Interaction Modes of Microsomal Cytochrome P450s with Its Reductase and the Role of Substrate Binding
Abstract
1. Introduction
2. Results
2.1. Characterization of the Membrane Fractions
2.2. Detailed Enzyme Activity
2.2.1. Reaction Velocities of the Different CPRmut:CYP Combinations
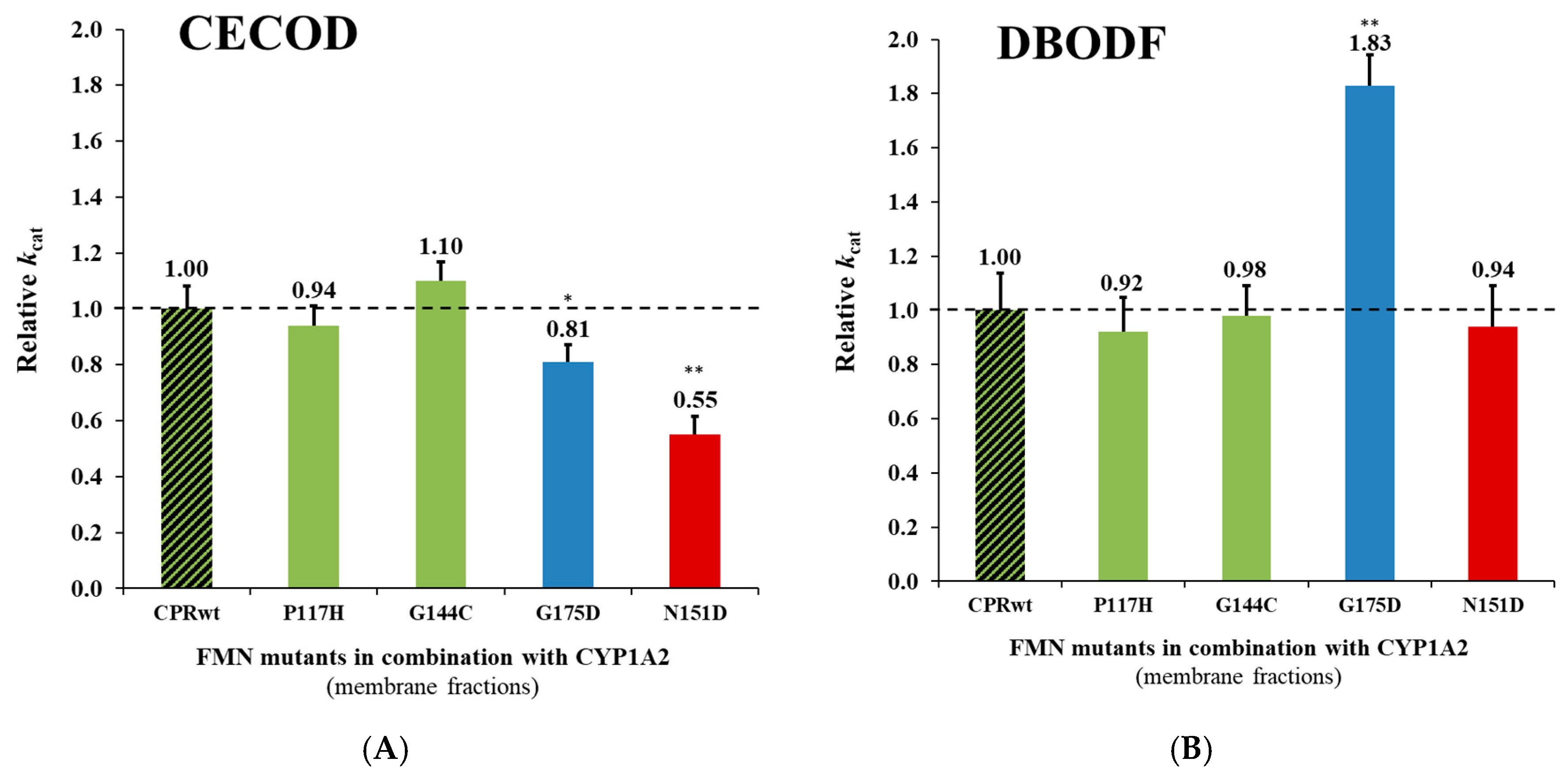
2.2.2. Reaction Velocities of CPRmut:CYP1A2 Using Different Substrates
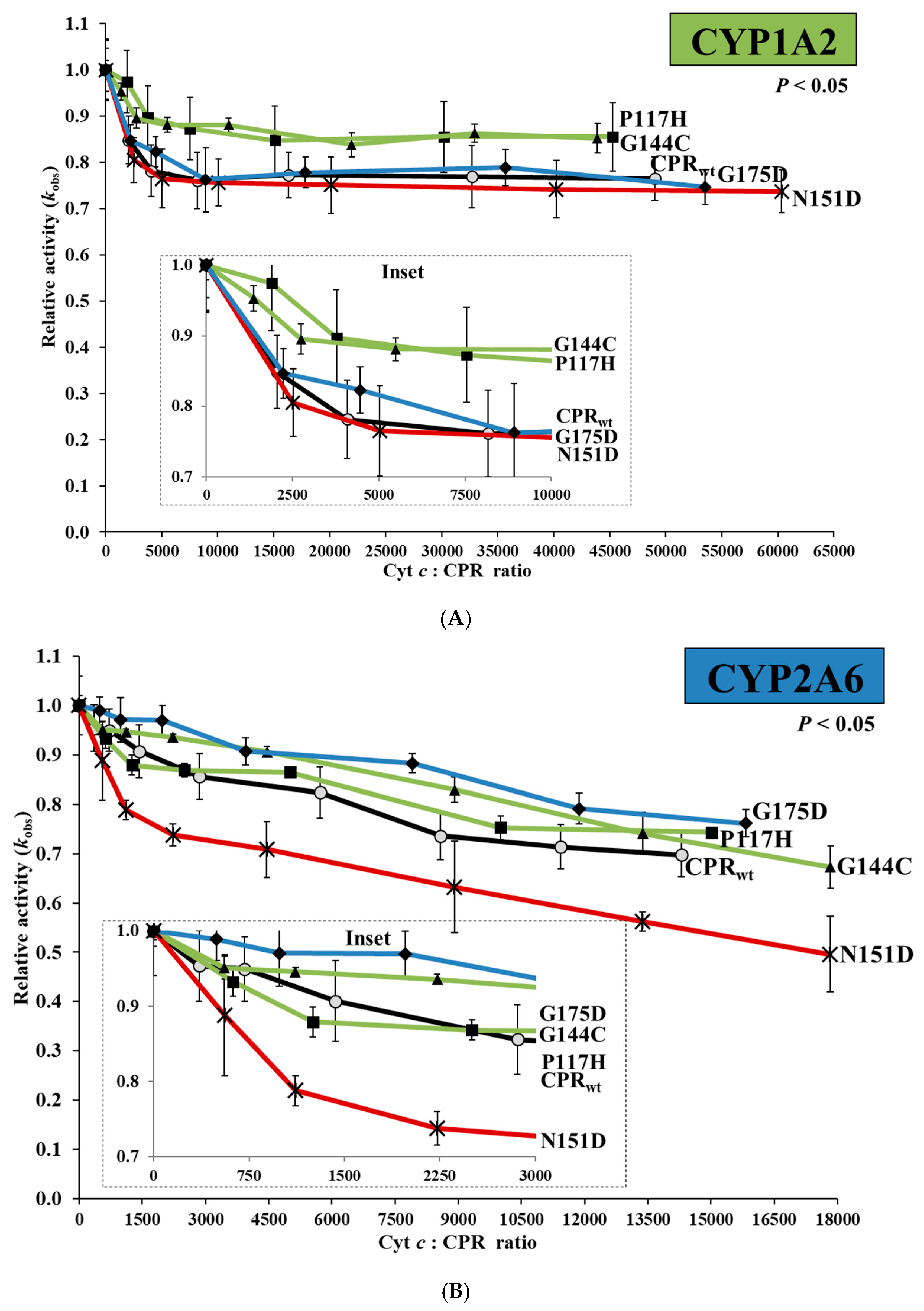
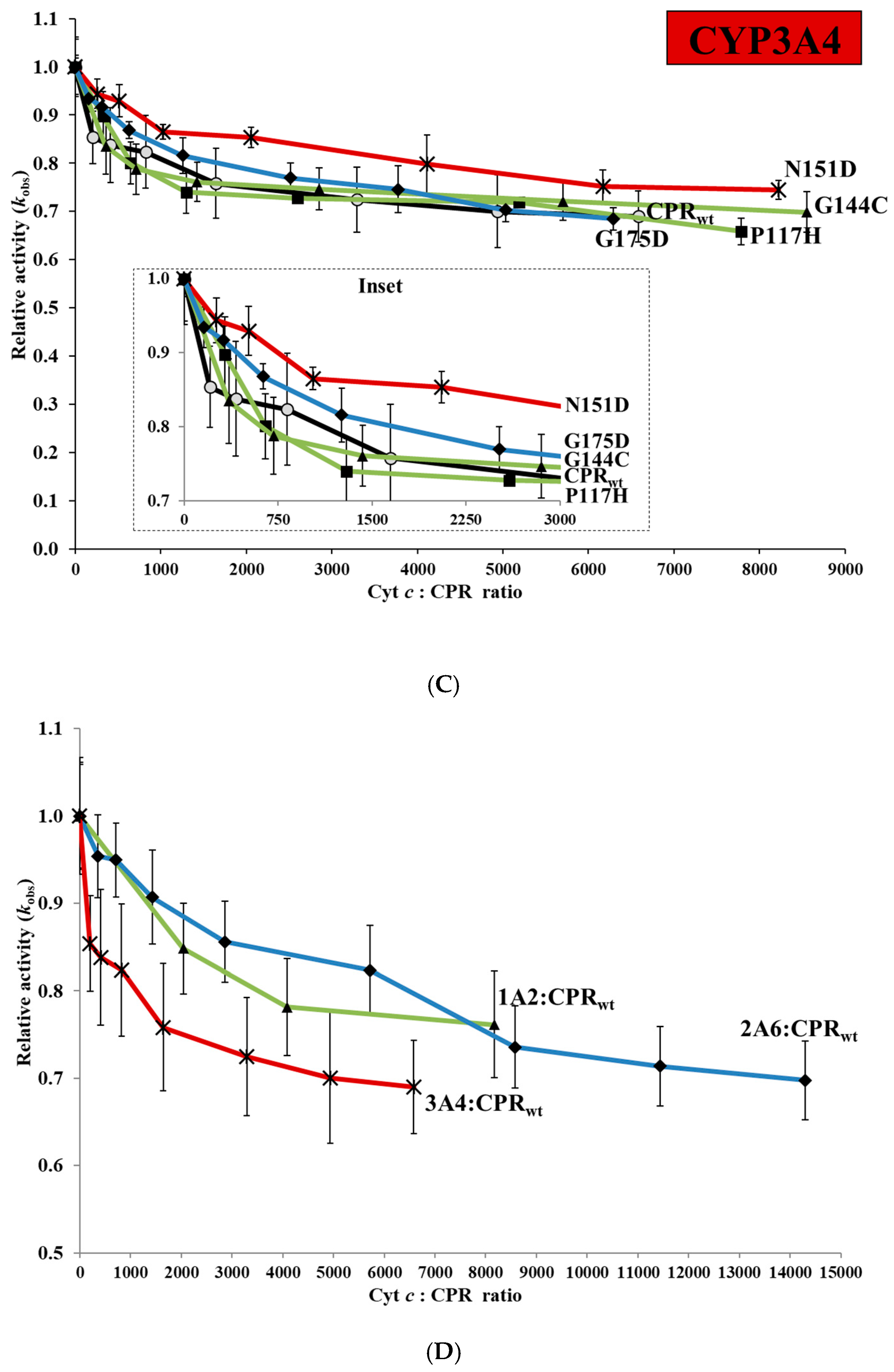
2.3. Cytochrome c Competition with CPRmut/CYP
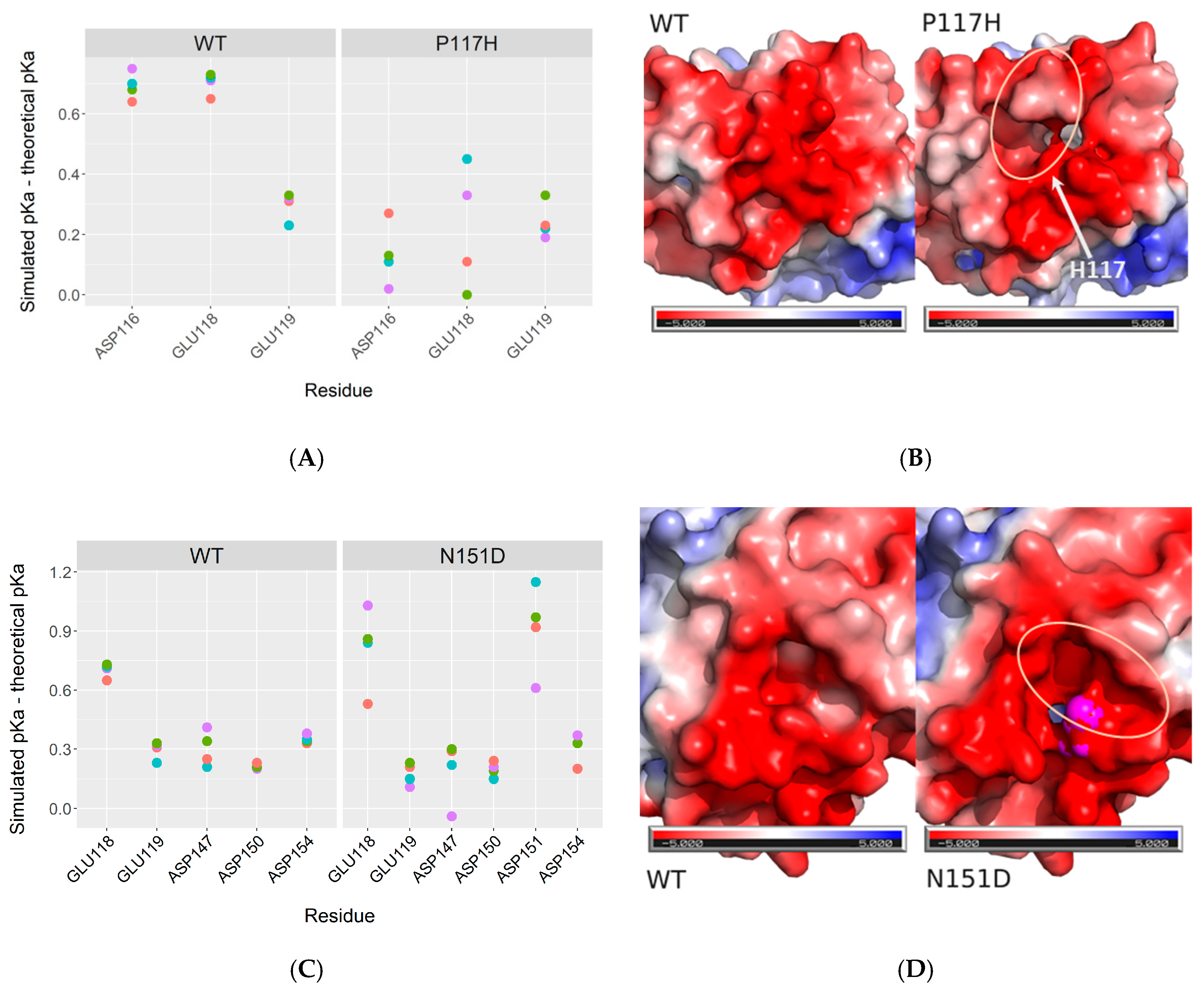
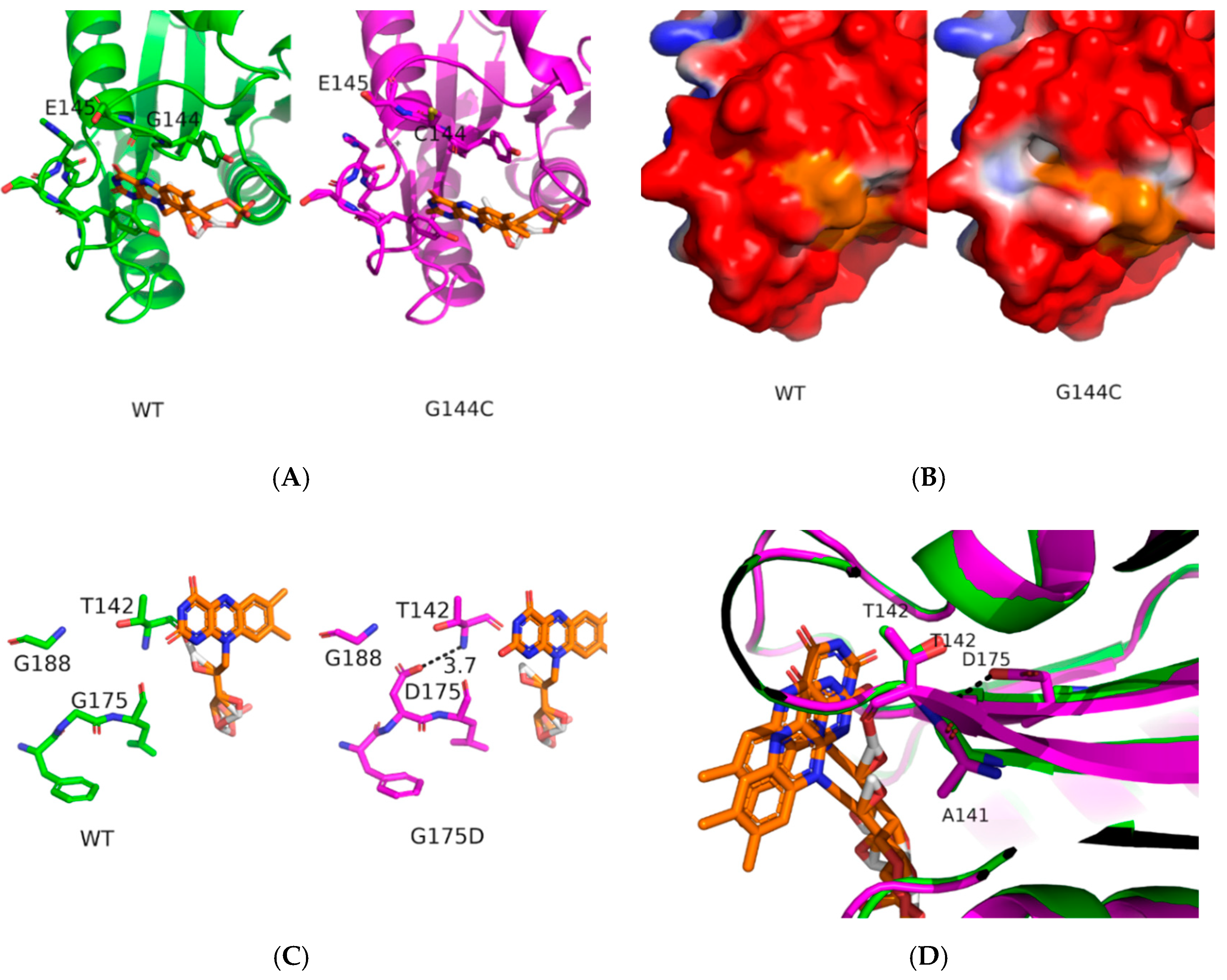
2.4. Structural Analysis of CPR Mutants
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Membrane Fractions Preparation
4.3. CYP Enzyme Activity Assays
4.4. Cytochrome c Competition Assays
4.5. Structural Analysis of CPR Mutants
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| C7H | Coumarin 7-hydroxylation |
| CEC | 3-cyano-7-ethoxycoumarin |
| CECOD | 3-cyano-7-ethoxycoumarin O-dealkylation |
| CPR | NADPH cytochrome P450 oxidoreductase |
| CYP | Cytochrome P450 |
| Cytochrome c | Cyt c |
| DBF | Dibenzylfluorescein |
| DBODF | Dibenzylfluorescein O-debenzylation |
| EROD | Ethoxyresorufin O-deethylation |
| ET | Electron transfer |
| EthR | Ethoxyresorufin |
| FAD | Flavin adenine dinucleotide |
| FD | FMN binding domain |
| FMN | Flavin mononucleotide |
References
- Guengerich, F.P. Human Cytochrome P450 Enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed.; Ortiz de Montellano, P.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 523–785. ISBN 978-3-319-12108-6. [Google Scholar]
- Hlavica, P. Mechanistic basis of electron transfer to cytochromes P450 by natural redox partners and artificial donor constructs. Adv. Exp. Med. Biol. 2015, 851, 247–297. [Google Scholar] [CrossRef] [PubMed]
- Strobel, H.W.; Hodgson, A.V.; Shen, S. NADPH Cytochrome P450 Reductase and Its Structural and Functional Domains. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 2nd ed.; Ortiz de Montellano, P.R.O., Ed.; Springer: Boston, MA, USA, 1995; pp. 225–244. ISBN 978-1-4757-2391-5. [Google Scholar]
