rapid Identification of Orthogonal Bioluminescent Tools. ACS Cent. Sci. 2017, 3, 1254–1261. [Google Scholar] [CrossRef]
- Keyaerts, M.; Caveliers, V.; Lahoutte, T. Bioluminescence imaging: Looking beyond the light. Trends Mol. Med. 2012, 18, 164–172. [Google Scholar] [CrossRef]
- Baba, S.; Cho, S.Y.; Ye, Z.; Cheng, L.; Engles, J.M.; Wahl, R.L. How Reproducible Is Bioluminescent Imaging of Tumor Cell Growth? Single Time Point versus the Dynamic Measurement Approach. Mol. Imaging 2007, 6. [Google Scholar] [CrossRef]
- Keyaerts, M.; Verschueren, J.; Bos, T.J.; Tchouate-Gainkam, L.O.; Peleman, C.; Breckpot, K.; Vanhove, C.; Caveliers, V.; Bossuyt, A.; Lahoutte, T. Dynamic bioluminescence imaging for quantitative tumour burden assessment using IV or IP administration of d-luciferin: Effect on intensity, time kinetics and repeatability of photon emission. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 999–1007. [Google Scholar] [CrossRef]
- Inoue, Y.; Kiryu, S.; Izawa, K.; Watanabe, M.; Tojo, A.; Ohtomo, K. Comparison of subcutaneous and intraperitoneal injection of d-luciferin for in vivo bioluminescence imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.T., Jr.; Miller, S.C. Beyond d-luciferin: Expanding the scope of bioluminescence imaging in vivo. Curr. Opin. Chem. Biol. 2014, 21, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Byun, S.S.; Paik, J.Y.; Lee, S.Y.; Song, S.H.; Choe, Y.S.; Kim, B.T. Cell uptake and tissue distribution of radioiodine labelled d-luciferin: Implications for luciferase based gene imaging. Nucl. Med. Commun. 2003, 24, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshian, J.; Wei, B.-R.; Chang, K.-E.; Shukla, S.; Ambudkar, S.V.; Simpson, R.M.; Gottesman, M.M.; Hall, M.D. Bioluminescent imaging of drug efflux at the blood–brain barrier mediated by the transporter Abcgproceedings. Natl. Acad. Sci. 2013, 110, 20801–20806. [Google Scholar] [CrossRef] [Green Version]
