Absorption and Emission Spectroscopic Investigation of the Thermal Dynamics of the Archaerhodopsin 3 Based Fluorescent Voltage Sensor Archon2
Abstract
:1. Introduction
2. Results
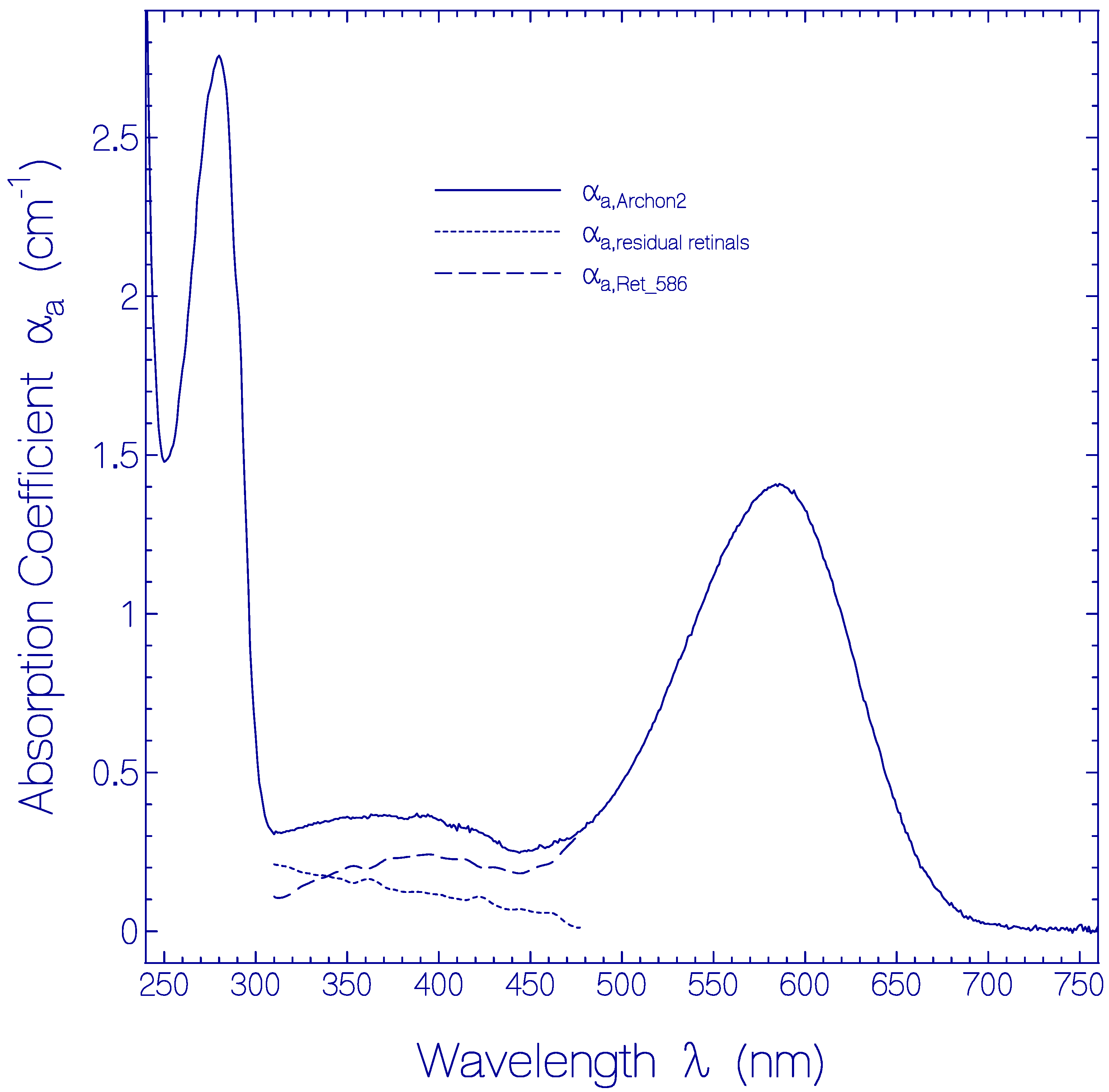
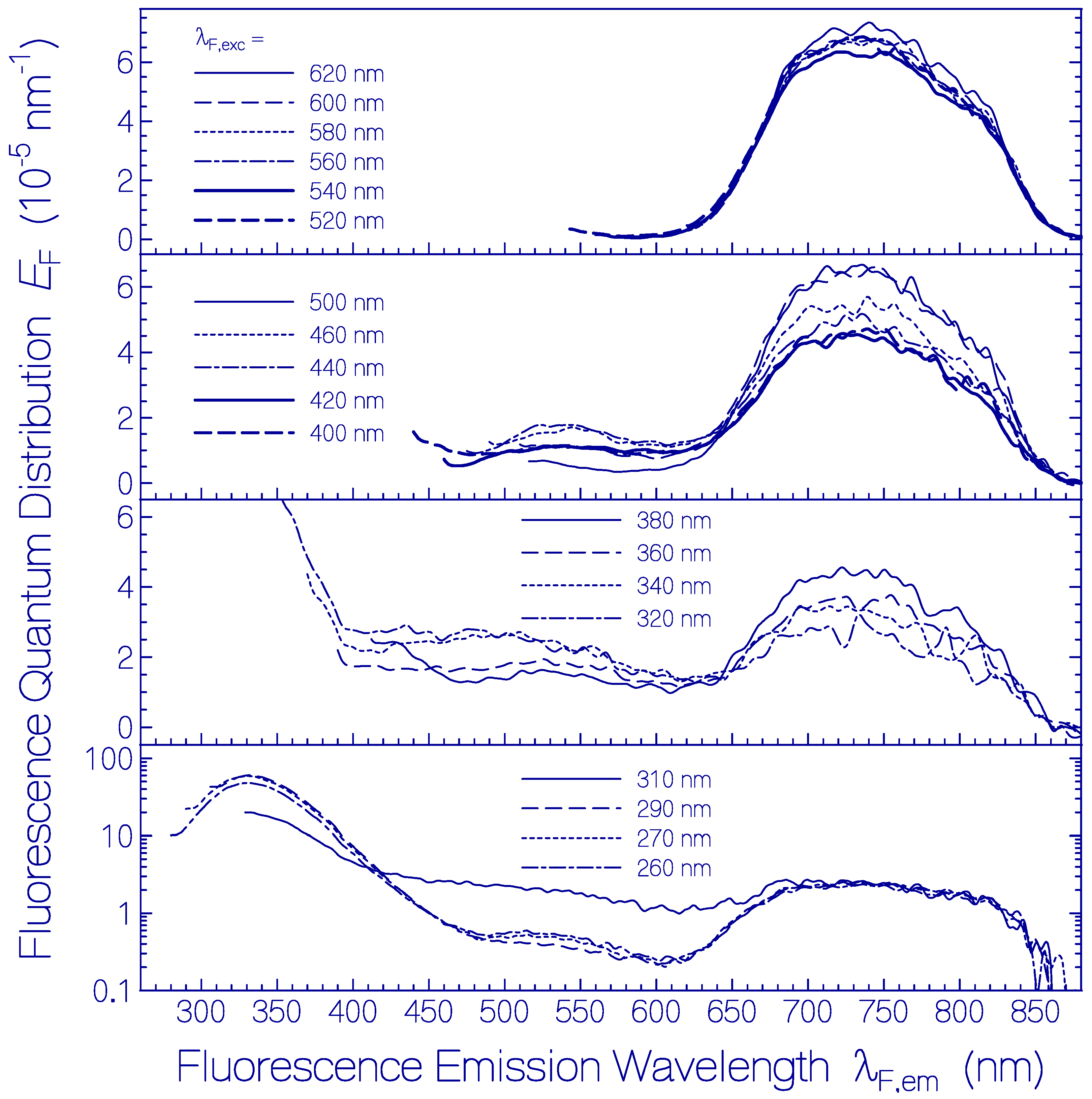
2.1. Absorption and Emission Behavior of Fresh Thawed Archon2 Samples
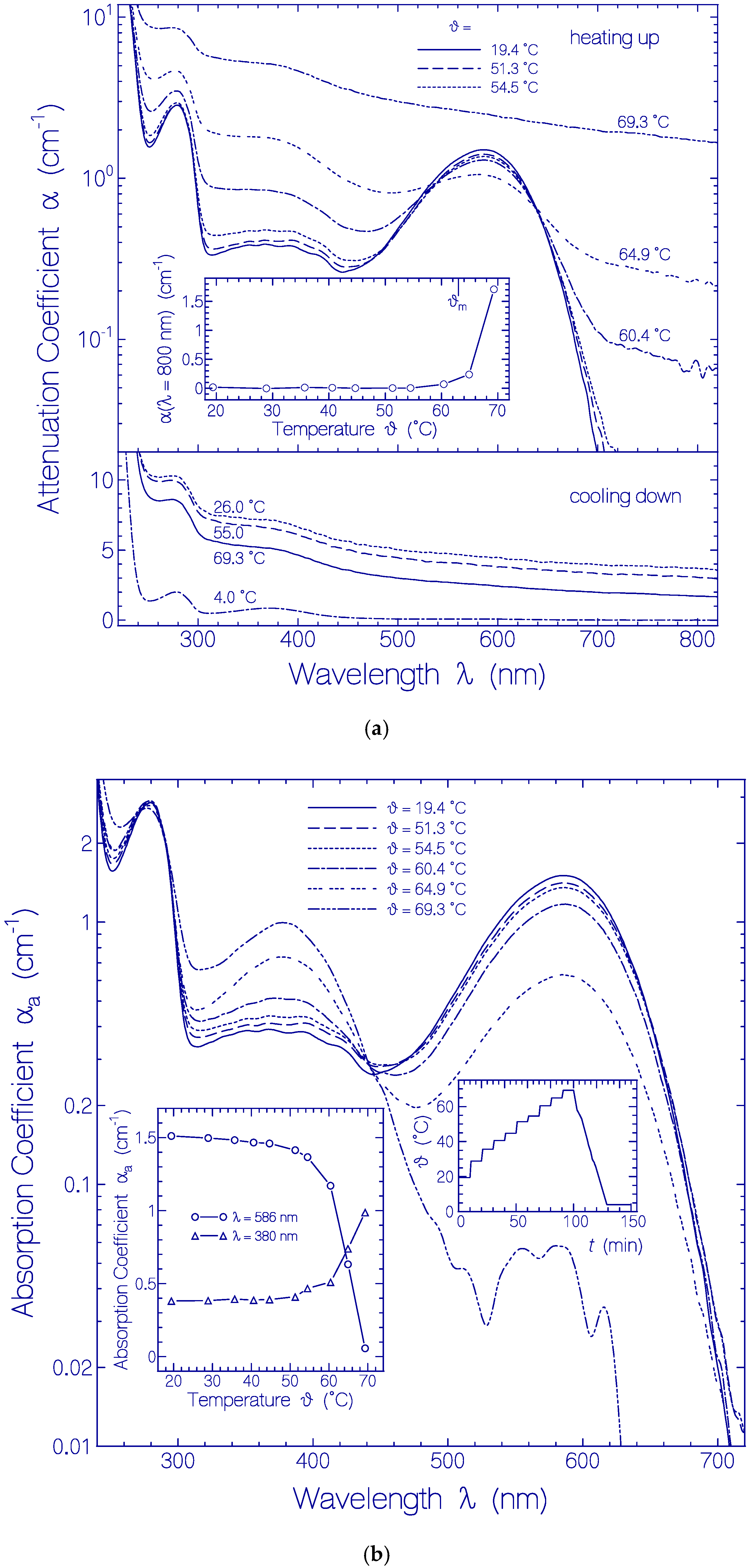
2.2. Heating-Cooling Cycle of a Fresh Thawed Archon2 Sample
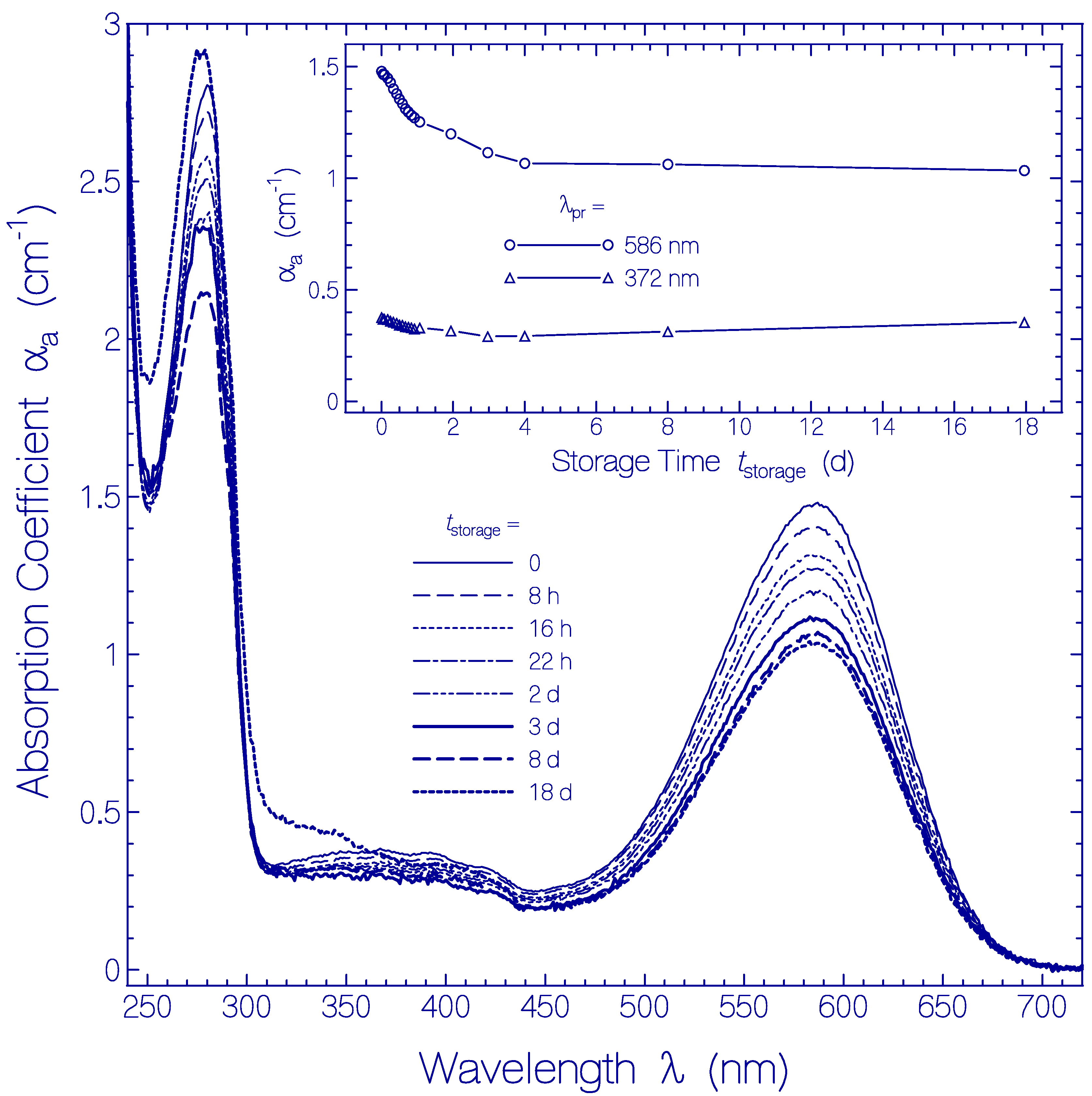
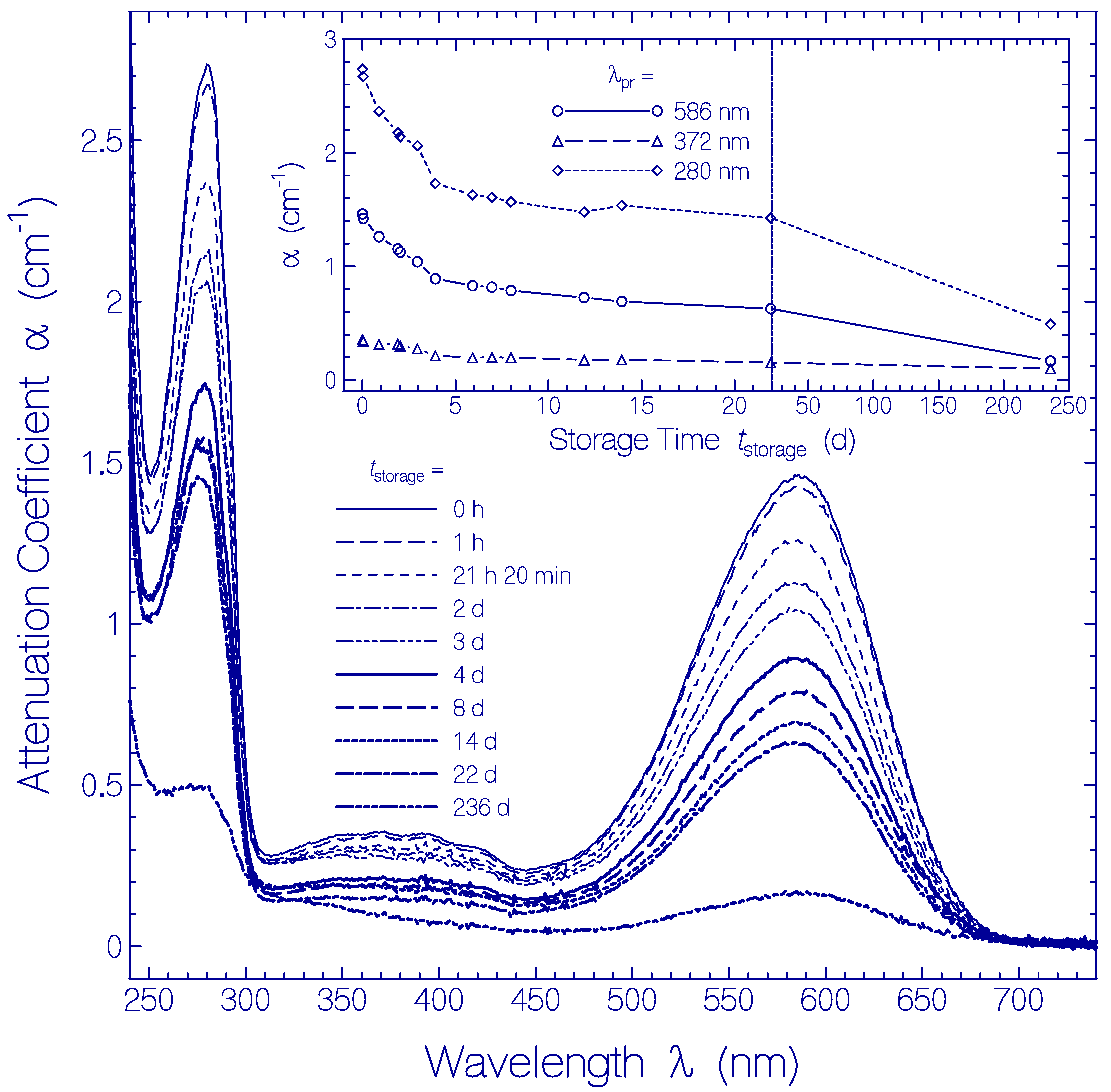
2.3. Temporal Development of Archon2 at Room Temperature
2.4. Temporal Development of Archon2 at Refrigerator Temperature
3. Discussion
3.1. Behavior of Fresh Thawed Archon2
3.2. Heat Denaturation of Archon2
3.3. Thermal Aging of Archon2
3.4. Comparison of Archon2 with QuasAr1
4. Materials and Methods
4.1. Sample Preparation
4.2. Spectroscopic Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Arch | Archaerhodopsin 3 from Halorubrum sodomense |
| Archon1 | Rhodopsin based on Arch with thirteen point mutations |
| Archon2 | Rhodopsin based on Arch with ten point mutations |
| FRET | Förster resonance energy transfer |
| GECI | Genetically encoded calcium indicator |
| GEVI | Genetically encoded voltage indicator |
| PRSB | Protonated retinal Schiff base |
| QuasAr | Quality superior to Arch |
| Ret_xxx | Retinal with absorption maximum approximately at xxx nm |
| RSB | Retinal Schiff base |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| VSD | Voltage sensing domain |
References
- Ainsworth, M.; Lee, S.; Cunningham, M.O.; Traub, R.D.; Kopell, N.J.; Whittington, M.A. Rates and rhythms: A synergistic view of frequency and temporal coding in neural networks. Neuron 2012, 75, 572–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoham, D.; Glaser, D.E.; Arieli, A.; Kenet, T.; Wijnbergen, C.; Toledo, Y.; Hildesheim, R.; Grinvald, A. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron 1999, 24, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, G.; Liu, P.; Naing, S.H.H.; Muller, V.R.; Miller, E.W. Synthesis of sulfonated carbofluoresceins for voltage imaging. J. Am. Chem. Soc. 2019, 141, 6631–6638. [Google Scholar] [CrossRef]
