Anti-PD-1 Therapy Does Not Influence Hearing Ability in the Most Sensitive Frequency Range, but Mitigates Outer Hair Cell Loss in the Basal Cochlear Region
Abstract
:1. Introduction
2. Results
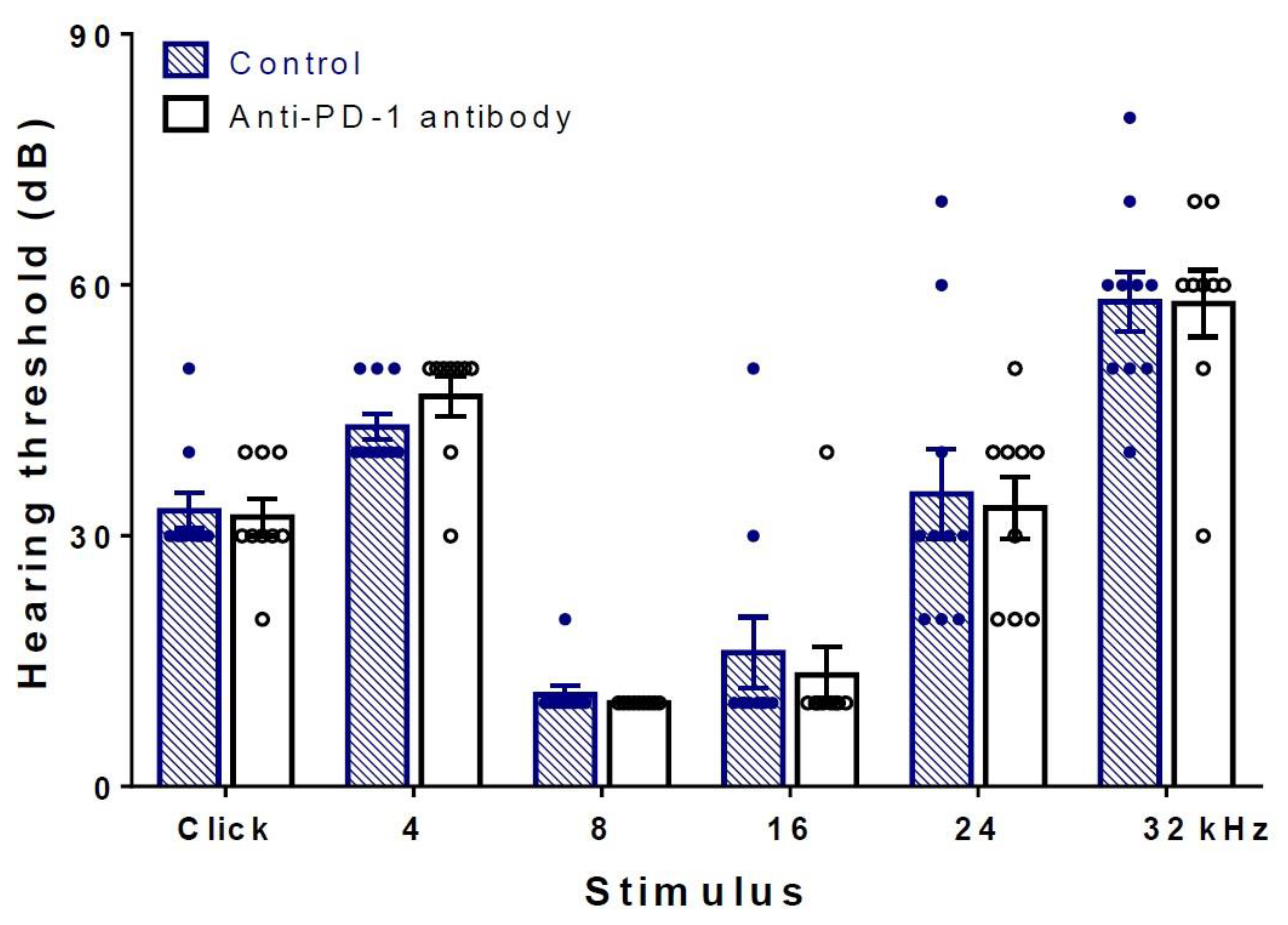
2.1. Differences in Hearing Thresholds between Treatment Groups Could Not Be Observed after PD-1 Inhibition
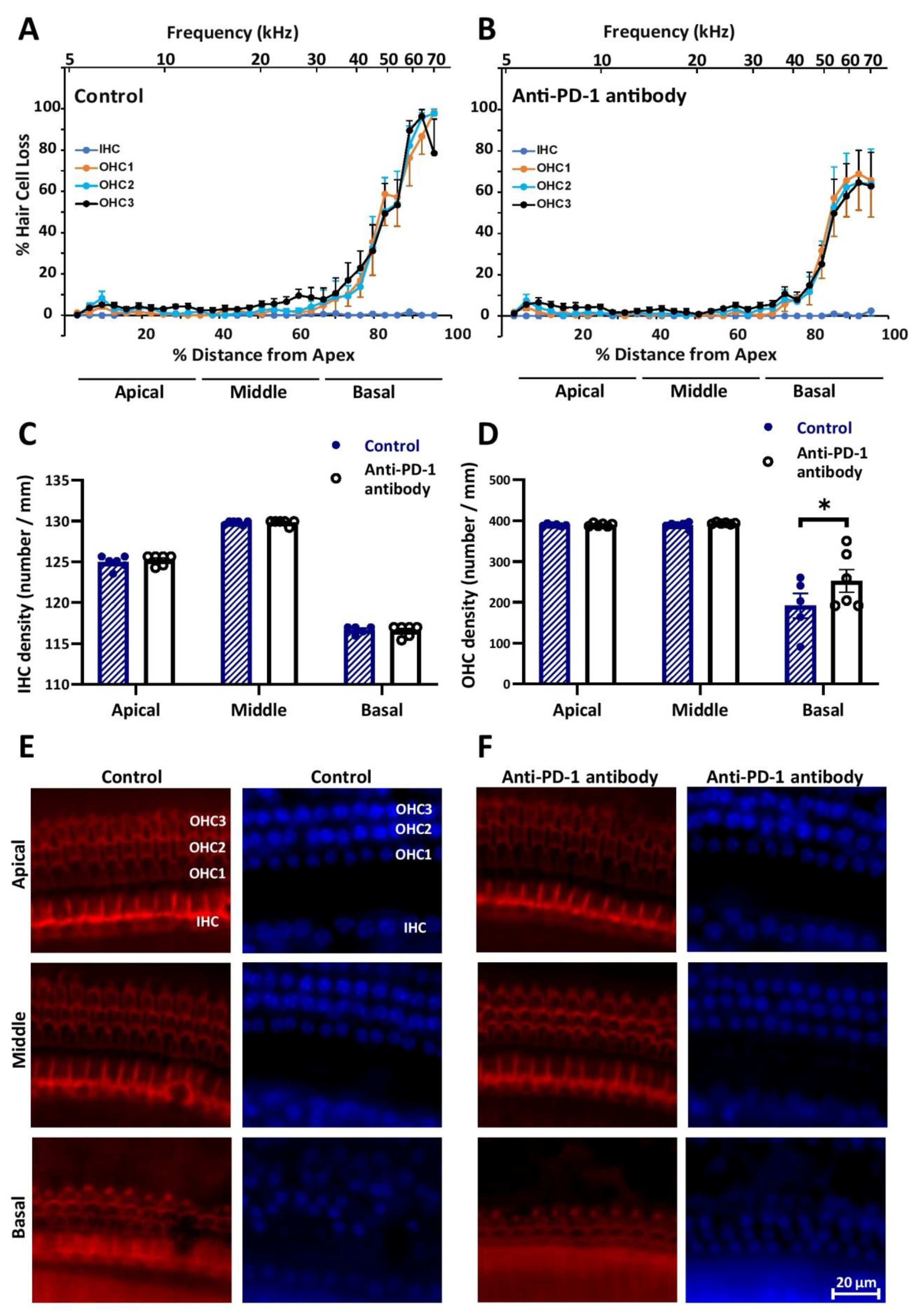
2.2. Anti-PD-1 Antibody Treatment Did Not Affect the Number of Hair Cells in the Apical and Middle Cochlear Turns, but Preserved OHCs in the Basal Turn
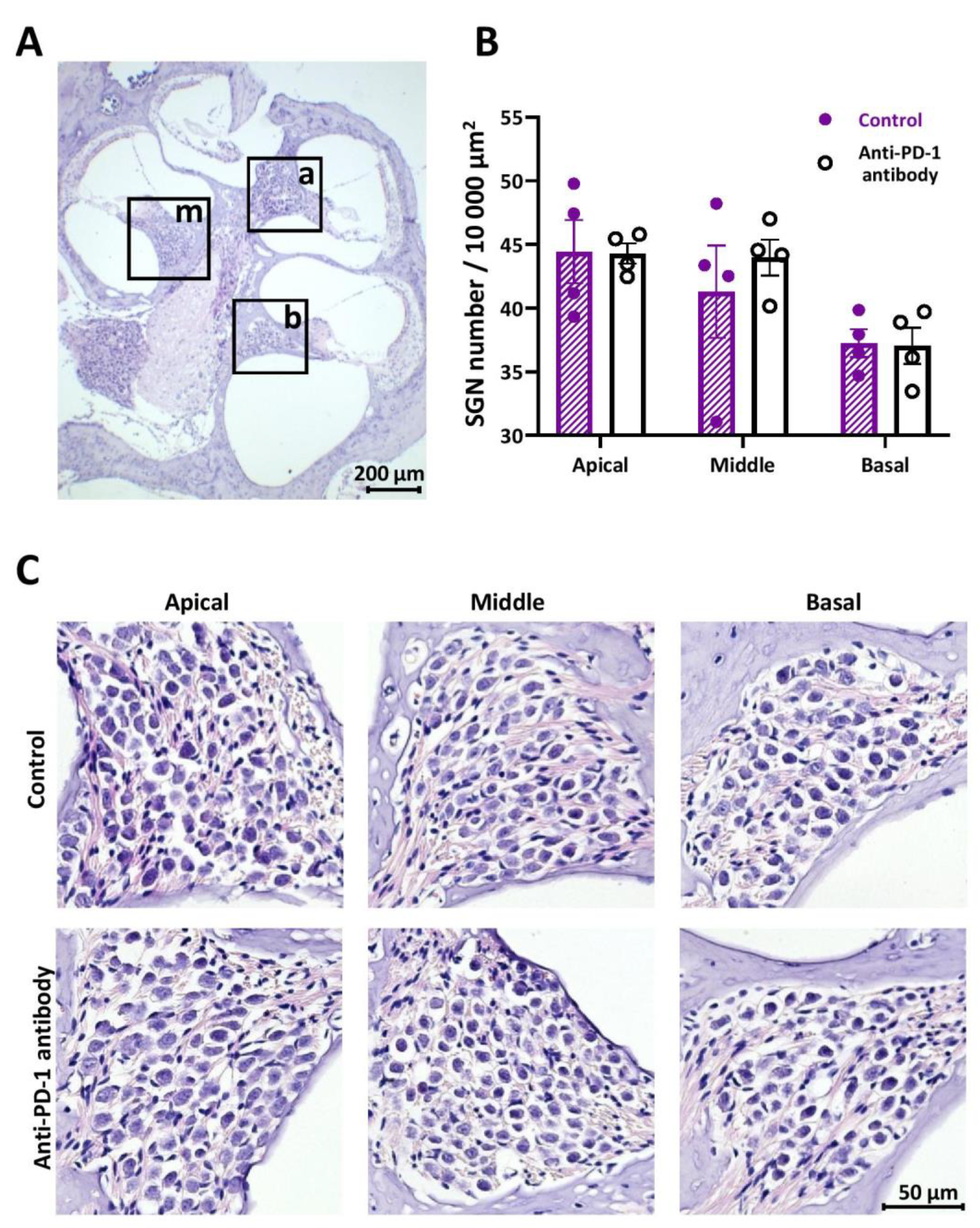
2.3. Number of SGNs Was Not Changed by Anti-PD-1 Antibody Treatment in Either Cochlear Turn
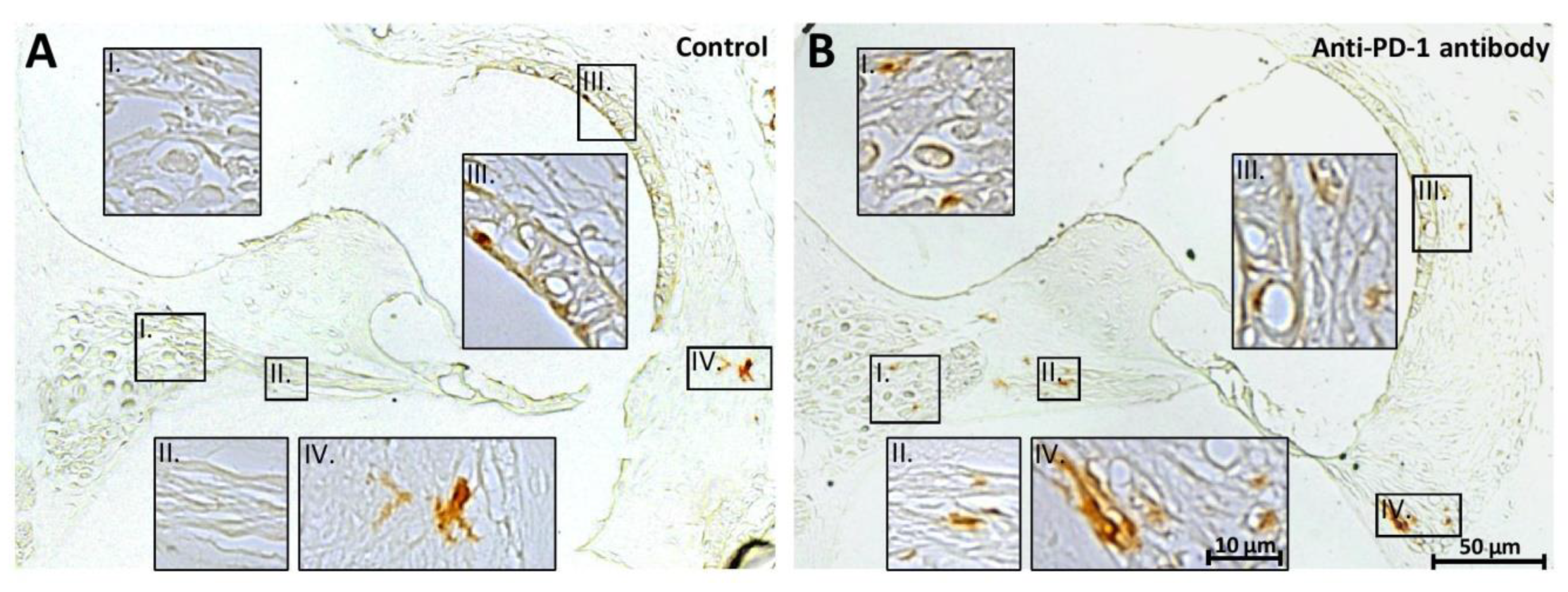
2.4. PD-1 Inhibition Did Not Affect the Number of Iba-1 Positive Macrophages in the Apical and Middle Cochlear Turns, but Increased It in the Basal Turn
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
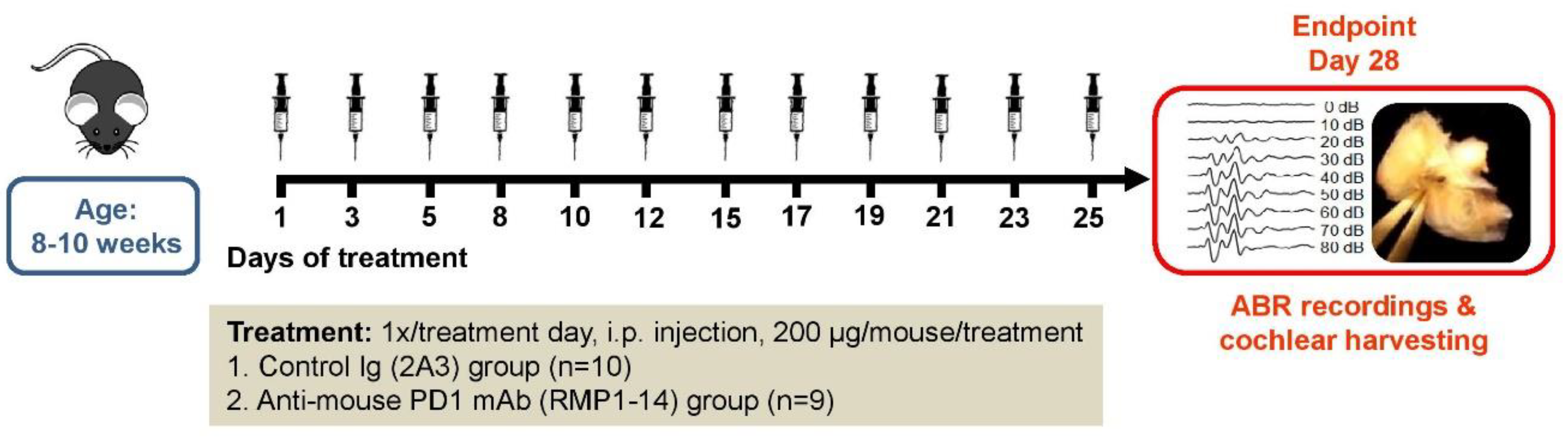
4.2. Experimental Groups and Drug Administration
4.3. Hearing Assessment
4.4. Tissue Preparation
4.5. Whole-Mount Cochlear Dissection and Phalloidin/DAPI Staining