- Kotlobay, A.A.; Sarkisyan, K.S.; Mokrushina, Y.A.; Marcet-Houben, M.; Serebrovskaya, E.O.; Markina, N.M.; Gonzalez Somermeyer, L.; Gorokhovatsky, A.Y.; Vvedensky, A.; Purtov, K.V.; et al. Genetically encodable bioluminescent system from fungi. Proc. Natl. Acad. Sci. USA 2018, 115, 12728–12732. [Google Scholar] [CrossRef] [Green Version]
- Gregor, C.; Pape, J.K.; Gwosch, K.C.; Gilat, T.; Sahl, S.J.; Hell, S.W. Autonomous bioluminescence imaging of single mammalian cells with the bacterial bioluminescence system. Proc. Natl. Acad. Sci. USA 2019, 116, 26491–26496. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Huang, L.; Zhou, Y.; Xue, T.; Chen, Z.; Han, G. Near-Infrared-Light Activatable Nanoparticles for Deep-Tissue-Penetrating Wireless Optogenetics. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef]

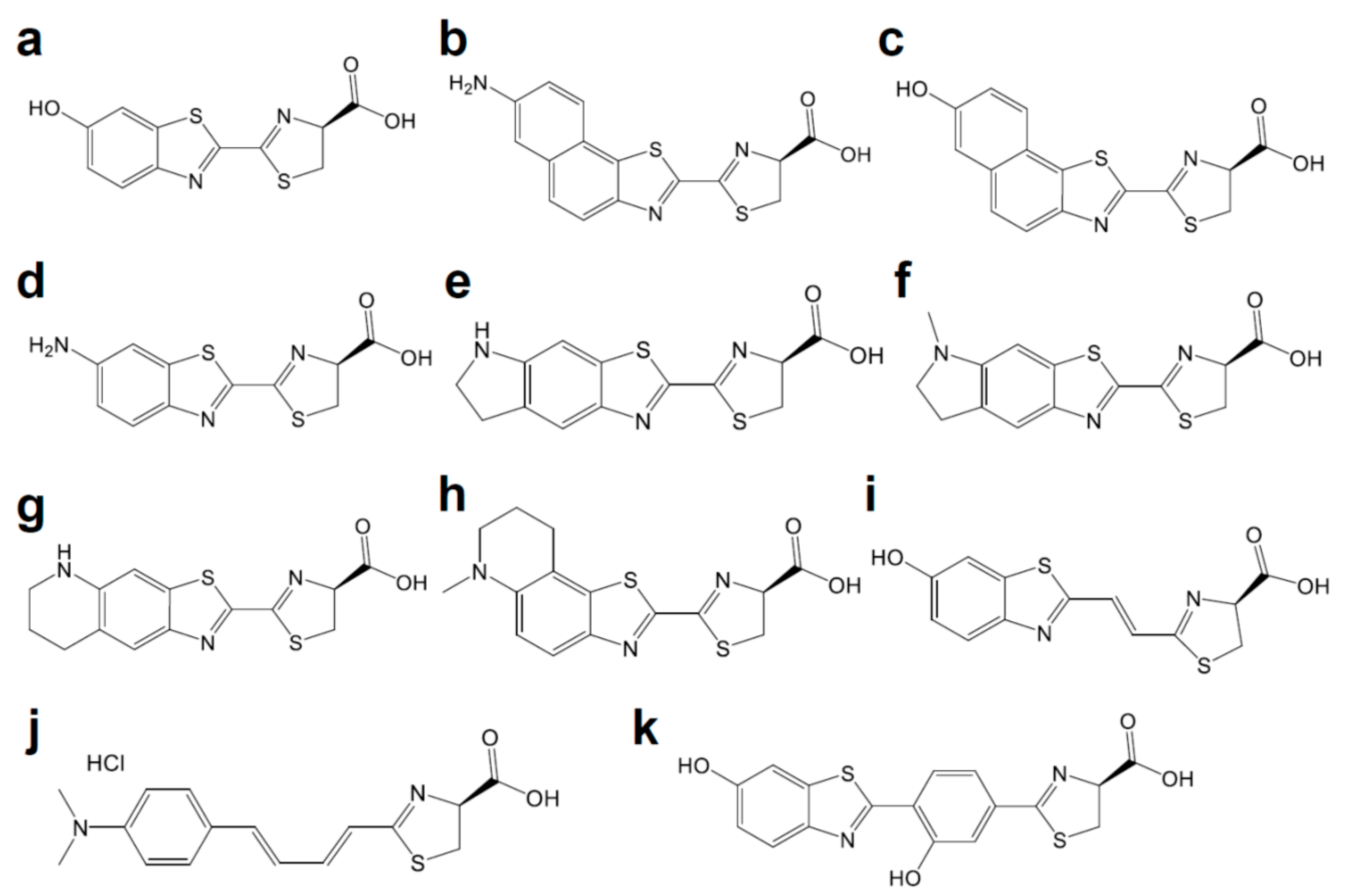
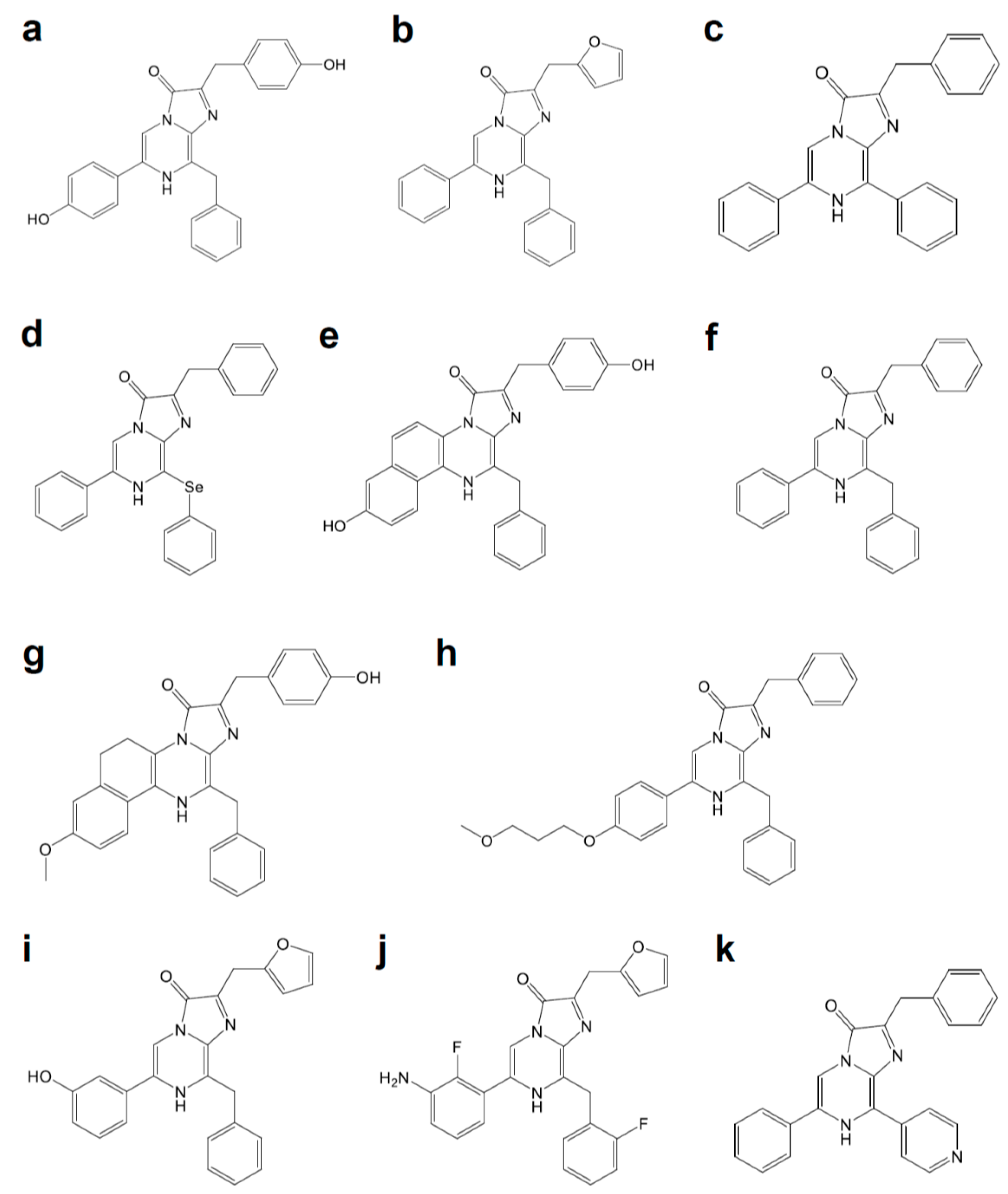
| Name | Origin | Modification | Luciferin | λmax (nm) | Improvement | Refs. |
|---|---|---|---|---|---|---|
| Mutant E | FLuc | T214A, A215L, I232A, F295L, E345K | d-luciferin | 560 | 27-fold more thermally stable than FLuc | [3] |
| LGR | FLuc | I423L, D436G, L530R | d-luciferin | 560 | 20-fold lower Km for ATP and d-luciferin, and 4-fold higher kcat than FLuc | [3] |
| YY5 | FLuc | T214A, A215L, I232A, F295L, E354K, I423L, D436G, L530R | d-Luciferin | 560 | 14-fold higher kcat than FLuc | [3] |
| x5 FLuc | FLuc | F14R, L35Q, V182K, I232K, and F465R | d-luciferin | 554 | Enhanced thermostability up to 45 °C with higher pH-tolerance | [4] |
| x5g | FLuc | F14R, L35Q, V182K, I232K, V241I, G246A, F250S, F465R | d-Luciferin | 560 | 3.69-fold brighter than FLuc | [5] |
| x12 FLuc | FLuc | F14R, L35Q, A105V, V182K, T214C, I232K, D234G, F295L, E354R, D357Y, S420T, and F465R | d-luciferin | 557 | Half-life of 15 min at 55 °C | [6] |
| - | FLuc | R218K | d-luciferin | 572 | [7] | |