- Mank, M.; Griesbeck, O. Genetically encoded calcium indicators. Chem. Rev. 2008, 108, 1550–1564. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Upadhyay, H.; Murphy, M.; Borja, G.; Rozsahegyi, E.M.; Barnett, A.; Brookings, T.; McManus, O.B.; Werley, C.A. Simultanous voltage and calcium imaging and optogenetic stimulation with high sensitivity and a wide field of view. Biomed. Opt. Express 2019, 10, 789–806. [Google Scholar] [CrossRef]
- Bando, Y.; Grimm, C.; Cornejo, V.H.; Yuste, R. Genetic voltage indicators. BMC Biol. 2019, 17, 71. [Google Scholar] [CrossRef]
- Panzera, L.C.; Hoppa, M.B. Genetically encoded voltage indicators are illuminating subcellular physiology of the axon. Front. Cell. Neurosci. 2019, 13, 52. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Iwasaki, H.; Sasaki, M.; Inaba, K.; Okamura, Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 2005, 435, 1239–1243. [Google Scholar] [CrossRef]
- Mutoh, H.; Akemann, W.; Knöpfel, T. Genetically engineered fluorescent voltage reporters. ACS Chem. Neurosci. 2012, 3, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.Z.; Schnitzer, M.J. Genetically encoded indicators of neuronal activity. Nat. Neurosci. 2016, 19, 1142–1153. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zou, P.; Cohen, A.E. Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol. 2017, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Sakamoto, M.; Kim, S.; Ayzenshtat, I.; Yuste, R. Comparative evaluation of genetically encoded voltage indicators. Cell Rep. 2019, 26, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.E.; Lee, S.; Baker, B.J. Optical consequences of a genetically-encoded voltage indicator with a pH sensitive fluorescent protein. Neurosci. Res. 2019, 146, 13–21. [Google Scholar] [CrossRef]