- Pandey, A.V.; Flück, C.E. NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef] [PubMed]
- Waskell, L.; Kim, J.-J.P. Electron Transfer Partners of Cytochrome P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed.; Ortiz de Montellano, P.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–68. ISBN 978-3-319-12108-6. [Google Scholar]
- Yablokov, E.O.; Sushko, T.A.; Ershov, P.V.; Florinskaya, A.V.; Gnedenko, O.V.; Shkel, T.V.; Grabovec, I.P.; Strushkevich, N.V.; Kaluzhskiy, L.A.; Usanov, S.A.; et al. A large-scale comparative analysis of affinity, thermodynamics and functional characteristics of interactions of twelve cytochrome P450 isoforms and their redox partners. Biochimie 2019, 162, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A.; Cao, C.; Waterman, M.R. An additional electrostatic interaction between adrenodoxin and P450c27 (CYP27A1) results in tighter binding than between adrenodoxin and p450scc (CYP11A1). J. Biol. Chem. 1999, 274, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Paine, M.; Wolf, C.R.; Scrutton, N.S.; Roberts, G.C.K. Relaxation kinetics of cytochrome P450 reductase: Internal electron transfer is limited by conformational change and regulated by coenzyme binding. Biochemistry 2002, 41, 4626–4637. [Google Scholar] [CrossRef]
- Hlavica, P.; Lehnerer, M. Oxidative biotransformation of fatty acids by cytochromes P450: Predicted key structural elements orchestrating substrate specificity, regioselectivity and catalytic efficiency. Curr. Drug Metab. 2010, 11, 85–104. [Google Scholar] [CrossRef]
- Kandel, S.E.; Lampe, J.N. Role of protein-protein interactions in cytochrome P450-mediated drug metabolism and toxicity. Chem. Res. Toxicol. 2014, 27, 1474–1486. [Google Scholar] [CrossRef]
- Campelo, D.; Esteves, F.; Brito Palma, B.; Costa Gomes, B.; Rueff, J.; Lautier, T.; Urban, P.; Truan, G.; Kranendonk, M. Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450. Int. J. Mol. Sci. 2018, 19, 3914. [Google Scholar] [CrossRef]
