Evaluation of Radioiodinated Fluoronicotinamide/Fluoropicolinamide-Benzamide Derivatives as Theranostic Agents for Melanoma
Abstract
1. Introduction
2. Results
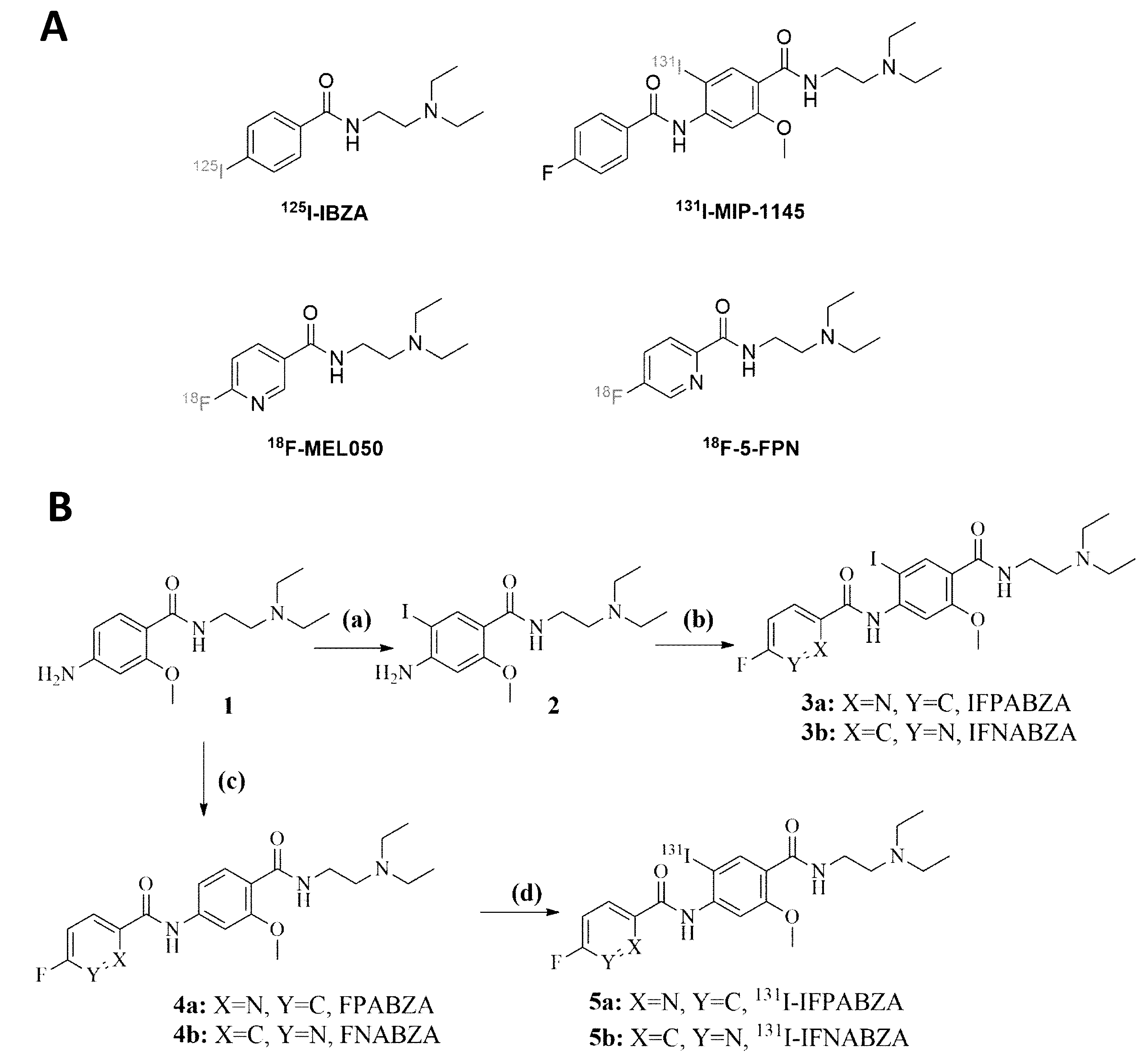
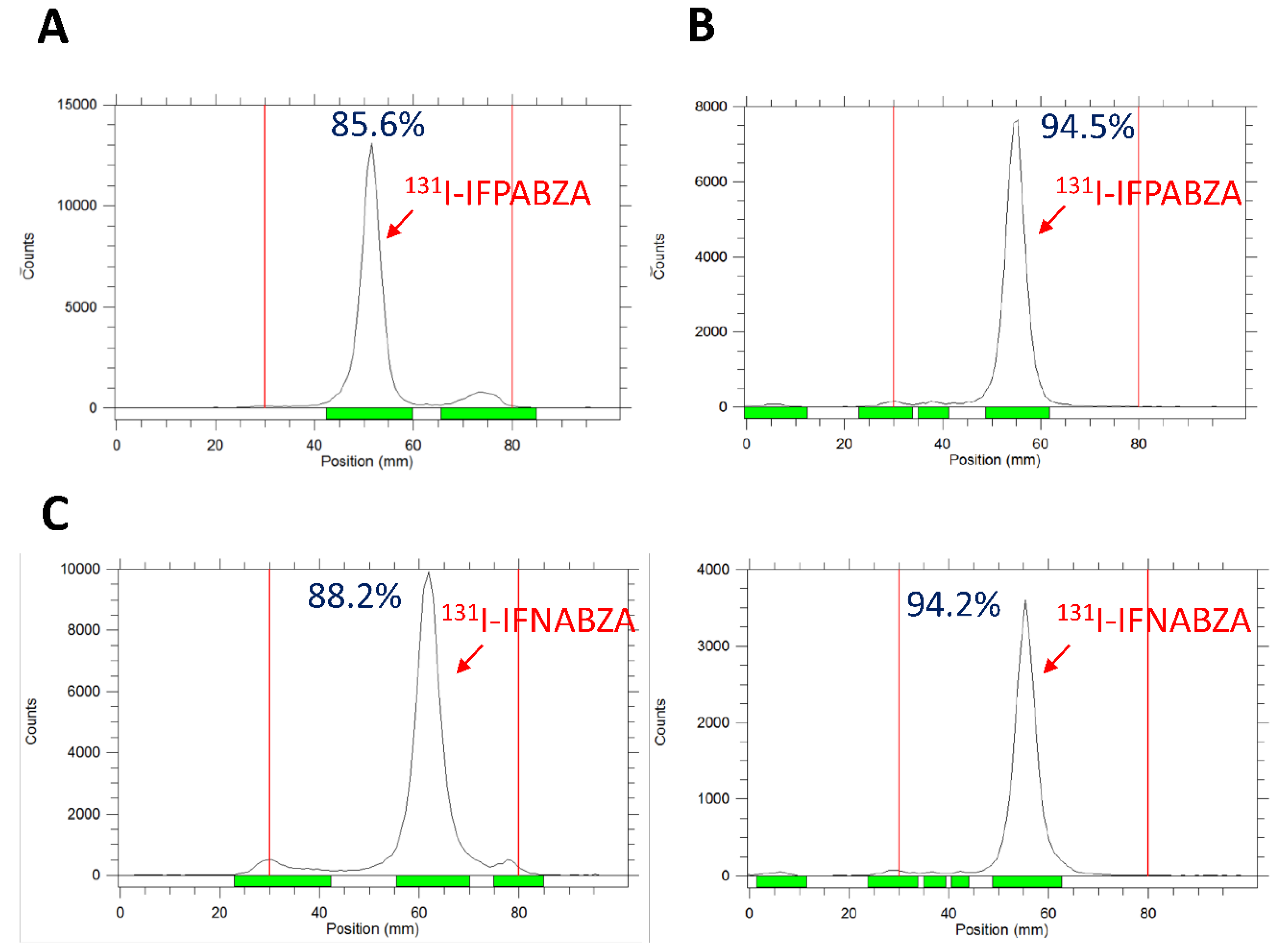
2.1. The Preparation of 131I-IFPABZA and 131I-IFNABZA
2.2. Partition Coefficient and In Vitro Stability of 131I-IFPABZA and 131I-IFNABZA
2.3. In Vitro Binding of 131I-IFPABZA and 131I-IFNABZA to Melanin
2.4. Assessment of In Vitro Cellular Uptake
2.5. Scintigraphic Imaging
2.6. Biodistribution Study
2.7. Estimated Absorption Dose Calculation
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Synthesis of 131I-IFPABZA and 131I-IFNABZA
4.2.1. Synthesis of 4-amino-N-(2-(diethylamino)ethyl)-2-methoxybenzamide (1)
4.2.2. Synthesis of 4-amino-N-(2-(diethylamino)ethyl)-5-iodo-2-methoxybenzamide (2)
4.2.3. Synthesis of N-(4-((2-(diethylamino)ethyl)carbamoyl)-2-iodo-5-methoxyphenyl)-5-fluoropico-linamide (3a) and N-(4-((2-(diethylamino)ethyl)carbamoyl)-2-iodo-5-methoxy-phenyl)-6-fluoronicotinamide (3b)
4.2.4. Synthesis of N-(4-((2-(diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-5-fluoropicolinamide (4a) and N-(4-((2-(diethylamino)ethyl)carbamoyl)-3-methoxy-phenyl)-5- fluoronicotinamide (4b)
4.2.5. Synthesis of 131I-N-(4-((2-(diethylamino)ethyl)carbamoyl)-2-iodo-5-methoxyphenyl)- 5-fluoropicolinamide (5a) and 131I-N-(4-((2-(diethylamino)ethyl)- carbamoyl)-2-iodo-5-methoxyphenyl)-6-fluoronicotinamide (5b)
4.3. Partition Coefficient of 131I-IFPABZA and 131I-IFNABZA
4.4. In Vitro Stability of 131I-IFPABZA and 131I-IFNABZA
4.5. Binding Affinity to Melanin
4.6. Cell Cultures and Xenograft Inoculation
4.7. In Vitro Cellular Uptake Assay
4.8. Scintigraphic Imaging
4.9. Biodistribution Study
4.10. Calculation of the Estimated Absorption Dose of Normal Organs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chia, A.L.; Simonova, G.; Dutta, B.; Lim, A.; Shumack, S. Melanoma diagnosis: Australian dermatologists’ number needed to treat. Australas. J. Dermatol. 