Role of Innate Immune Cells in Psoriasis
Abstract
:1. Introduction
2. Group 3 Innate Lymphoid Cells (ILC3s)
2.1. ILC3 Characteristics
2.2. ILC3s Contribute to the Development of Psoriasis via IL-22
2.3. Peripheral Blood ILC3s in Psoriasis Patients
2.4. ILC3s in Psoriasis Mouse Models
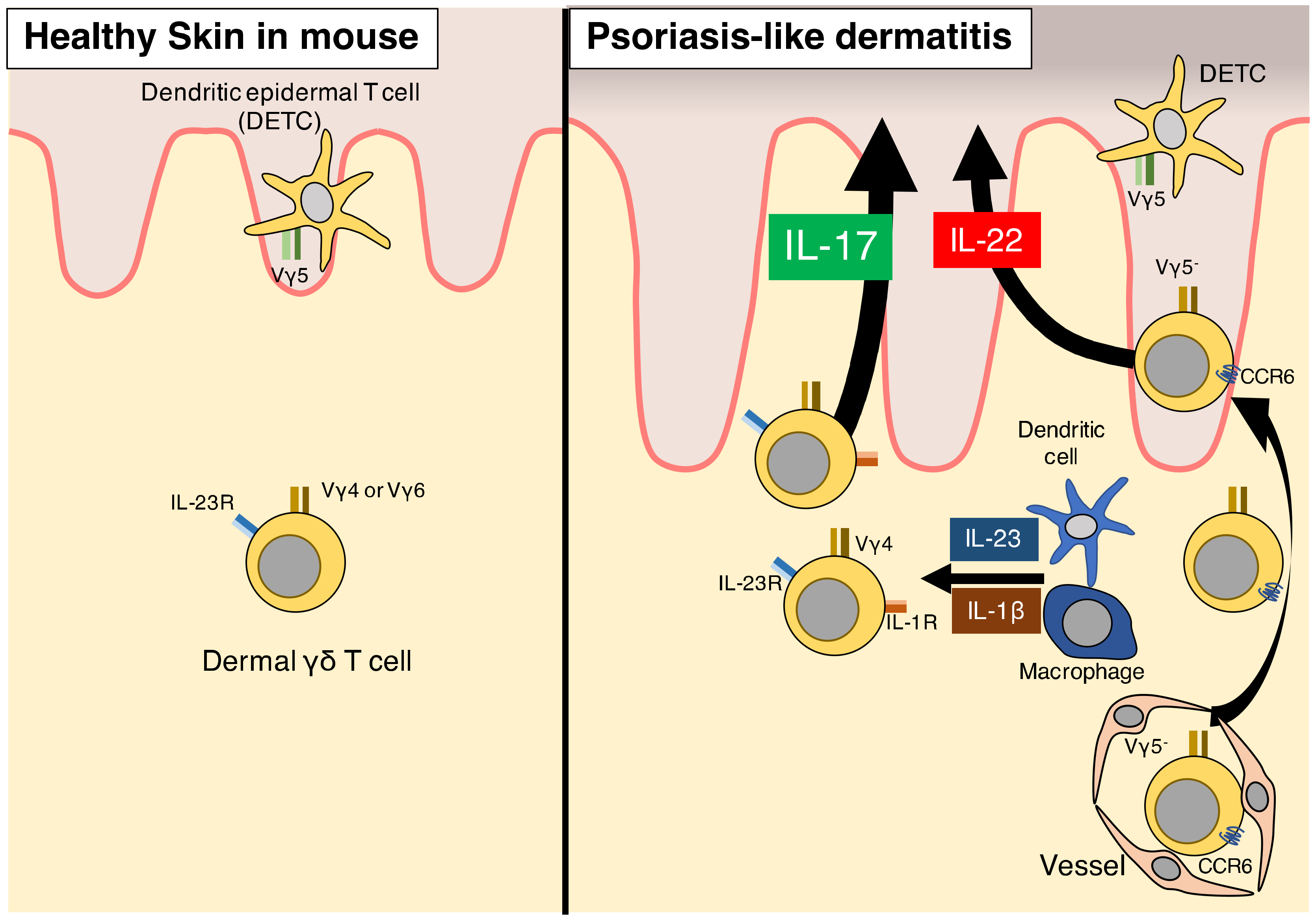
3. γδ T Cells
3.1. Characteristics of γδ T Cells as Innate Immune Cells
3.2. γδ T Cells in Humans
3.3. γδ T Cells in Psoriasis Patients
3.4. γδ T Cells in Mice
3.5. γδ T Cells Contribute to Psoriasis-Like Dermatitis in Murine Models via IL-17 Production
3.6. IL-1β Signaling Is Essential for γδ T-Cell Induction of Psoriasis
3.7. Memory Cell-Like Function of γδ T Cells in Psoriasis
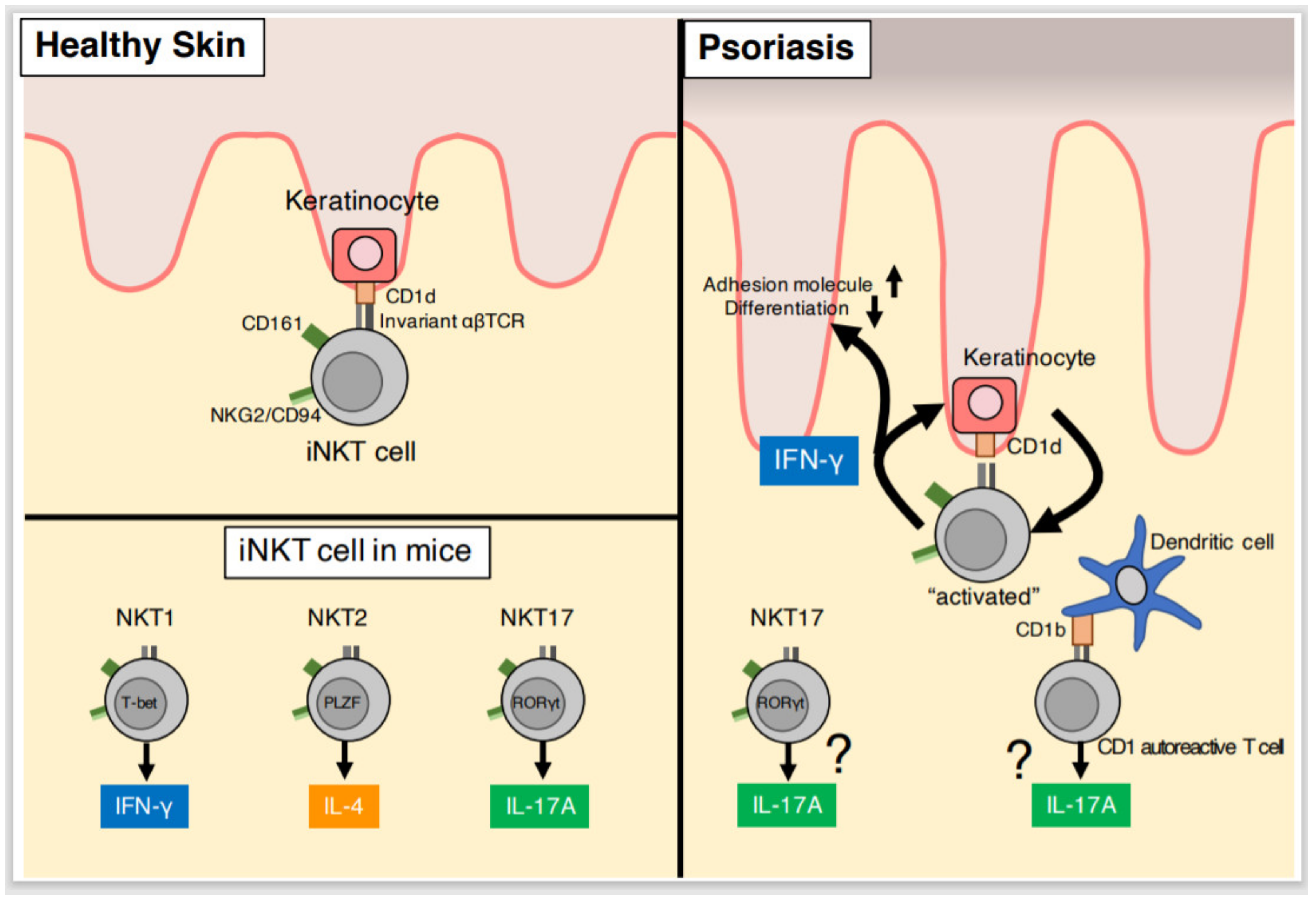
4. NKT Cells
4.1. NKT Cell Characteristics
4.2. NKT Cells in Psoriatic Lesions
4.3. Co-Activation of NKT Cells and Keratinocytes
4.4. NKT Cells and IL-17
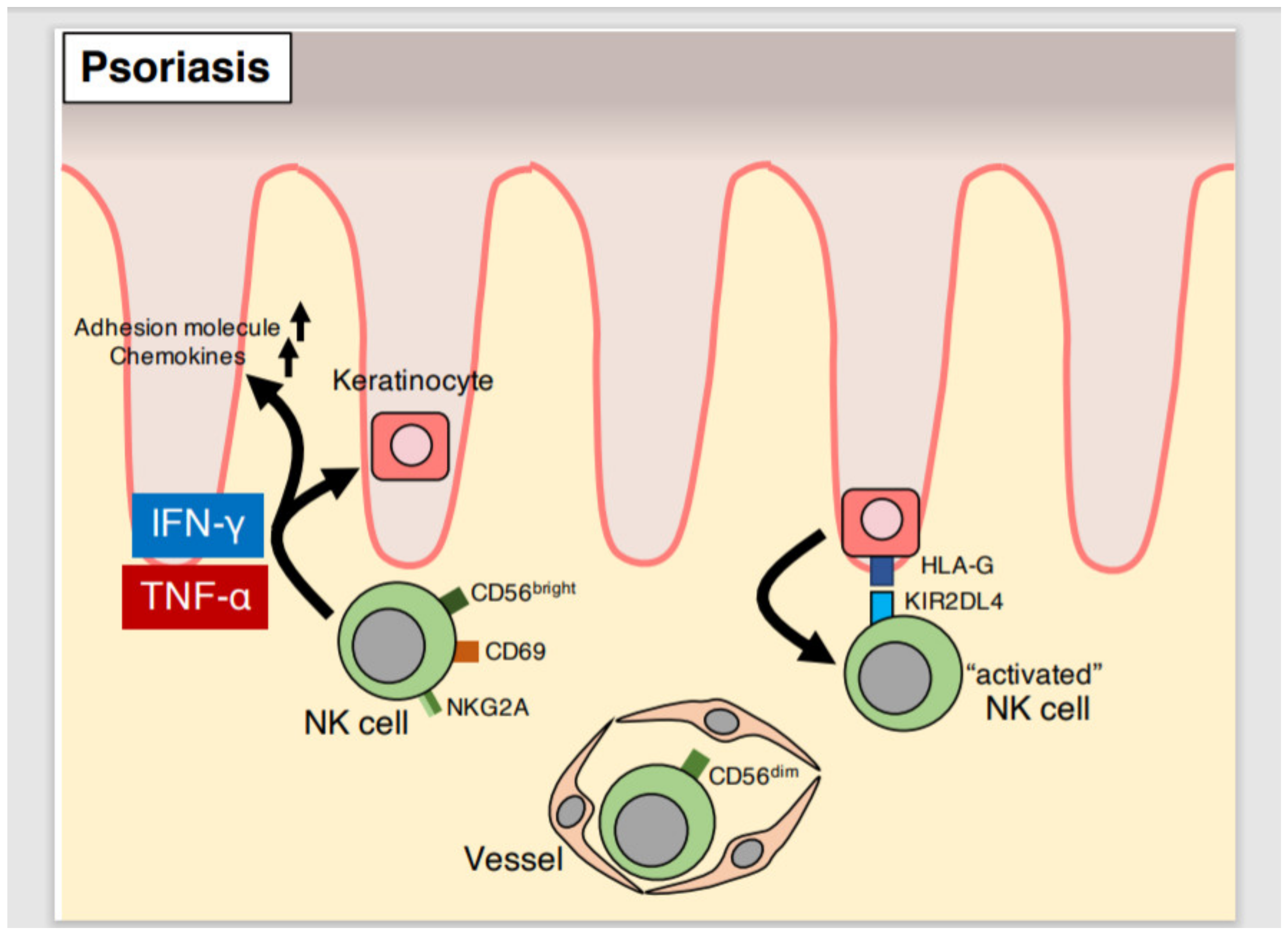
5. NK Cells
5.1. NK Cell Characteristics
5.2. Effector Function of NK Cells in Psoriatic Lesions
5.3. Effector Function of Peripheral Blood NK Cells in Psoriasis Patients
5.4. Cytotoxic Function of NK Cells in Psoriasis
5.5. Genetic Analyses of HLA and KIR in Psoriasis
5.6. NK Cells in Mouse Models of Psoriasis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mueller, W.; Herrmann, B. Cyclosporin A for psoriasis. N. Engl. J. Med. 1979, 301, 555. [Google Scholar] [CrossRef] [PubMed]
- Wrone-Smith, T.; Nickoloff, B.J. Dermal injection of immunocytes induces psoriasis. J. Clin. Investig. 1996, 98, 1878–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyman, O.; Hefti, H.P.; Conrad, C.; Nickoloff, B.J.; Suter, M.; Nestle, F.O. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 2004, 199, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, F.O.; Turka, L.A.; Nickoloff, B.J. Characterization of dermal dendritic cells in psoriasis: Autostimulation of T lymphocytes and induction of Th1 type cytokines. J. Clin. Investig. 1994, 94, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlaak, J.F.; Buslau, M.; Jochum, W.; Hermann, E.; Girndt, M.; Gallati, H.; Meyer zum Büschenfelde, K.H.; Fleischer, B. T cells involved in psoriasis vulgaris belong to the Th1 subset. J. Investig. Dermatol. 1994, 102, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, L.M.; Ozawa, M.; Kikuchi, T.; Walters, I.B.; Krueger, J.G. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: A type 1 differentiation bias is al. J. Investig. Dermatol. 1999, 113, 752–759. [Google Scholar] [CrossRef] [Green Version]
- Hancock, G.; Kaplan, G.; Cohn, Z. Keratinocyte growth regulation by the products of the immune cells. J. Exp. Med. 1988, 168, 1395–1402. [Google Scholar] [CrossRef]
- Detmar, M.; Orfanos, C.E. Tumor necrosis factor-alpha inhibits cell proliferation and induces class II antigens and cell adhesion molecules in cultured normal human keratinocytes in vitro. Arch. Dermatol. Res. 1990, 282, 238–245. [Google Scholar] [CrossRef]
- Krueger, G.; Callis, K. Potential of tumor necrosis factor inhibitors in psoriasis and psoriatic arthritis. Arch Dermatol. 2004, 140, 218–225. [Google Scholar] [CrossRef]