4.6. Cytocochleogram Plotting
4.7. Modiolar Sectioning for Hematoxylin-Eosin Staining and Immunohistochemistry
4.8. Hematoxylin/Eosin (HE) Staining and Counting Spiral Ganglion Neurons (SGNs)
4.9. Iba1 Immunohistochemistry
4.10. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICI | immune checkpoint inhibitor |
| PD-L1 | programmed death-ligand 1 |
| PD-1 | programmed cell death protein 1 |
| NSCLC | non-small-cell lung cancer |
| RCC | renal cell carcinoma |
| irAE | immune-related adverse event |
| ABR | auditory brainstem response |
| DAB | 3,3′-diaminobenzidine |
| HE | hematoxylin/eosin |
| SGN | spiral ganglion neuron |
| OHC | outer hair cell |
| IHC | inner hair cell |
| NIH | National Institutes of Health |
| PFA | paraformaldehyde |
| PBS | phosphate-buffered saline |
| EDTA | ethylenediamine-tetraacetate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| Iba1 | ionized calcium-binding adaptor molecule 1 |
| HRP | horseradish peroxidase |
| ANOVA | analysis of variance |
| SEM | standard error of the mean |
| Aβ | amyloid-β |
References
- Morello, S.; Pinto, A.; Blandizzi, C.; Antonioli, L. Myeloid cells in the tumor microenvironment: Role of adenosine. Oncoimmunology 2016, 5, e1108515. [Google Scholar] [CrossRef] [Green Version]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune Modulation in Cancer with Antibodies. Annu. Rev. Med. 2014, 65, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Yegutkin, G.G.; Pacher, P.; Blandizzi, C.; Haskó, G. Anti-CD73 in cancer immunotherapy: Awakening new opportunities. Trends Cancer 2016, 2, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.M.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’Donnell, P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef]
- Postow, M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. B. 2015, 35, 76–83. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Antoun, J.; Titah, C.; Cochereau, I. Ocular and orbital side-effects of checkpoint inhibitors. Curr. Opin. Oncol. 2016, 28, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Zibelman, M.; Pollak, N.; Olszanski, A.J. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J. Immunother. Cancer 2016, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobelmann, K.; Fitzgerald, D. A Case of Pembrolizumab Induced Autoimmune Sensorineural Hearing Loss. J. Otol. Rhinol. 2019. [Google Scholar] [CrossRef]
- Tampio, A.J.F.; Dhanireddy, S.; Sivapiragasam, A.; Nicholas, B.D. Bilateral Sensorineural Hearing Loss Associated With Nivolumab Therapy for Stage IV Malignant Melanoma. Ear Nose Throat J. 2020. [Google Scholar] [CrossRef]
- Raphael, Y. Cochlear pathology, sensory cell death and regeneration. Br. Med. Bull. 2002, 63, 25–38. [Google Scholar] [CrossRef]
- Tan, W.J.; Thorne, P.R.; Vlajkovic, S.M. Noise-induced cochlear inflammation. World J. Otorhinolaryngol. 2013, 3, 89–99. [Google Scholar] [CrossRef]
- Harris, J.P. Immunology of the inner ear: Response of the inner ear to antigen challenge. Otolaryngol. Neck Surg. 1983, 91, 18–23. [Google Scholar] [CrossRef]
- Okano, T.; Nakagawa, T.; Kita, T.; Kada, S.; Yoshimoto, M.; Nakahata, T.; Ito, J. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J. Neurosci. Res. 2008, 86, 1758–1767. [Google Scholar] [CrossRef]