2008, 49, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Linos, E.; Swetter, S.M.; Cockburn, M.G.; Colditz, G.A.; Clarke, C.A. Increasing burden of melanoma in the United States. J. Investig. Dermatol. 2009, 129, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Retsas, S. Cutaneous melanoma. Lancet 2005, 365, 2003–2004. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Cummins, D.L.; Cummins, J.M.; Pantle, H.; Silverman, M.A.; Leonard, A.L.; Chanmugam, A. Cutaneous malignant melanoma. Mayo Clin. Proc. 2006, 81, 500–507. [Google Scholar] [CrossRef]
- Tsao, H.; Atkins, M.B.; Sober, A.J. Management of cutaneous melanoma. N. Engl. J. Med. 2004, 351, 998–1012. [Google Scholar] [CrossRef]
- Jimbow, K.; Miyake, Y.; Homma, K.; Yasuda, K.; Izumi, Y.; Tsutsumi, A.; Ito, S. Characterization of melanogenesis and morphogenesis of melanosomes by physicochemical properties of melanin and melanosomes in malignant melanoma. Cancer Res. 1984, 44, 1128–1134. [Google Scholar]
- Pankovich, J.M.; Jimbow, K. Tyrosine transport in a human melanoma cell line as a basis for selective transport of cytotoxic analogues. Biochem. J. 1991, 280 Pt 3, 721–725. [Google Scholar] [CrossRef]
- Prota, G. Melanins, melanogenesis and melanocytes: Looking at their functional significance from the chemist’s viewpoint. Pigment. Cell Res. 2000, 13, 283–293. [Google Scholar] [CrossRef]
- Michelot, J.M.; Moreau, M.F.; Labarre, P.G.; Madelmont, J.C.; Veyre, A.J.; Papon, J.M.; Parry, D.F.; Bonafous, J.F.; Boire, J.Y.; Desplanches, G.G.; et al. Synthesis and evaluation of new iodine-125 radiopharmaceuticals as potential tracers for malignant melanoma. J. Nucl. Med. 1991, 32, 1573–1580. [Google Scholar]
- Michelot, J.M.; Moreau, M.F.; Veyre, A.J.; Bonafous, J.F.; Bacin, F.J.; Madelmont, J.C.; Bussiere, F.; Souteyrand, P.A.; Mauclaire, L.P.; Chossat, F.M.; et al. Phase II scintigraphic clinical trial of malignant melanoma and metastases with iodine-123-N-(2-diethylaminoethyl 4-iodobenzamide). J. Nucl. Med. 1993, 34, 1260–1266. [Google Scholar]