- Aigrain, L.; Pompon, D.; Moréra, S.; Truan, G. Structure of the open conformation of a functional chimeric NADPH cytochrome P450 reductase. EMBO Rep. 2009, 10, 742–747. [Google Scholar] [CrossRef]
- Ellis, J.; Gutierrez, A.; Barsukov, I.L.; Huang, W.-C.; Grossmann, J.G.; Roberts, G.C.K. Domain motion in cytochrome P450 reductase: Conformational equilibria revealed by NMR and small-angle x-ray scattering. J. Biol. Chem. 2009, 284, 36628–36637. [Google Scholar] [CrossRef]
- Hamdane, D.; Xia, C.; Im, S.-C.; Zhang, H.; Kim, J.-J.P.; Waskell, L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J. Biol. Chem. 2009, 284, 11374–11384. [Google Scholar] [CrossRef] [PubMed]
- Frances, O.; Fatemi, F.; Pompon, D.; Guittet, E.; Sizun, C.; Pérez, J.; Lescop, E.; Truan, G. A well-balanced preexisting equilibrium governs electron flux efficiency of a multidomain diflavin reductase. Biophys. J. 2015, 108, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Campelo, D.; Gomes, B.C.; Urban, P.; Bozonnet, S.; Lautier, T.; Rueff, J.; Truan, G.; Kranendonk, M. The Role of the FMN-Domain of Human Cytochrome P450 Oxidoreductase in Its Promiscuous Interactions with Structurally Diverse Redox Partners. Front. Pharmacol. 2020, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.L.; Kasper, C.B. Role of acidic residues in the interaction of NADPH-cytochrome P450 oxidoreductase with cytochrome P450 and cytochrome c. J. Biol. Chem. 1995, 270, 27475–27480. [Google Scholar] [CrossRef]
- Campelo, D.; Lautier, T.; Urban, P.; Esteves, F.; Bozonnet, S.; Truan, G.; Kranendonk, M. The Hinge Segment of Human NADPH-Cytochrome P450 Reductase in Conformational Switching: The Critical Role of Ionic Strength. Front. Pharmacol. 2017, 8, 755. [Google Scholar] [CrossRef]
- Barnaba, C.; Gentry, K.; Sumangala, N.; Ramamoorthy, A. The catalytic function of cytochrome P450 is entwined with its membrane-bound nature. F1000Research 2017, 6, 662. [Google Scholar] [CrossRef]