| - | FLuc | R218Q | d-luciferin | 608 | [7] | |
| - | FLuc | R218A | d-luciferin | 611 | [7] | |
| - | FLuc | R337K | d-luciferin | 595 | [7] | |
| - | FLuc | R337Q | d-Luciferin | 594 | [7] | |
| - | FLuc | H245A | d-luciferin | 604 | [8] | |
| - | FLuc | G315A | d-Luciferin | 607 | [8] | |
| - | FLuc | T343A | d-luciferin | 617 | [8] | |
| - | FLuc | A348V | d-luciferin | 610 | [8] | |
| - | FLuc | S284T | d-Luciferin | 615 | [9,10] | |
| Ppy RE9 | FLuc | T214A, A215L, I232A, S284T, F295L, I351V, R330G, E354I, and F465R | d-luciferin | 617 | ~11.5% respective light intensities compared to FLuc | [11] |
| - | FLuc | F247L | 6′-aminoluciferin | NA a | F247L induced 4.9-fold increase in light output | [8,12] |
| - | FLuc | R218K | CycLuc1 | 609 | [13] | |
| - | FLuc | R218K | CycLuc2 | 621 | [13] | |
| - | FLuc | R218K, L286M, and S347A | CycLuc7 | 623 | 46% of the initial rate of the pair between FLuc and d-luciferin | [14] |
| FLuc | R218K | CycLuc10 | 648 | [14] | ||
| - | FLuc | - | Infra-luciferin | 670 | [15] | |
| - | FLuc | F14R, L35Q, V182K, I232K, S284T and F465R | Infra-luciferin | 706 | [15] | |
| FLuc_green | FLuc | F14R, L35Q, A105V, V182K, T214C, I232K, D234G, V241I, G246A, F250S, E354R, D357Y, S420T, and F465R | Infra-luciferin | 700 | [16] | |
| FLuc_red | FLuc | (F14R, L35Q, A105V, V182K, T214C, I232K, S284T, D234G, E354I, D357Y, S420T, and F465R | Infra-luciferin | 720 | [16] | |
| - | FLuc | - | Akalumine-HCl | 677 | [17] | |
| AkaLuc | FLuc | T39A, E48Q, I51V, K68R, L86S, Q134R, I136V, Q147R, G175S, N229Y, I231N, F294C, F295L, N308S, H310R, H332R, S347N, I349V, L350M, D357R, A361S, D377V, S456G, N463Y, K524R, L526S, I540T, G545D | Akalumine-HCl | 650 | Improved luminescence intensity | [18] |
| Mutant G2 | FLuc | S220N, E311C, and A313G | PhOH-Luc | 608 | Photons in the 650–850 nm region accounted for ∼31% of the total emission | [19] |
| CBR2opt | CBR | R334S, G351R | d-Luciferin | 730 | [20] | |