- Fossiez, F. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef] [Green Version]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional Specialization of Interleukin-17 Family Members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Fitch, E.; Harper, E.; Skorcheva, I.; Kurtz, S.E.; Blauvelt, A. Pathophysiology of psoriasis: Recent advances on IL-23 and Th17 cytokines. Curr. Rheumatol. Rep. 2007, 9, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Kastelein, R.A.; Hunter, C.A.; Cua, D.J. Discovery and biology of IL-23 and IL-27: Related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007, 25, 221–242. [Google Scholar] [CrossRef] [Green Version]
- Stockinger, B.; Veldhoen, M. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 2007, 19, 281–286. [Google Scholar] [CrossRef]
- Piskin, G.; Sylva-Steenland, R.M.R.; Bos, J.D.; Teunissen, M.B.M. In Vitro and In Situ Expression of IL-23 by Keratinocytes in Healthy Skin and Psoriasis Lesions: Enhanced Expression in Psoriatic Skin. J. Immunol. 2006, 176, 1908–1915. [Google Scholar] [CrossRef]
- Amin, M.; Darji, K.; No, D.J.; Wu, J.J. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1627–1632. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Reich, K.; Lebwohl, M.; van de Kerkhof, P.; Paul, C.; Menter, A.; Cameron, G.S.; Erickson, J.; Zhang, L.; Secrest, R.J.; et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet (Lond. Engl.) 2015, 386, 541–551. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Keijsers, R.R.M.C.; Joosten, I.; van Erp, P.E.J.; Koenen, H.J.P.M.; van de Kerkhof, P.C.M. Cellular sources of IL-17 in psoriasis: A paradigm shift? Exp. Dermatol. 2014, 23, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Ahlfors, H.; Duarte, J.H.; Stockinger, B. Regulation and function of innate and adaptive interleukin-17-producing cells. EMBO Rep. 2012, 13, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, A.; Colonna, M.; Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 2014, 41, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Hashimoto-Hill, S.; Kim, M. Migration and Tissue Tropism of Innate Lymphoid Cells. Trends Immunol. 2016, 37, 68–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüggen, M.-C.; Bauer, W.M.; Reininger, B.; Clim, E.; Captarencu, C.; Steiner, G.E.; Brunner, P.M.; Meier, B.; French, L.E.; Stingl, G. In Situ Mapping of Innate Lymphoid Cells in Human Skin: Evidence for Remarkable Differences between Normal and Inflamed Skin. J. Investig. Dermatol. 2016, 136, 2396–2405. [Google Scholar] [CrossRef] [Green Version]
- Teunissen, M.B.M.; Munneke, J.M.; Bernink, J.H.; Spuls, P.I.; Res, P.C.M.; Te Velde, A.; Cheuk, S.; Brouwer, M.W.D.; Menting, S.P.; Eidsmo, L.; et al. Composition of innate lymphoid cell subsets in the human skin: Enrichment of NCR + ILC3 in lesional skin and blood of psoriasis patients. J. Investig. Dermatol. 2014, 134, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Villanova, F.; Flutter, B.; Tosi, I.; Grys, K.; Sreeneebus, H.; Perera, G.K.; Chapman, A.; Smith, C.H.; Di Meglio, P.; Nestle, F.O. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Investig. Dermatol. 2014, 134, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Boniface, K.; Bernard, F.-X.; Garcia, M.; Gurney, A.L.; Lecron, J.-C.; Morel, F. IL-22 Inhibits Epidermal Differentiation and Induces Proinflammatory Gene Expression and Migration of Human Keratinocytes. J. Immunol. 2005, 174, 3695–3702. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Trepicchio, W.L.; Oestreicher, J.L.; Pittman, D.; Wang, F.; Chamian, F.; Dhodapkar, M.; Krueger, J.G. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004, 199, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Keren, A.; Shemer, A.; Ginzburg, A.; Ullmann, Y.; Schrum, A.G.; Paus, R.; Gilhar, A. Innate lymphoid cells 3 induce psoriasis in xenotransplanted healthy human skin. J. Allergy Clin. Immunol. 2018, 142, 305–308.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyring-Andersen, B.; Geisler, C.; Agerbeck, C.; Lauritsen, J.P.H.; Gúdjonsdottir, S.D.; Skov, L.; Bonefeld, C.M. Increased number and frequency of group 3 innate lymphoid cells in nonlesional psoriatic skin. Br. J. Dermatol. 2014, 170, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Pantelyushin, S.; Haak, S.; Ingold, B.; Kulig, P.; Heppner, F.L.; Navarini, A.A.; Becher, B. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Investig. 2012, 122, 2252–2256. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Domingo, P.; Cupedo, T. Rorγt+ innate lymphoid cells in intestinal homeostasis and immunity. J. Innate Immun. 2011, 3, 577–584. [Google Scholar] [CrossRef]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef]
- Hedrick, M.N.; Lonsdorf, A.S.; Shirakawa, A.-K.; Richard Lee, C.-C.; Liao, F.; Singh, S.P.; Zhang, H.H.; Grinberg, A.; Love, P.E.; Hwang, S.T.; et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J. Clin. Investig. 2009, 119, 2317–2329. [Google Scholar] [CrossRef] [Green Version]
- Girardi, M. Immunosurveillance and immunoregulation by γδ T cells. J. Investig. Dermatol. 2006, 126, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Raulet, D.H. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu. Rev. Immunol. 1989, 7, 175–207. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. γ δ T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Witherden, D.A.; Johnson, M.D.; Havran, W.L. Coreceptors and their ligands in epithelial γδ T cell biology. Front. Immunol. 2018, 9, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Yang, W.; Pan, M.; Scully, E.; Girardi, M.; Augenlicht, L.H.; Craft, J.; Yin, Z. γδ T cells provide an early source of interferon γ in tumor immunity. J. Exp. Med. 2003, 198, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workalemahu, G.; Foerster, M.; Kroegel, C.; Braun, R.K. Human γδ-T Lymphocytes Express and Synthesize Connective Tissue Growth Factor: Effect of IL-15 and TGF-β1 and Comparison with αβ-T Lymphocytes. J. Immunol. 2003, 170, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Ebert, L.M.; Meuter, S.; Moser, B. Homing and function of human skin gammadelta T cells and NK cells: Relevance for tumor surveillance. J. Immunol. 2006, 176, 4331–4336. [Google Scholar] [CrossRef]
