Fingolimod (FTY720) Preserves High Energy Phosphates and Improves Cardiac Function in Heterotopic Heart Transplantation Model
Abstract
1. Introduction
2. Results
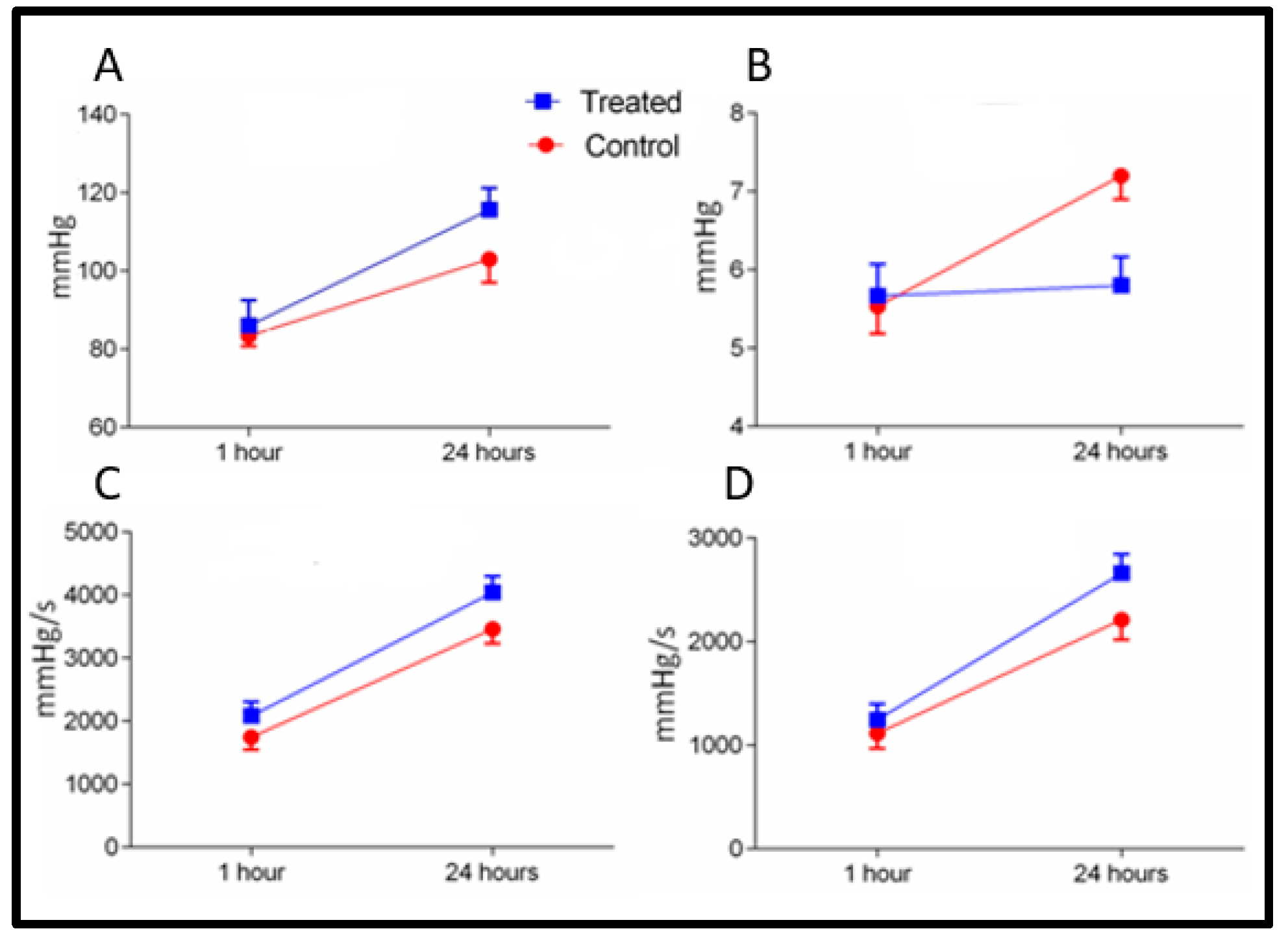
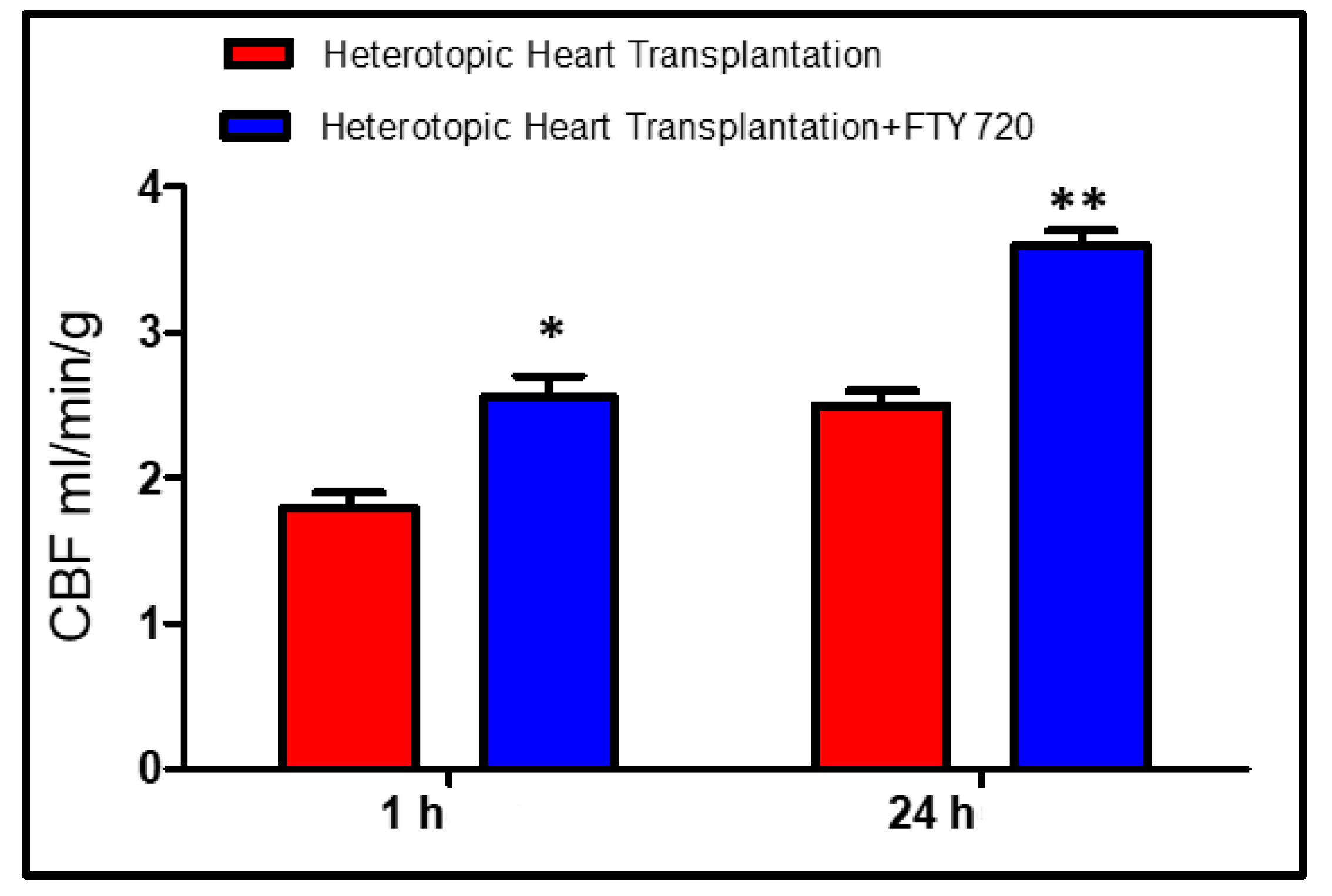
2.1. Early Reperfusion (Groups A and B)
2.2. Late Reperfusion (Groups C and D)
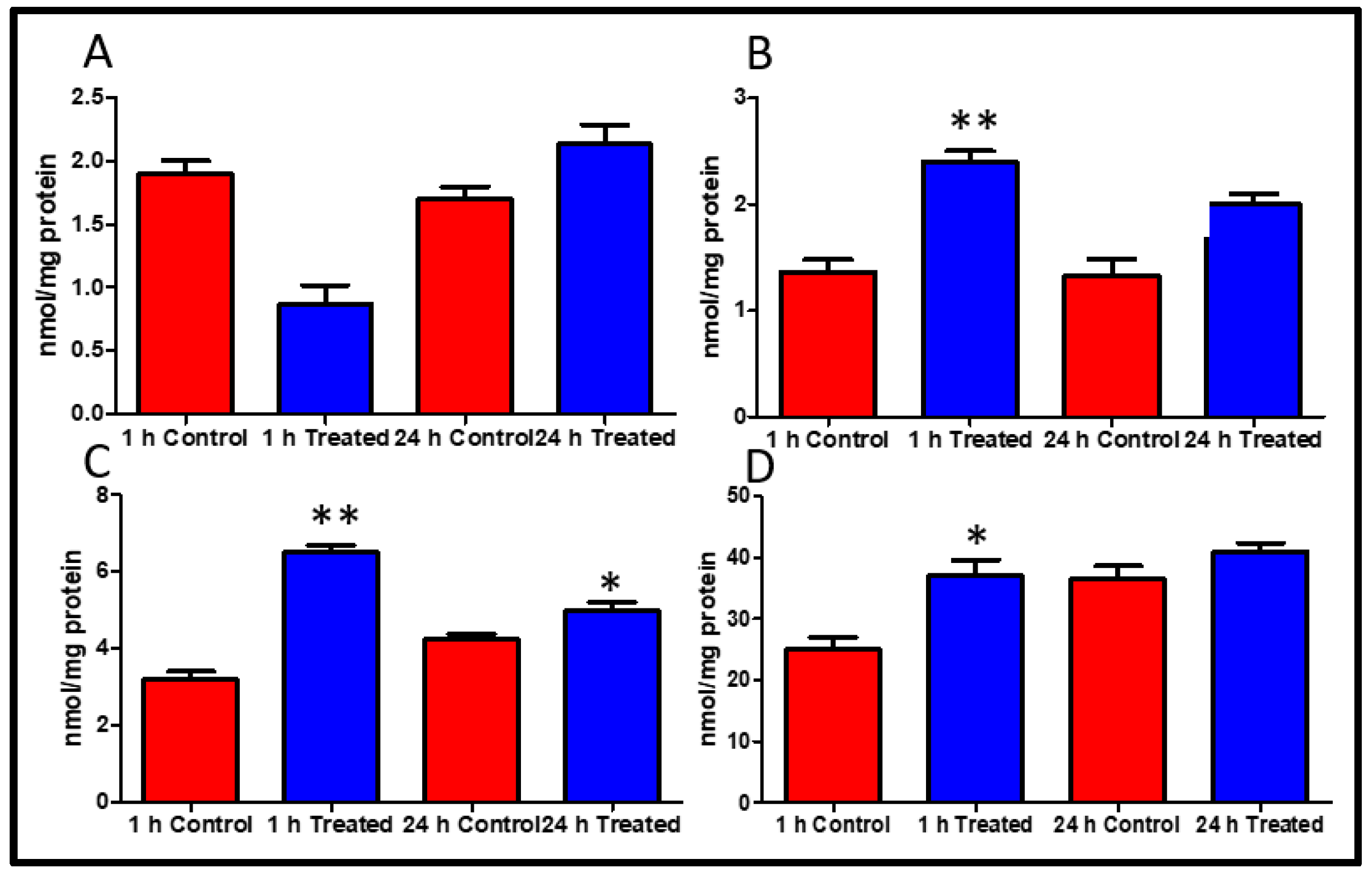
2.3. High-Energy Phosphates
2.4. Peroxynitrite Expression
2.5. Caspase 3 and PARP-1 Inhibition
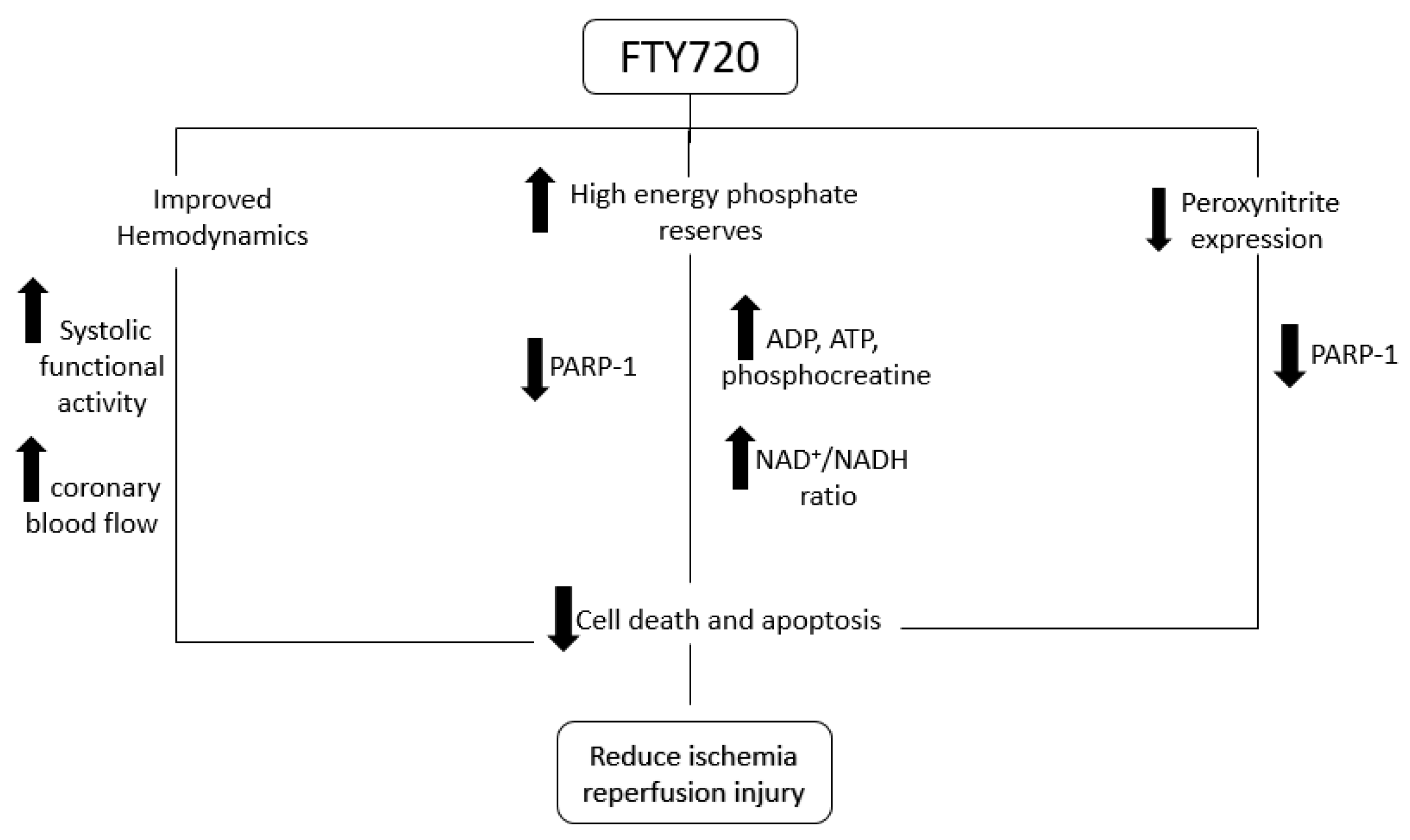
3. Discussion
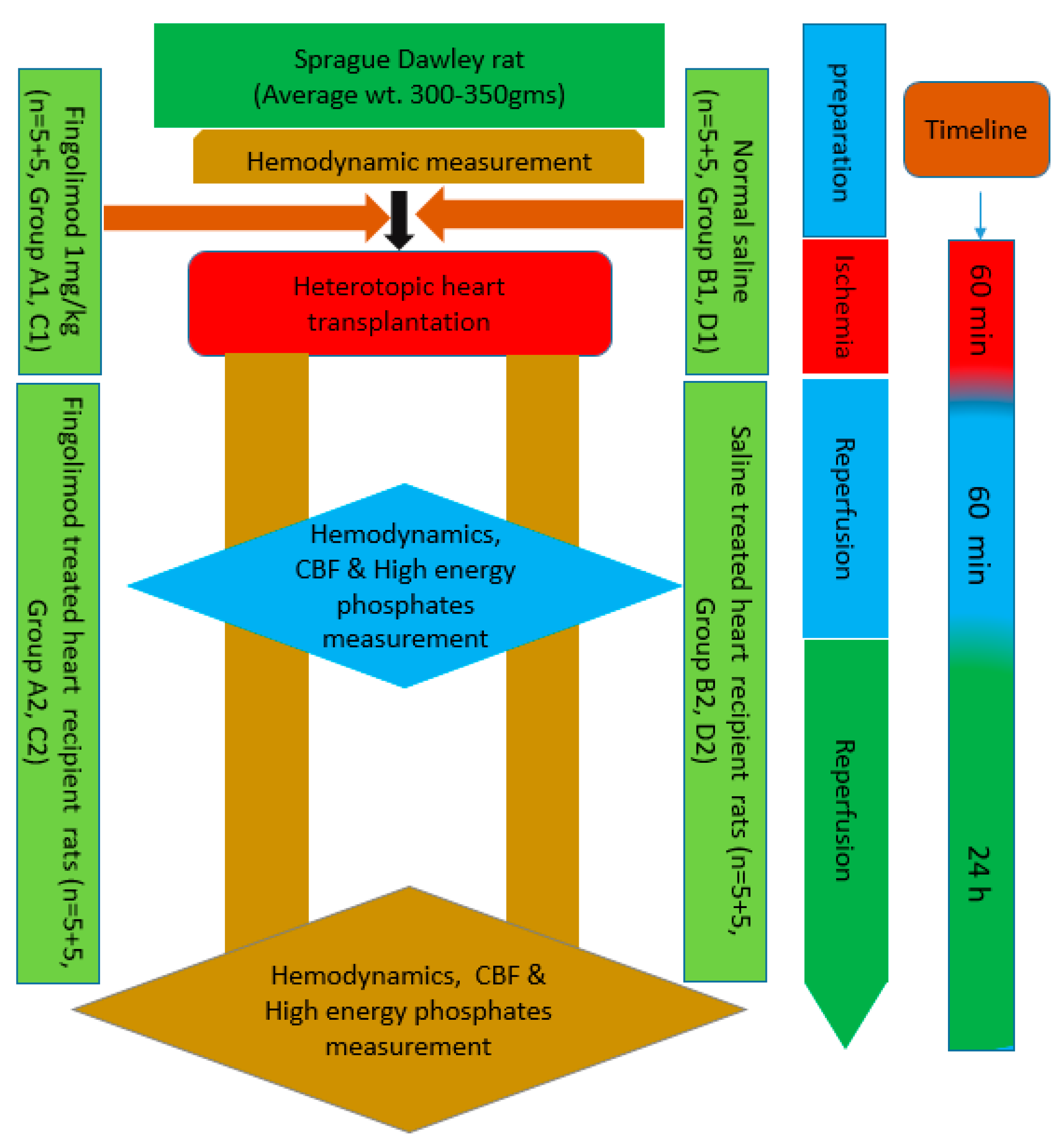
4. Materials and Methods
4.1. Functional Measurements of the Graft
4.2. High-Energy Phosphates Measurement
4.3. Measurement of Peroxynitrite Expression
4.4. Measurement of PARP and Caspase 3 Activation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Koerner, M.M.; Durand, J.-B.A.; Lafuente, J.; Noon, G.P.; Torre-Amione, G. Cardiac transplantation: The final therapeutic option for the treatment of heart failure. Curr. Opin. Cardiol. 2000, 15, 178–182. [Google Scholar] [CrossRef]
- Stoica, S.C. High-energy phosphates and the human donor heart. J. Hear. Lung Transplant. 2004, 23 (Suppl. 9), S244–S246. [Google Scholar] [CrossRef]
- Jennings, R.B.; Hawkins, H.K.; Lowe, J.E.; Hill, M.L.; Klotman, S.; Reimer, K.A. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am. J. Pathol. 1978, 92, 187–214. [Google Scholar]