| RLuc8 | RLuc | A55T, C124A, S130A, K136R, A143M, M185V, M253L, S287L | Coelenterazine | 487 | 4.3-fold a brighter than RLuc | [21] |
| RLuc8.6-535 | RLuc | A55T, A123S, C124A, S130A, K136R, A143M, D154M, E155G, D162E, I163L, V185L, M185V, M253L, S287L | Coelenterazine | 535 (570 b) | 6.0-fold brighter than RLuc | [22] |
| RLuc8.6-545 | RLuc | A55T, A123S, C124A, S130A, K136R, A143M, D154K, E155N, D162E, I163L, M185V, M253L, F261W, S287L | Coelenterazine | 545 | [22] | |
| RLuc8.6-547 | RLuc | A55T, A123S, C124A, S130A, K136R, A143M, D154A, E155G, D162E, I163V, M185V, M253L, F262W, S287L | Coelenterazine | 547 (588 b) | [22] | |
| C1A4E | OLuc | A4E, Q11R, A33K, V44I, A54F, P115E, Q124K, Y138I, N166R | Coelenterazine | NA a | 88,000-fold brighter than OLuc | [23] |
| NLuc | OLuc | A4E, Q11R, Q18L, L27V, A33N, K43R, V44I, A54I, F68D, L72Q, M75K, I90V, P115E, Q124K, Y138I, N166R | Furimazine | 460 | 150-fold brighter than either FLuc or RLuc | [23] |
| teLuc | OLuc | A4E, Q11R, Q18L, D19S, L27V, A33N, K43R, V44I, A54I, F68D, L72Q, M75K, D85N, I90V, P115E, Q124K, Y138I, C164H, N166R | Diphenylterazine | 502 | 2.6-fold brighter than NLuc | [24] |
| yeLuc | OLuc | F1L, A4E, Q11R, A14D, L27V, D19A, V27L, S28T, A33N, K43R, V44I, A54I, F68D, Q69R, L72Q, M75K, I90V, R112Q, P115E, Q124K, Y138I, L142R, C164S, and N166R | Selenoterazine | 527 | 11.5-fold brighter than NLuc | [24] |
| LumiLuc | OLuc | A4G, Q11R, D19A, S28T, A33N, K43R, V44I, A54I, G67C, F68D, G71A, L72Q, M75K, I90A, R112Q, P115E, V119K, Q124K, K136T, Y138I, C164H, and N166R | 8pyDTZ | 525 | 5-fold brighter than NLuc | [25] |
| Name | Bioluminescence Donor | Fluorescence Acceptor | Luciferin | λmax (nm) | Note | Refs. |
|---|---|---|---|---|---|---|
| BAF-Y | RLuc | EYFP | coelenterazine | 525 | 7-fold brighter than RLuc | [26] |
| eBAF-Y | RLuc8 | EYFP | coelenterazine | 525 | 25-fold brighter than RLuc | [26] |