- Kralj, J.M.; Hochbaum, D.R.; Douglass, A.D.; Cohen, A.E. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science 2011, 333, 345–348. [Google Scholar] [CrossRef]
- Gong, Y. The evolving capabilities of rhodopsin-based genetically encoded voltage indicators. Curr. Opin. Chem. Biol. 2015, 27, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, N.A.; Beergs, A.C.F.; Schüler, C.; Scheiwe, A.R.; Costa, W.S.; Bach, M.; Liewald, J.F.; Gottschalk, A. Rhodopsin-based voltage imaging tools for use in muscles and neurons of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2019, 116, 17051–17060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Zhao, Y.; Douglass, A.D.; Hochbaum, D.R.; Brinks, D.; Werley, C.A.; Harrison, D.J.; Campbell, R.E.; Cohen, A.E. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat. Commun. 2014, 5, 4625. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Wagner, M.J.; Li, Z.; Schnitzer, M.J. Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors. Nat. Commun. 2014, 5, 3674. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, C.; Li, J.Z.; Grewe, B.F.; Zhang, Y.; Eismann, S.; Schnitzer, M.J. High-speed recoding of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 2015, 350, 1361–1366. [Google Scholar] [CrossRef] [Green Version]
- González, J.E.; Tsien, R.Y. Voltage sensing by fluorescence resonance energy transfer in single cells. Biophys. J. 1995, 69, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Chanda, B.; Blunck, R.; Faria, L.C.; Schweizer, F.E.; Mody, I.; Bezanilla, F. A hybrid approach to measuring electrical activity in genetically specified neurons. Nat. Neurosci. 2005, 8, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.S.; Farhi, S.L.; Zhao, Y.; Brinks, D.; Zou, P.; Ruangkittisakul, A.; Platisa, J.; Pieribone, V.A.; Ballanyi, K.; Cohen, A.E.; et al. A bright and fast red fluorescent voltage indicator that reports neuronal activity in organotypic brain slices. J. Neurosci. 2016, 36, 2458–2472. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.; Grenier, V.; Hong, W.; Muller, V.R.; Miller, E.W. Fluorgenic targeting of voltage-sensitive dyes to neurons. J. Am. Chem. Soc. 2017, 139, 17334–17340. [Google Scholar]