- Huang, C.; Cui, Y.; Ji, L.; Zhang, W.; Li, R.; Ma, L.; Xing, W.; Zhou, H.; Chen, B.; Yu, J.; et al. Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult. Pharm. Biol. 2013, 51, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Behjati, M.; Etemadifar, M.; Esfahani, M.A. Cardiovascular effects of fingolimod: A review article. Iran. J. Neurol. 2014, 13, 119–126. [Google Scholar] [PubMed]
- Yopp, A.C.; Ochando, J.; Mao, M.; Ledgerwood, L.; Ding, Y.; Bromberg, J.S. Sphingosine 1-Phosphate Receptors Regulate Chemokine-Driven Transendothelial Migration of Lymph Node but Not Splenic T Cells. J. Immunol. 2005, 175, 2913–2924. [Google Scholar] [CrossRef]
- Aytan, N.; Choi, J.-K.; Carreras, I.; Brinkmann, V.; Kowall, N.W.; Jenkins, B.G.; Dedeoglu, A. Fingolimod modulates multiple neuroinflammatory markers in a mouse model of Alzheimer’s disease. Sci. Rep. 2016, 6, 24939. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Role of fingolimod in cardioprotection. In Pathophysiology of Ischemia Reperfusion Injury and Use of Fingolimod in Cardioprotection; Academic Press: Cambridge, MA, USA, 2019; pp. 101–121. [Google Scholar]