- Joyal, J.L.; Barrett, J.A.; Marquis, J.C.; Chen, J.; Hillier, S.M.; Maresca, K.P.; Boyd, M.; Gage, K.; Nimmagadda, S.; Kronauge, J.F.; et al. Preclinical evaluation of an 131I-labeled benzamide for targeted radiotherapy of metastatic melanoma. Cancer Res. 2010, 70, 4045–4053. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Greguric, I.; Roselt, P.; Neels, O.C.; Aide, N.; Taylor, S.R.; Katsifis, A.; Dorow, D.S.; Hicks, R.J. High-contrast PET of melanoma using (18F-MEL050, a selective probe for melanin with predominantly renal clearance. J. Nucl. Med. 2010, 51, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Greguric, I.; Taylor, S.R.; Denoyer, D.; Ballantyne, P.; Berghofer, P.; Roselt, P.; Pham, T.Q.; Mattner, F.; Bourdier, T.; Neels, O.C.; et al. Discovery of [18F]N-(2-(diethylamino)ethyl)-6-fluoronicotinamide: A melanoma positron emission tomography imaging radiotracer with high tumor to body contrast ratio and rapid renal clearance. J. Med. Chem. 2009, 52, 5299–5302. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Miao, Z.; Deng, Z.; Shen, B.; Hong, X.; Cheng, Z. Development of 18F-labeled picolinamide probes for PET imaging of malignant melanoma. J. Med. Chem. 2013, 56, 895–901. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, C.H.; Shen, C.C.; Chen, C.L.; Liu, R.S.; Lin, M.H.; Wang, H.E. Synthesis and evaluation of 123/131I-Iochlonicotinamide as a novel SPECT probe for malignant melanoma. Bioorg. Med. Chem. 2015, 23, 2261–2269. [Google Scholar] [CrossRef]
- Bonnet-Duquennoy, M.; Papon, J.; Mishellany, F.; Labarre, P.; Guerquin-Kern, J.L.; Wu, T.D.; Gardette, M.; Maublant, J.; Penault-Llorca, F.; Miot-Noirault, E.; et al. Targeted radionuclide therapy of melanoma: Anti-tumoural efficacy studies of a new 131I labelled potential agent. Int. J. Cancer 2009, 125, 708–716. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, L.; Gai, Y.; Liu, Q.; Yin, L.; Jiang, Y.; Wang, Y.; Zhang, Y.; Lan, X. Targeted radiotherapy of pigmented melanoma with 131I-5-IPN. J. Exp. Clin. Cancer Res. 2018, 37, 306. [Google Scholar] [CrossRef]