- Grenier, V.; Daws, B.R.; Liu, P.; Miller, E.W. Spying on neuronal membrane potential with genetically targetable voltage indicators. J. Am. Chem. Soc. 2019, 141, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Sundukova, M.; Prifti, E.; Bucci, A.; Krillova, K.; Serrao, J.; Reymond, L.; Umebayashi, M.; Hovius, R.; Riezman, H.; Johnsson, K.; et al. A chemogenetic approach for optical monitoring of voltage in neurons. Angew. Chem. Int. Ed. 2018, 58, 2341–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelfattah, A.S.; Kawashima, T.; Singh, A.; Novak, O.; Liu, H.; Shuai, Y.; Huang, Y.-C.; Campagnola, L.; Seeman, S.C.; Yu, J.; et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 2019, 364, 699–704. [Google Scholar] [CrossRef]
- Kralj, J.; Douglass, A.D.; Hochbaum, D.R.; Maclaurin, D.; Cohen, A.E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat. Methods 2012, 9, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Maclaurin, D.; Venkatachalam, V.; Lee, H.; Cohen, A.E. Mechanism of voltage-sensitive fluorescence in a microbial rhodopsin. Proc. Natl. Acad. Sci. USA 2013, 110, 5939–5944. [Google Scholar] [CrossRef] [Green Version]
- Flytzanis, N.C.; Bedbrook, C.N.; Engqvist, M.K.M.; Xiao, C.; Chan, K.Y.; Sternberg, P.W.; Arnold, F.H.; Gradinaru, V. Archaerhodopsin variants with enhanced voltage-sensitive fluorescence in mammalian and Caenorhabditis elegans neurons. Nat. Commun. 2014, 5, 4894. [Google Scholar] [CrossRef] [Green Version]
- Adam, Y.; Kim, J.J.; Lou, S.; Zhao, Y.; Brinks, D.; Wu, H.; Mostajo-Radji, M.A.; Kheifets, S.; Parot, V.; Chettih, S.; et al. All-optical electrophysiology reveals brain-state dependent changes in hippocampal subthreshold dynamics and