- Ahmed, N. Cardioprotective mechanism of FTY720 in ischemia reperfusion injury. J. Basic Clin. Physiol. Pharmacol. 2019, 30. [Google Scholar] [CrossRef]
- Szabó, G.; Liaudet, L.; Hagl, S.; Szabó, C. Poly (ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc. Res. 2004, 61, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Theilmeier, G.; Schmidt, C.; Herrmann, J.; Keul, P.; Schäfers, M.; Herrgott, I.; Mersmann, J.; Larmann, J.; Hermann, S.; Stypmann, J.; et al. High-Density Lipoproteins and Their Constituent, Sphingosine-1-Phosphate, Directly Protect the Heart Against Ischemia/Reperfusion Injury In Vivo via the S1P 3 Lysophospholipid Receptor. Circulation 2006, 114, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Cardioprotective Role of Sphingosine 1-Phosphate Receptor Agonist Fingolimod (FTY720) in Global Ischemia-Reperfusion Models; IRIS Verona: Verona, Italy, 2017; pp. 126–132. [Google Scholar]
- Ahmed, N.; Laghari, A.H.; Albkhoor, B.; Tabassum, S.; Meo, S.A.; Muhammad, N.; Linardi, D.; Al-Masri, A.A.; Fumagalli, G.; Luciani, G.B.; et al. Fingolimod Plays Role in Attenuation of Myocardial Injury Related to Experimental Model of Cardiac Arrest and Extracorporeal Life Support Resuscitation. Int. J. Mol. Sci. 2019, 20, 6237. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Linardi, D.; Muhammad, N.; Chiamulera, C.; Fumagalli, G.; Biagio, L.S.; Gebrie, M.A.; Aslam, M.; Luciani, G.B.; Faggian, G.; et al. Sphingosine 1-Phosphate Receptor Modulator Fingolimod (FTY720) Attenuates Myocardial Fibrosis in Post-heterotopic Heart Transplantation. Front. Pharmacol. 2017, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mehmood, A.; Linardi, D.; Sadiq, S.; Tessari, M.; Meo, S.A.; Rehman, R.; Hajjar, W.M.; Muhammad, N.; Iqbal, M.P.; et al. Cardioprotective Effects of Sphingosine-1-Phosphate Receptor Immunomodulator FTY720 in a Clinically Relevant Model of Cardioplegic Arrest and Cardiopulmonary Bypass. Front. Pharmacol. 2019, 10, 802. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef]
- Czubowicz, K.; Strosznajder, R. Ceramide in the Molecular Mechanisms of Neuronal Cell Death. The Role of Sphingosine-1-Phosphate. Mol. Neurobiol. 2014, 50, 26–37. [Google Scholar] [CrossRef]
- Booz, G.W. PARP Inhibitors and Heart Failure? Translational Medicine Caught in the Act. Congest. Hear. Fail. 2007, 13, 105–112. [Google Scholar] [CrossRef]
- Strosznajder, R.; Jęsko, H.; Zambrzycka, A. Poly(ADP-Ribose) Polymerase: The Nuclear Target in Signal Transduction and Its Role in Brain Ischemia–Reperfusion Injury. Mol. Neurobiol. 2005, 31, 149–168. [Google Scholar] [CrossRef]