- Barnaba, C.; Ramamoorthy, A. Picturing the Membrane-assisted Choreography of Cytochrome P450 with Lipid Nanodiscs. ChemPhysChem 2018, 19, 2603–2613. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Nanodiscs in Membrane Biochemistry and Biophysics. Chem. Rev. 2017, 117, 4669–4713. [Google Scholar] [CrossRef]
- Kranendonk, M.; Marohnic, C.C.; Panda, S.P.; Duarte, M.P.; Oliveira, J.S.; Masters, B.S.S.; Rueff, J. Impairment of human CYP1A2-mediated xenobiotic metabolism by Antley-Bixler syndrome variants of cytochrome P450 oxidoreductase. Arch. Biochem. Biophys. 2008, 475, 93–99. [Google Scholar] [CrossRef]
- Backes, W.L.; Kelley, R.W. Organization of multiple cytochrome P450s with NADPH-cytochrome P450 reductase in membranes. Pharmacol. Ther. 2003, 98, 221–233. [Google Scholar] [CrossRef]
- Shen, S.; Strobel, H.W. Probing the putative cytochrome P450- and cytochrome c-binding sites on NADPH-cytochrome P450 reductase by anti-peptide antibodies. Biochemistry 1994, 33, 8807–8812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Modi, S.; Smith, G.; Paine, M.; McDonagh, P.D.; Wolf, C.R.; Tew, D.; Lian, L.Y.; Roberts, G.C.; Driessen, H.P. Crystal structure of the FMN-binding domain of human cytochrome P450 reductase at 1.93 A resolution. Protein Sci. 1999, 8, 298–306. [Google Scholar] [CrossRef]
- Marohnic, C.C.; Panda, S.P.; McCammon, K.; Rueff, J.; Masters, B.S.S.; Kranendonk, M. Human cytochrome P450 oxidoreductase deficiency caused by the Y181D mutation: Molecular consequences and rescue of defect. Drug Metab. Dispos. Biol. Fate Chem. 2010, 38, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R.; Backes, W.L. The functional effects of physical interactions involving cytochromes P450: Putative mechanisms of action and the extent of these effects in biological membranes. Drug Metab. Rev. 2016, 48, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.C.; McKinnon, R.A.; Miners, J.O. Cytochrome P450 structure-function: Insights from molecular dynamics simulations. Drug Metab. Rev. 2016, 48, 434–452. [Google Scholar] [CrossRef]