excitability. bioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Piatkevich, K.D.; Jung, E.E.; Straub, C.; Linghu, C.; Park, D.; Suk, H.J.; Hochbaum, D.R.; Goodwin, D.; Pnevmatikakis, E.; Pak, N.; et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 2018, 14, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Förster, T. Fluoreszenz Organischer Verbindungen; Vandenhoeck und Ruprecht: Göttingen, Germany, 1951; pp. 83–86. [Google Scholar]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 213–261. [Google Scholar]
- Gong, Y.; Li, J.Z.; Schnitzer, M.J. Enhanced Archaerhodopsin fluorescent protein voltage indicators. PLoS ONE 2013, 8, e66959. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Vasan, G.; Huang, C.; Haziza, S.; Li, J.Z.; Inan, H.; Schnitzer, M.J.; Pieribone, V.A. Fast, in vivo voltage imaging using a red fluorescent indicator. Nat. Methods 2018, 15, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Piatkevich, K.D.; Bensussen, S.; Tseng, H.-A.; Shroff, S.N.; Lopez-Huerta, V.G.; Park, G.; Jung, E.E.; Shemesh, O.A.; Straub, C.; Gritton, H.J.; et al. Population imaging of neural activity in awake behaving mice. Nature 2019, 574, 413–417. [Google Scholar] [CrossRef]
- Kandori, H.; Shichida, Y.; Yoshizawa, T. Photoisomerization in rhodopsin. Biochemistry 2001, 66, 1197–1209. [Google Scholar] [CrossRef]
- Wand, A.; Gdor, I.; Zhu, J.; Sheves, M.; Ruhman, S. Shedding new light on retinal protein photochemistry. Annu. Rev. Phys. Chem. 2013, 64, 437–458. [Google Scholar] [CrossRef]
- Alexiev, U.; Farrens, D.L. Fluorescence spectroscopy of rhodopsins: Insights and approaches. Biochim. Biophys. Acta 2014, 1837, 694–709. [Google Scholar] [CrossRef] [Green Version]
- Ernst, O.P.; Lodowski, D.T.; Elstner, M.; Hegemann, P.; Brown, L.S.; Kandori, H. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem. Rev. 2014, 114, 126–163. [Google Scholar] [CrossRef]
- Penzkofer, A.; Hegemann, P.; Kateriya, S. Organic dyes in optogenetics. In Organic Lasers and Organic Photonics; Duarte, F.J., Ed.; IOP Publishing: Bristol, UK, 2018; pp. 13-1–13-114. [Google Scholar]