- Carlucci, F.; Tabucchi, A.; Biagioli, B.; Simeone, F.; Scolletta, S.; Rosi, F.; Marinello, E. Cardiac surgery: Myocardial energy balance, antioxidant status and endothelial function after ischemia–reperfusion. Biomed. Pharmacother. 2002, 56, 483–491. [Google Scholar] [CrossRef]
- Szabo, G.; Stumpf, N.; Radovits, T.; Sonnenberg, K.; Gerö, D.; Hagl, S.; Szabó, C.; Bährle, S. Effects of inosine on reperfusion injury after heart transplantation. Eur. J. Cardio-Thorac. Surg. 2006, 30, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, H.; Yue, X.; Squier, M.; Lorek, A.; Cady, E.; Penrice, J.; Sarraf, C.; Wylezinska, M.; Kirkbride, V.; Cooper, C.E.; et al. Increased apoptosis in the cingulate sulcus of newborn piglets following transient hypoxia-ischaemia is related to the degree of high energy phosphate depletion during the insult. Neurosci. Lett. 1994, 181, 121–125. [Google Scholar] [CrossRef]
- Desrois, M.; Piccardo, A.; Zogheib, E.; Dalmasso, C.; Lan, C.; Fourré, D.; Cozzone, P.; Caus, T.; Bernard, M. Heart Donation After Cardiac Death: Preliminary Study on an Isolated, Perfused Swine Heart After 20 Minutes of Normothermic Ischemia. Transplant. Proc. 2014, 46, 3314–3318. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Stiegler, P.; Taeubl, P.; Sereinigg, M.; Puntschart, A.; Bradatsch, A.; Curcic, P.; Seifert-Held, T.; Zmugg, G.; Stojakovic, T.; et al. Energy status of pig donor organs after ischemia is independent of donor type. J. Surg. Res. 2013, 180, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Chen, S.-Q.; Li, Z.-P.; Zhang, J.; Xue, J.-K.; Wang, W.-T.; Huang, W.-J.; Cheng, J.-Y.; Li, H.-P. Effect of exogenous phosphocreatine on cardiomycytic apoptosis and expression of Bcl-2 and Bax after cardiopulmonary resuscitation in rats. World J. Emerg. Med. 2011, 2, 291–295. [Google Scholar] [CrossRef]
- Szabó, G.; Bährle, S.; Stumpf, N.; Sonnenberg, K.; Szabó, E.; Pacher, P.; Csont, T.; Schulz, R.; Dengler, T.J.; Liaudet, L.; et al. Poly(ADP-Ribose) Polymerase Inhibition Reduces Reperfusion Injury After Heart Transplantation. Circ. Res. 2002, 90, 100–106. [Google Scholar] [CrossRef]
- Koh, D.W.; Dawson, T.M.; Dawson, V.L. Mediation of cell death by poly(ADP-ribose) polymerase-1. Pharmacol. Res. 2005, 52, 5–14. [Google Scholar] [CrossRef]
- Szabo, G.; Soós, P.; Heger, U.; Flechtenmacher, C.; Bährle, S.; Zsengellér, Z.; Szabo, C.; Hagl, S. Poly(ADP-ribose) polymerase inhibition attenuates biventricular reperfusion injury after orthotopic heart transplantation? Eur. J. Cardio-Thorac. Surg. 2005, 27, 226–234. [Google Scholar] [CrossRef]