- Gentry, K.A.; Anantharamaiah, G.M.; Ramamoorthy, A. Probing protein-protein and protein-substrate interactions in the dynamic membrane-associated ternary complex of cytochromes P450, b5, and reductase. Chem. Commun. 2019, 55, 13422–13425. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.-H.; Chuo, S.-W.; Qiu, Y.; Wang, L.-P.; Goodin, D.B. Linkage between Proximal and Distal Movements of P450cam Induced by Putidaredoxin. Biochemistry 2020, 59, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Šrejber, M.; Navrátilová, V.; Paloncýová, M.; Bazgier, V.; Berka, K.; Anzenbacher, P.; Otyepka, M. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J. Inorg. Biochem. 2018, 183, 117–136. [Google Scholar] [CrossRef]
- Burkhard, F.Z.; Parween, S.; Udhane, S.S.; Flück, C.E.; Pandey, A.V. P450 Oxidoreductase deficiency: Analysis of mutations and polymorphisms. J. Steroid Biochem. Mol. Biol. 2017, 165, 38–50. [Google Scholar] [CrossRef]
- Moutinho, D.; Marohnic, C.C.; Panda, S.P.; Rueff, J.; Masters, B.S.; Kranendonk, M. Altered human CYP3A4 activity caused by Antley-Bixler syndrome-related variants of NADPH-cytochrome P450 oxidoreductase measured in a robust in vitro system. Drug Metab. Dispos. Biol. Fate Chem. 2012, 40, 754–760. [Google Scholar] [CrossRef]
- Huang, N.; Agrawal, V.; Giacomini, K.M.; Miller, W.L. Genetics of P450 oxidoreductase: Sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc. Natl. Acad. Sci. USA 2008, 105, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Udhane, S.S.; Parween, S.; Kagawa, N.; Pandey, A.V. Altered CYP19A1 and CYP3A4 Activities Due to Mutations A115V, T142A, Q153R and P284L in the Human P450 Oxidoreductase. Front. Pharmacol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Palma, B.B.; Gilep, A.A.; Laires, A.; Oliveira, J.S.; Usanov, S.A.; Rueff, J.; Kranendonk, M. The stimulatory role of human cytochrome b5 in the bioactivation activities of human CYP1A2, 2A6 and 2E1: A new cell expression system to study cytochrome P450-mediated biotransformation (a corrigendum report on Duarte et al. (2005) Mutagenesis 20, 93–100). Mutagenesis 2007, 22, 75–81. [Google Scholar] [CrossRef]