- Cordova, A.; Toia, F.; La Mendola, C.; Orlando, V.; Meraviglia, S.; Rinaldi, G.; Todaro, M.; Cicero, G.; Zichichi, L.; Donni, P.L.; et al. Characterization of human γδ T lymphocytes infiltrating primary malignant melanomas. PLoS ONE 2012, 7, e49878. [Google Scholar] [CrossRef] [Green Version]
- Toulon, A.; Breton, L.; Taylor, K.R.; Tenenhaus, M.; Bhavsar, D.; Lanigan, C.; Rudolph, R.; Jameson, J.; Havran, W.L. A role for human skin-resident T cells in wound healing. J. Exp. Med. 2009, 206, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.S.; Diamond, A.; Russell, A.; Jameson, J.M. Human αβ and γδ T Cells in Skin Immunity and Disease. Front. Immunol. 2018, 9, 1304. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.; Wang, T.; Zheng, J.; et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 2011, 35, 596–610. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Valle, F.; Gray, E.E.; Cyster, J.G. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc. Natl. Acad. Sci. USA 2015, 112, 8046–8051. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Xue, F.; Quan, C.; Qu, M.; Liu, N.; Zhang, Y.; Fleming, C.; Hu, X.; Zhang, H.-G.; Weichselbaum, R.; et al. A Critical Role of the IL-1β-IL-1R Signaling Pathway in Skin Inflammation and Psoriasis Pathogenesis. J. Investig. Dermatol. 2019, 139, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laggner, U.; Di Meglio, P.; Perera, G.K.; Hundhausen, C.; Lacy, K.E.; Ali, N.; Smith, C.H.; Hayday, A.C.; Nickoloff, B.J.; Nestle, F.O. Identification of a Novel Proinflammatory Human Skin-Homing Vγ9Vδ2 T Cell Subset with a Potential Role in Psoriasis. J. Immunol. 2011, 187, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.R.; O’Malley, J.T.; Lowry, E.L.; Hamm, D.; Kirsch, I.R.; Robins, H.S.; Kupper, T.S.; Krueger, J.G.; Clark, R.A. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J. Clin. Investig. 2017, 127, 4031–4041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumaria, N.; Roediger, B.; Ng, L.G.; Qin, J.; Pinto, R.; Cavanagh, L.L.; Shklovskaya, E.; Barbara, B.F.; Triccas, J.A.; Weninger, W. Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J. Exp. Med. 2011, 208, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Jorquera, A.; Msallam, R.; Wienert, S.; Klauschen, F.; Ginhoux, F.; Bajénoff, M. Epidermal γδ T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J. Exp. Med. 2018, 215, 2994–3005. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Xue, F.; Fleming, C.; Yang, J.; Ding, C.; Ma, Y.; Liu, M.; Zhang, H.; Zheng, J.; Xiong, N.; et al. Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat. Commun. 2014. [Google Scholar] [CrossRef]
- Gray, E.E.; Suzuki, K.; Cyster, J.G. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J. Immunol. 2011, 186, 6091–6095. [Google Scholar] [CrossRef] [Green Version]
- Mabuchi, T.; Takekoshi, T.; Hwang, S.T. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J. Immunol. 2011, 187, 5026–5031. [Google Scholar] [CrossRef] [Green Version]
- Patel, U.; Mark, N.M.; Machler, B.C.; Levine, V.J. Imiquimod 5% cream induced psoriasis: A case report, summary of the literature and mechanism. Br. J. Dermatol. 2011, 164, 670–672. [Google Scholar] [CrossRef]