- Adam, Y.; Kim, J.J.; Lou, S.; Zhao, Y.; Xie, M.E.; Brinks, D.; Wu, H.; Mostajo-Radji, M.A.; Kheifets, S.; Parot, V.; et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 2019, 569, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Penzkofer, A.; Silapetere, A.; Hegemann, P. Absorption and emission spectroscopic investigation of the thermal dynamics of the Archaerhodopsin 3 based fluorescent voltage sensor QuasAr1. Int. J. Mol. Sci. 2019, 20, 4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penzkofer, A.; Silapetere, A.; Hegemann, P. Photocycle dynamics of the Archaerhodopsin 3 based fluorescent voltage sensor QuasAr1. Int. J. Mol. Sci. 2020, 21, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, S.; Adam, Y.; Weinstein, E.N.; Williams, E.; Williams, K.; Parot, V.; Kavokine, N.; Liberles, S.; Madisen, L.; Zeng, H.; et al. Genetically targeted all-optical electrophysiology with a transgenic Cre-dependent Optopatch mouse. J. Neurosci. 2016, 36, 11059–11073. [Google Scholar] [CrossRef] [PubMed]
- Penzkofer, A.; Luck, M.; Mathes, T.; Hegemann, P. Bistable retinal Schiff base photodynamics of histidine kinase rhodopsin HKR1 from Chlamydomonas reinhardtii. Photochem. Photobiol. 2014, 90, 773–785. [Google Scholar] [PubMed]
- Chen, R.F. Fluorescence quantum yields of tryptophan and tyrosine. Anal. Lett. 1967, 1, 35–42. [Google Scholar] [CrossRef]
- Eisinger, J.; Navon, G. Fluorescence quenching and isotope effect of tryptophan. J. Chem. Phys. 1969, 50, 2069–2077. [Google Scholar] [CrossRef]
- Kirby, E.P.; Steiner, R.F. The influence of solvent and temperature upon the fluorescence of indole derivatives. J. Phys. Chem. 1970, 74, 4480–4490. [Google Scholar] [CrossRef]
- Reineck, P.; Francis, A.; Orth, A.; Lau, D.W.M.; Nixon-Luke, R.D.V.; Rastogi, I.D.; Razali, W.A.W.; Cordina, N.M.; Parker, L.M.; Sreenivasan, V.K.A.; et al. Brightness and photostability of emerging red and near-IR fluorescent nanomaterials for bioimaging. Adv. Opt. Mater. 2016, 4, 1549–1557. [Google Scholar] [CrossRef]