| BRET1 | RLuc | eYFP | coelenterazine | 527 | [27] | |
| BRET2 | RLuc | GFP2 | DeepBlueC | 410 | [28] | |
| BRET3 | RLuc8 | mOrange | coelenterazine | 564 | [29] | |
| BRET3.1 | RLuc8 | mOrange | coelenterazine-v | 564 | Decreased BRET efficiency | [30] |
| BRET4 | RLuc8 | TagRFP | coelenterazine | 584 | [31] | |
| BRET5 | RLuc8.6 | TagRFP | coelenterazine | 584 | [30] | |
| BRET6 | RLuc8.6 | TurboFP | coelenterazine | 635 | [30] | |
| BRET6.1 | RLuc8.6 | TurboFP | coelenterazine-v | 635 | Increased BRET efficiency | [30] |
| - | RLuc8 | iRFP670 | methoxy-eCoelenterazine | 643 | [32] | |
| - | RLuc8 | iRFP720 | methoxy-eCoelenterazine | 702 | [32] | |
| iRFP-RLuc8.6-535SG | RLuc8.6-535 (S257G) | iRFP | BBlue2.3 | 717 | [33] | |
| Nano-lantern (YNL) | RLuc8∆N3(S257G) | Venus∆C10 | coelenterazine | 530 | 2.9-fold brighter than eBAF-Y | [34] |
| CNL | RLuc8∆N3 (S257G) | mTurquoise2∆C10 | coelenterazine | 470 | [35] | |
| ONL | RLuc8.6-535∆N3 | mKusabiraOrange2 | coelenterazine | 560 | [35] | |
| - | NLuc | YFP | furimazine | 530 | [36] | |
| - | NLuc | Venus | furimazine | 535 | [37] | |
| - | NLuc | mKate2 | furimazine | 633 | [38] | |
| CeNL | NLuc∆N3 | mTurquoise2∆C10 | furimazine | 475 | [39] | |
| GeNL | NLuc∆N5 | mNeonGreen∆C10 | furimazine | 520 | [39] | |
| YeNL | NLuc∆N4 | Venus∆C12 | furimazine | 530 | [39] | |
| ReNL | NLuc∆N5 | tdTomato∆C9 | furimazine | 585 | [39] | |
| OeNL | NLuc | mKOκ | furimazine | 565 | [39] | |
| GpNLuc LumiFluor | NLuc | EGFP | furimazine | 509 | 45-fold brighter than Nano-lantern | [40] |
| OgNLuc LumiFluor | NLuc | LSS mOrange | furimazine | 572 | [40] | |
| Antares | NLuc | CyOFP1 | furimazine, hydrofurimazine, or fluorofurimazine | 583 | Similar or higher brightness in vivo compared to AkaBLI with hydrofurimazine, or fluorofurimazine | [41,42] |
| Antares2 | teLuc | CyOFP1 | diphenylterazine | 583 | Additional 35–90% signal increase over teLuc, | [24] |