- Eisenhut, M.; Hull, W.E.; Mohammed, A.; Mier, W.; Lay, D.; Just, W.; Gorgas, K.; Lehmann, W.D.; Haberkorn, U. Radioiodinated N-(2-diethylaminoethyl)benzamide derivatives with high melanoma uptake: Structure-affinity relationships, metabolic fate, and intracellular localization. J. Med. Chem. 2000, 43, 3913–3922. [Google Scholar] [CrossRef]
- Pham, T.Q.; Greguric, I.; Liu, X.; Berghofer, P.; Ballantyne, P.; Chapman, J.; Mattner, F.; Dikic, B.; Jackson, T.; Loc’h, C.; et al. Synthesis and evaluation of novel radioiodinated benzamides for malignant melanoma. J. Med. Chem. 2007, 50, 3561–3572. [Google Scholar] [CrossRef]
- Pham, T.Q.; Berghofer, P.; Liu, X.; Greguric, I.; Dikic, B.; Ballantyne, P.; Mattner, F.; Nguyen, V.; Loc’h, C.; Katsifis, A. Preparation and biologic evaluation of a novel radioiodinated benzylpiperazine, 123I-MEL037, for malignant melanoma. J. Nucl. Med. 2007, 48, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Comeau, A.; Bowen, W.D.; Mach, R.H.; Ross, B.D.; Hong, H.; Van Dort, M.E. Design and Investigation of a [18F]-Labeled Benzamide Derivative as a High Affinity Dual Sigma Receptor Subtype Radioligand for Prostate Tumor Imaging. Mol. Pharm. 2017, 14, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Huang, S.P.; Lo, Y.C.; Liu, R.S.; Wang, S.J.; Lin, W.J.; Shen, C.C.; Wang, H.E. Synthesis and preclinical characterization of [18F]FPBZA: A novel PET probe for melanoma. Biomed. Res. Int. 2014, 2014, 912498. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chang, C.H.; Lo, Y.H.; Lin, M.H.; Shen, C.C.; Liu, R.S.; Wang, H.E.; Chen, C.L. Preparation and characterization of a novel Al18F-NOTA-BZA conjugate for melanin-targeted imaging of malignant melanoma. Bioorg. Med. Chem. Lett. 2016, 26, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Nicholl, C.; Mohammed, A.; Hull, W.E.; Bubeck, B.; Eisenhut, M. Pharmacokinetics of iodine-123-IMBA for melanoma imaging. J. Nucl. Med. 1997, 38, 127–133. [Google Scholar]
- Chang, C.C.; Chang, C.H.; Shen, C.C.; Chen, C.L.; Liu, R.S.; Lin, M.H.; Wang, H.E. Synthesis and characterization of a novel radioiodinated phenylacetamide and its homolog as theranostic agents for malignant melanoma. Eur. J. Pharm. Sci. 2016, 81, 201–209. [Google Scholar] [CrossRef]