- Penzkofer, A.; Scheib, U.; Hegemann, P.; Stehfest, K. Absorption and emission spectroscopic investigation of thermal dynamics and photo-dynamics of the rhodopsin domain of the rhodopsin-guanylyl cyclase from the aquatic fungus Blastocladiella emersonii. BAOJ Phys. 2016, 2, 6. [Google Scholar]
- Penzkofer, A.; Stierl, M.; Hegemann, P.; Kateriya, S. Thermal protein unfolding in photo-activated adenylate cyclase nano-clusters from the amoeboflagellate Naegleria gruberi NEG-M strain. J. Photochem. Photobiol. A Chem. 2011, 225, 42–51. [Google Scholar] [CrossRef]
- Weigand, R.; Rotermund, F.; Penzkofer, A. Aggregation dependent absorption reduction of indocyanine green. J. Phys. Chem. A 1997, 101, 7729–7734. [Google Scholar] [CrossRef]
- Penzkofer, A.; Shirdel, J.; Zirak, P.; Breitkreuz, H.; Wolf, E. Protein aggregation studied by forward light scattering and light transmission analysis. Chem. Phys. 2007, 342, 55–63. [Google Scholar] [CrossRef]
- Holzer, W.; Pichlmaier, M.; Penzkofer, A.; Bradley, D.D.C.; Blau, W.J. Fluorescence spectroscopic behavior of neat and blended polymer thin films. Chem. Phys. 1999, 246, 445–462. [Google Scholar] [CrossRef]
- Penzkofer, A. Photoluminescence behavior of riboflavin and lumiflavin in liquid solutions and solid films. Chem. Phys. 2012, 400, 142–153. [Google Scholar] [CrossRef]
- Madge, D.; Wong, R.; Seybold, P.G. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: Improved absolute standards for quantum yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar]
- Birkmann, C.; Penzkofer, A.; Tsuboi, T. Fluorescence excitation spectroscopic characterization of colour centres in a LiF crystal. Appl. Phys. B 2003, 77, 625–632. [Google Scholar] [CrossRef]
- Dörr, F. Spectroscopy with polarized light. Angew. Chem. Int. Ed. 1966, 5, 478–495. [Google Scholar] [CrossRef]
- Lindsey, J. PhotochemCAD Spectra by Category. Available online: https://omlc.org/spectra/PhotochemCAD/html/ (accessed on 7 July 2019).