- Slauenwhite, D.; Johnston, B. Regulation of NKT cell localization in homeostasis and infection. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.H.; Papadopoulos, M.; Pant, H.; Tumes, D.J. The role of invariant T cells in inflammation of the skin and airways. Semin. Immunopathol. 2019, 41, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Wang, H.; Starrett, G.J.; Phuong, V.; Jameson, S.C.; Hogquist, K.A. Tissue-Specific Distribution of iNKT Cells Impacts Their Cytokine Response. Immunity 2015, 43, 566–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortesi, F.; Delfanti, G.; Casorati, G.; Dellabona, P. The pathophysiological relevance of the iNKT cell/mononuclear phagocyte crosstalk in tissues. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bonish, B.; Jullien, D.; Dutronc, Y.; Huang, B.B.; Modlin, R.; Spada, F.M.; Porcelli, S.A.; Nickoloff, B.J. Overexpression of CD1d by Keratinocytes in Psoriasis and CD1d-Dependent IFN-γ Production by NK-T Cells. J. Immunol. 2000, 165, 4076–4085. [Google Scholar] [CrossRef] [Green Version]
- Vissers, W.H.P.M.; Berends, M.; Muys, L.; van Erp, P.E.J.; de Jong, E.M.G.J.; van de Kerkhof, P.C.M. The effect of the combination of calcipotriol and betamethasone dipropionate versus both monotherapies on epidermal proliferation, keratinization and T-cell subsets in chronic plaque psoriasis. Exp. Dermatol. 2004, 13, 106–112. [Google Scholar] [CrossRef]
- Bovenschen, H.J.; Gerritsen, W.J.; Van Rens, D.W.A.; Seyger, M.M.B.; De Jong, E.M.G.J.; Van De Kerkhof, P.C.M. Explorative immunohistochemical study to evaluate the addition of a topical corticosteroid in the early phase of alefacept treatment for psoriasis. Arch. Dermatol. Res. 2007, 298, 457–463. [Google Scholar] [CrossRef]
- Chan, W.L.; Pejnovic, N.; Liew, T.V.; Lee, C.A.; Groves, R.; Hamilton, H. NKT cell subsets in infection and inflammation. Immunol. Lett. 2003, 85, 159–163. [Google Scholar] [CrossRef]
- Van Der Vliet, H.J.J.; Von Blomberg, B.M.E.; Nishi, N.; Reijm, M.; Voskuyl, A.E.; Van Bodegraven, A.A.; Polman, C.H.; Rustemeyer, T.; Lips, P.; Van Den Eertwegh, A.J.M.; et al. Circulating Vα24+ Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 2001, 100, 144–148. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Wrone-Smith, T.; Bonish, B.; Porcelli, S.A. Response of Murine and Normal Human Skin to Injection of Allogeneic Blood-Derived Psoriatic Immunocytes. Arch. Dermatol. 1999, 135, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Gilhar, A.; Ullmann, Y.; Kerner, H.; Assy, B.; Shalaginov, R.; Serafimovich, S.; Kalish, R.S. Psoriasis is mediated by a cutaneous defect triggered by activated immunocytes: Induction of psoriasis by cells with natural killer receptors. J. Investig. Dermatol. 2002, 119, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickoloff, B.J.; Bonish, B.; Huang, B.B.; Porcelli, S.A. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J. Dermatol. Sci. 2000, 24, 212–225. [Google Scholar] [CrossRef]