- Chezal, J.M.; Papon, J.; Labarre, P.; Lartigue, C.; Galmier, M.J.; Decombat, C.; Chavignon, O.; Maublant, J.; Teulade, J.C.; Madelmont, J.C.; et al. Evaluation of radiolabeled (hetero)aromatic analogues of N-(2-diethylaminoethyl)-4-iodobenzamide for imaging and targeted radionuclide therapy of melanoma. J. Med. Chem. 2008, 51, 3133–3144. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.Y.; Park, J.H.; Yang, S.D.; Hur, M.G.; Min, J.J.; Yu, K.H. Synthesis and characterization of a 68Ga-labeled N-(2-diethylaminoethyl)benzamide derivative as potential PET probe for malignant melanoma. Bioorg. Med. Chem. 2012, 20, 4915–4920. [Google Scholar] [CrossRef]
- Ren, G.; Miao, Z.; Liu, H.; Jiang, L.; Limpa-Amara, N.; Mahmood, A.; Gambhir, S.S.; Cheng, Z. Melanin-targeted preclinical PET imaging of melanoma metastasis. J. Nucl. Med. 2009, 50, 1692–1699. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chang, C.H.; Li, J.J.; Stabin, M.G.; Chang, Y.J.; Chen, L.C.; Lin, M.H.; Tseng, Y.L.; Lin, W.J.; Lee, T.W.; et al. Pharmacokinetics and dosimetry of 111In/188Re-labeled PEGylated liposomal drugs in two colon carcinoma-bearing mouse models. Cancer Biother. Radiopharm. 2011, 26, 373–380. [Google Scholar] [CrossRef]
- Molina-Trinidad, E.M.; de Murphy, C.A.; Ferro-Flores, G.; Murphy-Stack, E.; Jung-Cook, H. Radiopharmacokinetic and dosimetric parameters of 188Re-lanreotide in athymic mice with induced human cancer tumors. Int. J. Pharm. 2006, 310, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Stabin, M.G.; Siegel, J.A. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003, 85, 294–310. [Google Scholar] [CrossRef] [PubMed]




| Organ | 5 min | 15 min | 1 h | 4 h | 24 h | 48 h | 96 h |
|---|---|---|---|---|---|---|---|
| Blood | 1.70 ± 0.15 | 1.24 ± 0.27 | 0.75 ± 0.19 | 0.27 ± 0.04 | 0.06 ± 0.04 | 0.01 ± 0.00 | 0.00 ± 0.00 |
| Heart | 7.97 ± 0.30 | 3.76 ± 1.09 | 1.69 ± 0.51 | 0.60 ± 0.15 | 0.25 ± 0.04 | 0.17 ± 0.05 | 0.11 ± 0.03 |
| Lung | 23.49 ± 2.20 | 13.87 ± 2.64 | 5.50 ± 1.57 | 2.34 ± 0.96 | 0.42 ± 0.33 | 0.09 ± 0.03 | 0.05 ± 0.01 |