- Palma, B.B.; Silva E Sousa, M.; Urban, P.; Rueff, J.; Kranendonk, M. Functional characterization of eight human CYP1A2 variants: The role of cytochrome b5. Pharmacogenet. Genom. 2013, 23, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Campelo, D.; Urban, P.; Bozonnet, S.; Lautier, T.; Rueff, J.; Truan, G.; Kranendonk, M. Human cytochrome P450 expression in bacteria: Whole-cell high-throughput activity assay for CYP1A2, 2A6 and 3A4. Biochem. Pharmacol. 2018, 158, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Palma, B.B.; Laires, A.; Oliveira, J.S.; Rueff, J.; Kranendonk, M. Escherichia coli BTC, a human cytochrome P450 competent tester strain with a high sensitivity towards alkylating agents: Involvement of alkyltransferases in the repair of DNA damage induced by aromatic amines. Mutagenesis 2005, 20, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.F.; Khalighi, M.; Fisher, J.M.; Shen, D.D.; Kunze, K.L.; Marsh, C.L.; Perkins, J.D.; Thummel, K.E. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J. Pharmacol. Exp. Ther. 1997, 283, 1552–1562. [Google Scholar]
- Venkatakrishnan, K.; von Moltke, L.L.; Court, M.H.; Harmatz, J.S.; Crespi, C.L.; Greenblatt, D.J. Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: Ratios of accessory proteins as sources of discrepancies between the approaches. Drug Metab. Dispos. 2000, 28, 1493–1504. [Google Scholar]
- Scott, E.E.; Wolf, C.R.; Otyepka, M.; Humphreys, S.C.; Reed, J.R.; Henderson, C.J.; McLaughlin, L.A.; Paloncýová, M.; Navrátilová, V.; Berka, K.; et al. The Role of Protein-Protein and Protein-Membrane Interactions on P450 Function. Drug Metab. Dispos. Biol. Fate Chem. 2016, 44, 576–590. [Google Scholar] [CrossRef]
- Jang, H.-H.; Jamakhandi, A.P.; Sullivan, S.Z.; Yun, C.-H.; Hollenberg, P.F.; Miller, G.P. Beta sheet 2-alpha helix C loop of cytochrome P450 reductase serves as a docking site for redox partners. Biochim. Biophys. Acta 2010, 1804, 1285–1293. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, M.; Rwere, F.; Waskell, L.; Ramamoorthy, A. Kinetic and structural characterization of the interaction between the FMN binding domain of cytochrome P450 reductase and cytochrome c. J. Biol. Chem. 2015, 290, 4843–4855. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Roberts, D.L.; Paschke, R.; Shea, T.M.; Masters, B.S.; Kim, J.J. Three-dimensional structure of NADPH-cytochrome P450 reductase: Prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411–8416. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, H.; Yuan, Y.-C.; Chen, S. Sequence-function correlation of aromatase and its interaction with reductase. J. Steroid Biochem. Mol. Biol. 2010, 118, 203–206. [Google Scholar] [CrossRef][Green Version]
- Nicolo, C.; Flück, C.E.; Mullis, P.E.; Pandey, A.V. Restoration of mutant cytochrome P450 reductase activity by external flavin. Mol. Cell. Endocrinol. 2010, 321, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y. Localization of cytochrome c-binding domain on NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1986, 261, 14232–14239. [Google Scholar] [PubMed]
- Allorge, D.; Bréant, D.; Harlow, J.; Chowdry, J.; Lo-Guidice, J.-M.; Chevalier, D.; Cauffiez, C.; Lhermitte, M.; Blaney, F.E.; Tucker, G.T.; et al. Functional analysis of CYP2D6.31 variant: Homology modeling suggests possible disruption of redox partner interaction by Arg440His substitution. Proteins 2005, 59, 339–346. [Google Scholar] [CrossRef]
- Sündermann, A.; Oostenbrink, C. Molecular dynamics simulations give insight into the conformational change, complex formation, and electron transfer pathway for cytochrome P450 reductase. Protein Sci. Publ. Protein Soc. 2013, 22, 1183–1195. [Google Scholar] [CrossRef]
- Huang, N.; Pandey, A.V.; Agrawal, V.; Reardon, W.; Lapunzina, P.D.; Mowat, D.; Jabs, E.W.; Van Vliet, G.; Sack, J.; Flück, C.E.; et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am. J. Hum. Genet. 2005, 76, 729–749. [Google Scholar] [CrossRef]
- Xia, C.; Panda, S.P.; Marohnic, C.C.; Martásek, P.; Masters, B.S.; Kim, J.-J.P. Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc. Natl. Acad. Sci. USA 2011, 108, 13486–13491. [Google Scholar] [CrossRef]