- Coquet, J.M.; Chakravarti, S.; Kyparissoudis, K.; McNab, F.W.; Pitt, L.A.; McKenzie, B.S.; Berzins, S.P.; Smyth, M.J.; Godfrey, D.I. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. USA 2008, 105, 11287–11292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doisne, J.-M.; Soulard, V.; Bécourt, C.; Amniai, L.; Henrot, P.; Havenar-Daughton, C.; Blanchet, C.; Zitvogel, L.; Ryffel, B.; Cavaillon, J.-M.; et al. Cutting edge: Crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J. Immunol. 2011, 186, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Doisne, J.-M.; Becourt, C.; Amniai, L.; Duarte, N.; Le Luduec, J.-B.; Eberl, G.; Benlagha, K. Skin and Peripheral Lymph Node Invariant NKT Cells Are Mainly Retinoic Acid Receptor-Related Orphan Receptor γt + and Respond Preferentially under Inflammatory Conditions. J. Immunol. 2009, 183, 2142–2149. [Google Scholar] [CrossRef] [Green Version]
- Webster, K.E.; Kim, H.-O.; Kyparissoudis, K.; Corpuz, T.M.; Pinget, G.V.; Uldrich, A.P.; Brink, R.; Belz, G.T.; Cho, J.-H.; Godfrey, D.I.; et al. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 2014, 7, 1058–1067. [Google Scholar] [CrossRef] [Green Version]
- Milosavljevic, N.; Gazdic, M.; Simovic Markovic, B.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Djonov, V.; Lukic, M.L.; Volarevic, V. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. 2017, 23, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, S.; He, Y.; Zhang, H.; Cao, L.; Van Rhijn, I.; Moody, D.B.; Gudjonsson, J.E.; Wang, C.R. CD1b-autoreactive T cells contribute to hyperlipidemia-induced skin inflammation in mice. J. Clin. Investig. 2017, 127, 2339–2352. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. NATURAL KILLER CELLS IN ANTIVIRAL DEFENSE: Function and Regulation by Innate Cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef]
- Moretta, L.; Bottino, C.; Pende, D.; Mingari, M.C.; Biassoni, R.; Moretta, A. Human natural killer cells: Their origin, receptors and function. Eur. J. Immunol. 2002, 32, 1205–1211. [Google Scholar] [CrossRef]
- Belizário, J.E.; Neyra, J.M.; Setúbal Destro Rodrigues, M.F. When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection. Innate Immun. 2018, 24, 452–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-Bustamante, P.; Keşmir, C.; de Boer, R.J. The evolution of natural killer cell receptors. Immunogenetics 2016, 68, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Trowsdale, J. Genetic and Functional Relationships between MHC and NK Receptor Genes HLA class I and NK receptors are encoded within. Immunity 2001, 15, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Barten, R.; Torkar, M.; Haude, A.; Trowsdale, J.; Wilson, M.J. Divergent and convergent evolution of NK-cell receptors. Trends Immunol. 2001, 22, 52–57. [Google Scholar] [CrossRef]