- Ahmed, N. Experimental models for ischemia–reperfusion injury. In Pathophysiology of Ischemia Reperfusion Injury and Use of Fingolimod in Cardioprotection; Ahmed, N., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 87–100. [Google Scholar]
- Hallström, S.; Gasser, H.; Neumayer, C.; Fügl, A.; Nanobashvili, J.; Jakubowski, A.; Huk, I.; Schlag, G.; Malinski, T. S-Nitroso Human Serum Albumin Treatment Reduces Ischemia/Reperfusion Injury in Skeletal Muscle via Nitric Oxide Release. Circulation 2002, 105, 3032–3038. [Google Scholar] [CrossRef][Green Version]


| Hemodynamic Variables | A1 and C1 (FTY720 Group) Baseline | B1 and D1 (Control Group) Baseline | p Value |
|---|---|---|---|
| Aortic systolic pressure (mmHg) | 114.4 ± 1.9 | 116.6 ± 2.8 | 0.2783 |
| Aortic diastolic pressure (mmHg) | 90.6 ± 3.1 | 93.7 ± 3.7 | 0.4210 |
| Body weight (g) | 319 ± 8.2 | 327 ± 11.4 | 0.3136 |
| Heart rate/min | 364 ± 7.6 | 359 ± 11.5 | 0.5398 |
| Left ventricular end-systolic pressure (mmHg) | 116.6 ± 5.1 | 115.6 ± 4.9 | 0.4298 |
| Left ventricular end-diastolic pressure (mmHg) | 7.6 ± 0.8 | 7.4 ± 0.5 | 0.3746 |
| Left ventricular dP/dTmax (mmHg/s) | 7246 ± 189.2 | 6987 ± 158.8 | 0.4330 |