- Tsuboi, T.; Penzkofer, A.; Lammel, O. Oscillator strength of F2- colour centres in LiF crystal. Opt. Quantum Electron. 2003, 35, 267–274. [Google Scholar] [CrossRef]
- Strickler, S.J.; Berg, R.A. Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Birks, J.B.; Dyson, D.J. The relations between the fluorescence and absorption properties of organic molecules. Proc. R. Soc. Lond. Ser. A 1963, 275, 135–148. [Google Scholar]
- Deshpande, A.V.; Beidoun, A.; Penzkofer, A.; Wagenblast, G. Absorption and emission spectroscopic investigation of cyanovinyldiethylaniline dye vapors. Chem. Phys. 1990, 142, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Weigand, R.; Rotermund, F.; Penzkofer, A. Degree of aggregation of indocyanine green in aqueous solutions determined by Mie scattering. Chem. Phys. 1997, 220, 373–384. [Google Scholar] [CrossRef]
- Bohren, G.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley and Sons: Hoboken, NJ, USA, 1983; pp. 3–429. [Google Scholar]
- Gratz, H.; Penzkofer, A.; Weidner, P. Nanometer particle size, pore size, and specific surface determination of colloidal suspensions and porous glasses by Rayleigh light scattering. J. Non Cryst. Solids 1995, 189, 50–54. [Google Scholar] [CrossRef]
- Fleming, G.R. Chemical Applications in Ultrafast Spectroscopy; Oxford University Press: New York, NY, USA, 1986; pp. 179–195. [Google Scholar]
- Voet, D.; Voet, J.G. Biochemistry, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 482–505. [Google Scholar]

| Parameter | Archon2 | QuasAr1 |
|---|---|---|
| C (mol dm−3) | 2.01 × 10−5 | 2.76 × 10−5 |
| Fresh Thawed Sample | ||
| Dominant PRSB | Ret_586 | Ret_580 |
| λa,max (nm) | 586 | 580 |
| κ | 0.784 | 0.862 |
| σa(λa,max) (cm2) | 1.485 × 10−16 | 1.593 × 10−16 |
| σa,int (cm) | 5.75 × 10−13 | 6.1 × 10−13 |
| f | 0.546 | 0.58 |
| ϕF | (1.05 ± 0.15) × 10−2 | (6.5 ± 0.5) × 10−3 |
| Bmax (M−1cm−1) | 408 ± 60 | 270 ± 20 |
| τrad (ns) | 10.1 | 9.32 |
| τF,SB (ps) | 106 | 61.5 |
| Heat Denatured Sample | ||
| ϑm (°C) | 63 ± 3 | 65 ± 3 |
| Dominant RSB | Ret_380 | Ret_380 |
| λa,max (nm) | 380 | 380 |
| σa(λa,max) (cm2) | 9.08 × 10−17 | 7.4 × 10−17 |
| σa,int (cm) | 5.95 × 10−13 | 5.2 × 10−13 |
| f | 0.56 | 0.49 |
| ϕF | 0.008 ± 0.001 | ≈0.04 |
| τrad (ns) | 3.31 | 3.74 |
| τF (ps) | 26.5 | 150 |
| Aging at Room Temperature | ||
| ϑ (°C) | 21 ± 1 | 21–25 |
| tonset of light scattering (h) | ≈48 | ≈12 |
| (d−1) | ≈0.14 | ≈0 |
| Ground-state isomerization | no | yes |
| Aging at Refrigerator Temperature | ||
| ϑ (°C) | 3 ± 1 | 2.5 ± 0.5 |
| tonset of light scattering (d) | >22 | ≈50 |
| (d−1) | ≈0.15 | 0.054 |
| Ground-state isomerization | no | small |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penzkofer, A.; Silapetere, A.; Hegemann, P. Absorption and Emission Spectroscopic Investigation of the Thermal Dynamics of the Archaerhodopsin 3 Based Fluorescent Voltage Sensor Archon2. Int. J. Mol. Sci. 2020, 21, 6576. https://doi.org/10.3390/ijms21186576
Penzkofer A, Silapetere A, Hegemann P. Absorption and Emission Spectroscopic Investigation of the Thermal Dynamics of the Archaerhodopsin 3 Based Fluorescent Voltage Sensor Archon2. International Journal of Molecular Sciences. 2020; 21(18):6576. https://doi.org/10.3390/ijms21186576
Chicago/Turabian StylePenzkofer, Alfons, Arita Silapetere, and Peter Hegemann. 2020. "Absorption and Emission Spectroscopic Investigation of the Thermal Dynamics of the Archaerhodopsin 3 Based Fluorescent Voltage Sensor Archon2" International Journal of Molecular Sciences 21, no. 18: 6576. https://doi.org/10.3390/ijms21186576