- Vivier, E.; Nunès, J.A.; Vély, F. Natural killer cell signaling pathways. Science 2004, 306, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Borrego, F.; Masilamani, M.; Marusina, A.I.; Tang, X.; Coligan, J.E. The CD94/NKG2 family of receptors: From molecules and cells to clinical relevance. Immunol. Res. 2006, 35, 263–277. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Hudspeth, K.; Donadon, M.; Cimino, M.; Pontarini, E.; Tentorio, P.; Preti, M.; Hong, M.; Bertoletti, A.; Bicciato, S.; Invernizzi, P.; et al. Human liver-resident CD56bright/CD16neg NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016, 66, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Di Santo, J.P. Functionally distinct NK-cell subsets: Developmental origins and biological implications. Eur. J. Immunol. 2008, 38, 2948–2951. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Z. Diversity of tissue-resident NK cells. Semin. Immunol. 2017, 31, 3–10. [Google Scholar] [CrossRef]
- Cameron, A.; Kirby, B.; Fei, W.; Griffiths, C.E.M. Natural killer and natural killer-T cells in psoriasis. Arch. Dermatol. Res. 2002, 294, 363–369. [Google Scholar] [CrossRef]
- Vissers, W.H.P.M.; Arndtz, C.H.M.; Muys, L.; Van Erp, P.E.J.; de Jong, E.M.G.; van de Kerkhof, P.C.M. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late. Br. J. Dermatol. 2004, 150, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, C.; Nasorri, F.; Bedini, C.; de Pità, O.; Girolomoni, G.; Cavani, A. CD56brightCD16- NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur. J. Immunol. 2006, 36, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cardili, R.N.; Alves, T.G.; Freitas, J.C.O.C.; Soares, C.P.; Mendes-Junior, C.T.; Soares, E.G.; Donadi, E.A.; Silva-Souza, C. Expression of human leucocyte antigen-G primarily targets affected skin of patients with psoriasis. Br. J. Dermatol. 2010, 163, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Long, E.O. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front. Immunol. 2012, 3, 258. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, A.C.; Chaurasia, S.; Mishra, S.K.; Aggarwal, A.; Misra, R. IL-17 and IFN-γ producing NK and γδ-T cells are preferentially expanded in synovial fluid of patients with reactive arthritis and undifferentiated spondyloarthritis. Clin. Immunol. 2017, 183, 207–212. [Google Scholar] [CrossRef]
- Cella, M.; Fuchs, A.; Vermi, W.; Facchetti, F.; Otero, K.; Lennerz, J.K.M.; Doherty, J.M.; Mills, J.C.; Colonna, M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457, 722–725. [Google Scholar] [CrossRef]
- Cameron, A.L.; Kirby, B.; Griffiths, C.E.M. Circulating natural killer cells in psoriasis. Br. J. Dermatol. 2003, 149, 160–164. [Google Scholar] [CrossRef]
- Gambichler, T.; Zhang, Y.; Höxtermann, S.; Kreuter, A. Natural killer cells and B lymphocytes in peripheral blood of patients with psoriasis. Br. J. Dermatol. 2013, 168, 894–896. [Google Scholar] [CrossRef]
- Dunphy, S.E.; Sweeney, C.M.; Kelly, G.; Tobin, A.M.; Kirby, B.; Gardiner, C.M. Natural killer cells from psoriasis vulgaris patients have reduced levels of cytotoxicity associated degranulation and cytokine production. Clin. Immunol. 2017, 177, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Vičić, M.; Kaštelan, M.; Sotošek Tokmadžić, V.; Prpić Massari, L. Systemic and Local Increase of Granulysin Expression in Cytotoxic Lymphocytes in Severe Psoriasis. Acta Derm. Venereol. 2019, 99, 1136–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermis, E.; Celik, S.K.; Solak, N.; Genc, G.C.; Dursun, A. The role of GNLY gene polymorphisms in psoriasis pathogenesis. An. Bras. Dermatol. 2019, 94, 198–203. [Google Scholar] [CrossRef]