| Left ventricular dP/dTmin (mmHg/s) | −9002 ± 387.3 | −9180 ± 299.6 | 0.5673 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, N.; Farooq, J.; Sadiq, S.; Meo, S.A.; Jan, A.; Cheema, F.H.; Faggian, G.; Rungatscher, A. Fingolimod (FTY720) Preserves High Energy Phosphates and Improves Cardiac Function in Heterotopic Heart Transplantation Model. Int. J. Mol. Sci. 2020, 21, 6548. https://doi.org/10.3390/ijms21186548
Ahmed N, Farooq J, Sadiq S, Meo SA, Jan A, Cheema FH, Faggian G, Rungatscher A. Fingolimod (FTY720) Preserves High Energy Phosphates and Improves Cardiac Function in Heterotopic Heart Transplantation Model. International Journal of Molecular Sciences. 2020; 21(18):6548. https://doi.org/10.3390/ijms21186548
Chicago/Turabian StyleAhmed, Naseer, Javeria Farooq, Soban Sadiq, Sultan Ayoub Meo, Azam Jan, Faisal H. Cheema, Giuseppe Faggian, and Alessio Rungatscher. 2020. "Fingolimod (FTY720) Preserves High Energy Phosphates and Improves Cardiac Function in Heterotopic Heart Transplantation Model" International Journal of Molecular Sciences 21, no. 18: 6548. https://doi.org/10.3390/ijms21186548
APA StyleAhmed, N., Farooq, J., Sadiq, S., Meo, S. A., Jan, A., Cheema, F. H., Faggian, G., & Rungatscher, A. (2020). Fingolimod (FTY720) Preserves High Energy Phosphates and Improves Cardiac Function in Heterotopic Heart Transplantation Model. International Journal of Molecular Sciences, 21(18), 6548. https://doi.org/10.3390/ijms21186548