| Membrane Fractions | Protein Contents | |||
|---|---|---|---|---|
| Cyp Isoform | CPR Form | CYP | CPR | CPR/CYP |
| (pmol/mg Protein) | Ratios a | |||
| CYP1A2 | wt a | 54 ± 1 | 4.1 ± 1.5 | 1:13 |
| P117H b | 148 ± 1 | 12.2 ± 0.5 | 1:12 | |
| G144C b | 124 ± 2 | 14.1 ± 0.2 | 1:9 | |
| G175D | 379 ± 2 | 26.5 ± 0.4 | 1:14 | |
| N151D | 446 ± 4 | 27.7 ± 0.5 | 1:16 | |
| CPRnull b | 91 ± 6 | NA | NA | |
| CYP2A6 | wt b | 139 ± 1 | 10.5 ± 1.3 | 1:13 |
| P117H | 205 ± 3 | 16.5 ± 0.9 | 1:12 | |
| G144C | 315 ± 8 | 28.3 ± 0.8 | 1:11 | |
| G175D b | 112 ± 6 | 11.3 ± 0.5 | 1:10 | |
| N151D | 221 ± 1 | 19.7 ± 0.8 | 1:11 | |
| CPRnull b | 178 ± 4 | NA | NA | |
| CYP3A4 | wt a | 83 ± 3 | 19.8 ± 0.2 | 1:4 |
| P117H | 230 ± 9 | 35.5 ± 0.8 | 1:6 | |
| G144C | 189 ± 11 | 26.5 ± 0.3 | 1:7 | |
| G175D | 143 ± 5 | 31.5 ± 0.5 | 1:5 | |
| N151D b | 45 ± 1 | 12.7 ± 0.6 | 1:4 | |
| CPRnull b | 85 ± 3 | NA | NA | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, F.; Urban, P.; Rueff, J.; Truan, G.; Kranendonk, M. Interaction Modes of Microsomal Cytochrome P450s with Its Reductase and the Role of Substrate Binding. Int. J. Mol. Sci. 2020, 21, 6669. https://doi.org/10.3390/ijms21186669
Esteves F, Urban P, Rueff J, Truan G, Kranendonk M. Interaction Modes of Microsomal Cytochrome P450s with Its Reductase and the Role of Substrate Binding. International Journal of Molecular Sciences. 2020; 21(18):6669. https://doi.org/10.3390/ijms21186669
Chicago/Turabian StyleEsteves, Francisco, Philippe Urban, José Rueff, Gilles Truan, and Michel Kranendonk. 2020. "Interaction Modes of Microsomal Cytochrome P450s with Its Reductase and the Role of Substrate Binding" International Journal of Molecular Sciences 21, no. 18: 6669. https://doi.org/10.3390/ijms21186669
APA StyleEsteves, F., Urban, P., Rueff, J., Truan, G., & Kranendonk, M. (2020). Interaction Modes of Microsomal Cytochrome P450s with Its Reductase and the Role of Substrate Binding. International Journal of Molecular Sciences, 21(18), 6669. https://doi.org/10.3390/ijms21186669