| Liver | 11.91 ± 0.93 | 12.90 ± 0.99 | 11.12 ± 3.43 | 4.69 ± 1.66 | 1.93 ± 0.33 | 1.59 ± 0.37 | 0.92 ± 0.11 |
| Stomach | 3.60 ± 0.49 | 3.62 ± 0.78 | 3.20 ± 1.67 | 2.11 ± 1.60 | 0.33 ± 0.13 | 0.08 ± 0.07 | 0.03 ± 0.01 |
| Small int. | 6.85 ± 2.39 | 14.27 ± 3.04 | 9.38 ± 3.10 | 1.00 ± 0.22 | 0.24 ± 0.08 | 0.05 ± 0.03 | 0.03 ± 0.01 |
| Large int. | 4.14 ± 0.39 | 3.89 ± 0.73 | 3.10 ± 1.29 | 2.36 ± 1.16 | 0.21 ± 0.06 | 0.04 ± 0.02 | 0.01 ± 0.01 |
| Spleen | 10.09 ± 0.92 | 11.83 ± 1.53 | 9.19 ± 1.49 | 7.32 ± 3.53 | 3.12 ± 1.28 | 3.06 ± 1.07 | 1.16 ± 0.64 |
| Pancreas | 9.36 ± 0.55 | 6.97 ± 1.15 | 5.26 ± 2.75 | 1.60 ± 0.48 | 0.24 ± 0.10 | 0.10 ± 0.06 | 0.04 ± 0.01 |
| Bone | 3.18 ± 0.19 | 2.56 ± 0.51 | 1.43 ± 0.33 | 0.61 ± 0.22 | 0.19 ± 0.05 | 0.15 ± 0.03 | 0.09 ± 0.04 |
| Muscle | 2.89 ± 0.18 | 1.88 ± 0.34 | 0.59 ± 0.12 | 0.15 ± 0.02 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Tumor | 4.29 ± 0.93 | 4.90 ± 1.24 | 5.84 ± 1.80 | 5.19 ± 2.84 | 5.06 ± 2.09 | 5.17 ± 1.53 | 1.51 ± 0.34 |
| Brain | 0.95 ± 0.11 | 0.65 ± 0.13 | 0.18 ± 0.04 | 0.03 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Kidneys | 26.80 ± 2.32 | 23.28 ± 2.78 | 13.61 ± 3.32 | 6.33 ± 1.76 | 1.04 ± 0.25 | 0.48 ± 0.32 | 0.21 ± 0.02 |
| Eye ball | 7.67 ± 0.84 | 10.57 ± 1.80 | 15.57 ± 4.36 | 17.25 ± 1.11 | 14.69 ± 3.35 | 15.45 ± 2.41 | 14.06 ± 1.93 |
| Urine | 2.66 ± 1.56 | 32.36 ± 25.47 | 79.37 ± 60.45 | 20.57 ± 7.97 | 1.88 ± 1.24 | 0.15 ± 0.08 | 0.07 ± 0.02 |
| Feces | 1.19 ± 0.24 | 2.02 ± 0.51 | 9.14 ± 4.48 | 244.2 ± 50.65 | 21.77 ± 11.19 | 3.35 ± 3.36 | 0.46 ± 0.16 |
| Bladder | 1.78 ± 0.66 | 2.55 ± 0.34 | 4.04 ± 2.87 | 4.21 ± 1.97 | 0.21 ± 0.16 | 0.02 ± 0.01 | 0.01 ± 0.00 |
| Ratios | |||||||
| Tumor/muscle | 1.48 ± 0.33 | 2.61 ± 0.81 | 9.90 ± 3.66 | 34.60 ± 19.49 | 168.7 ± 89.52 | 258.5 ± 76.50 | 151.0 ± 34.00 |
| Tumor/blood | 2.52 ± 0.59 | 3.95 ± 1.32 | 7.79 ± 3.11 | 19.22 ± 10.90 | 84.33 ± 66.14 | 517.0 ± 153.0 | N/A |
| Tumor/liver | 0.36 ± 0.08 | 0.38 ± 0.10 | 0.53 ± 0.23 | 1.11 ± 0.72 | 2.62 ± 1.17 | 3.25 ± 1.22 | 1.64 ± 0.42 |
| Organ | Estimated Dose (mSv/MBq−1) |