- Holm, S.J.; Sakuraba, K.; Mallbris, L.; Wolk, K.; Ståhle, M.; Sánchez, F.O. Distinct HLA-C/KIR genotype profile associates with guttate psoriasis. J. Investig. Dermatol. 2005, 125, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hayashi, G.; Lai, O.Y.; Dilthey, A.; Kuebler, P.J.; Wong, T.V.; Martin, M.P.; Fernandez Vina, M.A.; McVean, G.; Wabl, M.; et al. Psoriasis patients are enriched for genetic variants that protect against HIV-1 disease. PLoS Genet. 2012, 8, e1002514. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Chen, H.; Gupta, R.; Paz-Altschul, O.; Bowcock, A.M.; Liao, W. Deletion of the activating NKG2C receptor and a functional polymorphism in its ligand HLA-E in psoriasis susceptibility. Exp. Dermatol. 2013, 22, 679–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhrberg, M. The KIR gene family: Life in the fast lane of evolution. Eur. J. Immunol. 2005, 35, 10–15. [Google Scholar] [CrossRef]
- Surcel, M.; Huică, R.-I.; Munteanu, A.; Isvoranu, G.; Pîrvu, I.; Ciotaru, D.; Constantin, C.; Bratu, O.; Căruntu, C.; Neagu, M.; et al. Phenotypic changes of lymphocyte populations in psoriasiform dermatitis animal model. Exp. Ther. Med. 2018, 17, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Surcel, M.; Munteanu, A.N.; Huică, R.-I.; Isvoranu, G.; Pîrvu, I.R.; Constantin, C.; Bratu, O.; Căruntu, C.; Zaharescu, I.; Sima, L.; et al. Reinforcing involvement of NK cells in psoriasiform dermatitis animal model. Exp. Ther. Med. 2019, 18, 4956–4966. [Google Scholar] [CrossRef] [Green Version]

| Cell Type | Tissue | Healthy Condition | Psoriasis | |
|---|---|---|---|---|
| ILC3 | Number | Skin | Presence | Increase |
| PB | Presence | Increase * | ||
| Phenotype | Skin, PB | NCR- | NCR+ | |
| Skin, PB | IL-22 (+) | |||
| γδT cell | Number | Skin | Presence | Increase |
| PB | Presence | Decrease | ||
| Phenotype | Skin | IL-17 (+), IL-22 (+) | ||
| NKT cell | Number | Skin | Presence | Increase? |
| PB | Presence | Presence | ||
| Phenotype | Skin | IFN-g (+) | ||
| NK cell | Number | Skin | Presence | Increase |
| PB | Presence | Decrease | ||
| Phenotype | Skin | N.A. | CD69+ | |
| Skin | IFN-γ (+), TNF-α (+) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Ogawa, E.; Okuyama, R. Role of Innate Immune Cells in Psoriasis. Int. J. Mol. Sci. 2020, 21, 6604. https://doi.org/10.3390/ijms21186604
Sato Y, Ogawa E, Okuyama R. Role of Innate Immune Cells in Psoriasis. International Journal of Molecular Sciences. 2020; 21(18):6604. https://doi.org/10.3390/ijms21186604
Chicago/Turabian StyleSato, Yuki, Eisaku Ogawa, and Ryuhei Okuyama. 2020. "Role of Innate Immune Cells in Psoriasis" International Journal of Molecular Sciences 21, no. 18: 6604. https://doi.org/10.3390/ijms21186604
APA StyleSato, Y., Ogawa, E., & Okuyama, R. (2020). Role of Innate Immune Cells in Psoriasis. International Journal of Molecular Sciences, 21(18), 6604. https://doi.org/10.3390/ijms21186604