| - | LumiLuc (teLuc with E4G, L18Q, S19A, V27L, S28T, G67C, G71A, N85D, V90A, R112Q, V119K, and K136T mutations) | mScarlet | 8pyDTZ | 600 | [25] | |
| - | FLuc | DsRed | d-luciferin | 583 | [43] | |
| - | mCherry | d-luciferin | 610 | [44] | ||
| - | mKate | d-luciferin | 635 | [45] | ||
| - | Cy3.5 | d-luciferin | 596 | [46] | ||
| - | Ppy RE10 | Alexa Fluor 680 | d-luciferin | 705 | BRET ratio: 34.0 | [47] |
| - | Alexa Fluor 750 | d-luciferin | 783 | BRET ratio: 4.0 | [47] | |
| - | NLuc | Alexa Fluor 488 | furimazine | 525 | Via SNAP-tag or HaloTag7 | [48] |
| - | TMR | furimazine | 585 | Via SNAP-tag or HaloTag7 | [48] | |
| - | CPY | furimazine | 645 | Via SNAP-tag or HaloTag7 | [48] | |
| SiR | furimazine | 670 | Via SNAP-tag or HaloTag7 | [48] | ||
| - | RLuc8 | QD605 | coelenterazine | 605 | BRET ratio: 0.70 | [49] |
| - | QD655 | coelenterazine | 655 | BRET ratio: 1.2 | [49,50,51] | |
| - | QD705 | coelenterazine | 705 | BRET ratio: 2.3 | [49] | |
| - | QD800 | coelenterazine | 800 | BRET ratio: 1.32 | [49] | |
| - | NLuc | QD705 | furimazine | 705 | BRET ratio: 13.3 | [52] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, M.; Ozawa, T. Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission. Int. J. Mol. Sci. 2020, 21, 6538. https://doi.org/10.3390/ijms21186538
Endo M, Ozawa T. Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission. International Journal of Molecular Sciences. 2020; 21(18):6538. https://doi.org/10.3390/ijms21186538
Chicago/Turabian StyleEndo, Mizuki, and Takeaki Ozawa. 2020. "Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission" International Journal of Molecular Sciences 21, no. 18: 6538. https://doi.org/10.3390/ijms21186538
APA StyleEndo, M., & Ozawa, T. (2020). Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission. International Journal of Molecular Sciences, 21(18), 6538. https://doi.org/10.3390/ijms21186538