|---|---|
| Adrenals | 2.76 × 10−2 |
| Brain | 3.88 × 10−3 |
| Breasts | 1.82 × 10−2 |
| Gallbladder Wall | 3.33 × 10−2 |
| LLI Wall | 2.91 × 10−2 |
| Small Intestine | 2.79 × 10−2 |
| Stomach Wall | 2.72 × 10−2 |
| ULI Wall | 2.51 × 10−2 |
| Heart Wall | 1.48 × 10−2 |
| Kidneys | 6.27 × 10−2 |
| Liver | 1.22 × 10−1 |
| Lungs | 2.25 × 10−2 |
| Muscle | 1.01 × 10−2 |
| Ovaries | 2.29 × 10−2 |
| Pancreas | 2.58 × 10−2 |
| Red Marrow | 1.80 × 10−2 |
| Osteogenic Cells | 4.92 × 10−2 |
| Skin | 1.58 × 10−2 |
| Spleen | 1.51 × 10−1 |
| Testes | 1.87 × 10−2 |
| Thymus | 1.94 × 10−2 |
| Thyroid | 1.89 × 10−2 |
| Urinary Bladder Wall | 2.18 × 10−2 |
| Uterus | 2.33 × 10−2 |
| Whole Body | 2.37 × 10−2 |
| Effective Dose | 3.02 × 10−2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Chen, Y.-Y.; Lo, Y.-H.; Lin, M.-H.; Chang, C.-H.; Chen, C.-L.; Wang, H.-E.; Wu, C.-Y. Evaluation of Radioiodinated Fluoronicotinamide/Fluoropicolinamide-Benzamide Derivatives as Theranostic Agents for Melanoma. Int. J. Mol. Sci. 2020, 21, 6597. https://doi.org/10.3390/ijms21186597
Chen C-C, Chen Y-Y, Lo Y-H, Lin M-H, Chang C-H, Chen C-L, Wang H-E, Wu C-Y. Evaluation of Radioiodinated Fluoronicotinamide/Fluoropicolinamide-Benzamide Derivatives as Theranostic Agents for Melanoma. International Journal of Molecular Sciences. 2020; 21(18):6597. https://doi.org/10.3390/ijms21186597
Chicago/Turabian StyleChen, Chao-Cheng, Yang-Yi Chen, Yi-Hsuan Lo, Ming-Hsien Lin, Chih-Hsien Chang, Chuan-Lin Chen, Hsin-Ell Wang, and Chun-Yi Wu. 2020. "Evaluation of Radioiodinated Fluoronicotinamide/Fluoropicolinamide-Benzamide Derivatives as Theranostic Agents for Melanoma" International Journal of Molecular Sciences 21, no. 18: 6597. https://doi.org/10.3390/ijms21186597
APA StyleChen, C.-C., Chen, Y.-Y., Lo, Y.-H., Lin, M.-H., Chang, C.-H., Chen, C.-L., Wang, H.-E., & Wu, C.-Y. (2020). Evaluation of Radioiodinated Fluoronicotinamide/Fluoropicolinamide-Benzamide Derivatives as Theranostic Agents for Melanoma. International Journal of Molecular Sciences, 21(18), 6597. https://doi.org/10.3390/ijms21186597