- Köles, L.; Szepesy, J.; Berekméri, E.; Zelles, T. Purinergic Signaling and Cochlear Injury-Targeting the Immune System? Int. J. Mol. Sci. 2019, 20, 2979. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Vethanayagam, R.R.; Dong, Y.; Cai, Q.; Hu, B.H. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience 2015, 303, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.H.; Zhang, C.; Frye, M.D. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear. Res. 2018, 362, 14–24. [Google Scholar] [CrossRef]
- Wood, M.B.; Zuo, J. The Contribution of Immune Infiltrates to Ototoxicity and Cochlear Hair Cell Loss. Front. Cell. Neurosci. 2017, 11, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.J.T.; Thorne, P.R.; Vlajkovic, S.M. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem. Cell Biol. 2016, 146, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yamashita, D.; Uehara, N.; Inokuchi, G.; Hasegawa, S.; Otsuki, N.; Nibu, K.I. A high-fat diet delays age-related hearing loss progression in C57BL/6J mice. PLoS ONE 2015, 10, e0117547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinec, G.M.; Lomberk, G.; Urrutia, R.A.; Kalinec, F. Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss. Front. Cell. Neurosci. 2017, 11, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujioka, M.; Okano, H.; Ogawa, K. Inflammatory and immune responses in the cochlea: Potential therapeutic targets for sensorineural hearing loss. Front. Pharmacol. 2014, 5, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frye, M.D.; Yang, W.; Zhang, C.; Xiong, B.; Hu, B.H. Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae. Hear. Res. 2017, 344, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Dai, M.; Fridberger, A.; Hassan, A.; DeGagne, J.; Neng, L.; Zhang, F.; He, W.; Ren, T.; Trune, D.; et al. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. USA 2012, 109, 10388–10393. [Google Scholar] [CrossRef] [Green Version]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005, 489, 180–194. [Google Scholar] [CrossRef]
- Koo, J.-W.; Quintanilla-Dieck, L.; Jiang, M.; Liu, J.; Urdang, Z.D.; Allensworth, J.J.; Cross, C.P.; Li, H.; Steyger, P.S. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci. Transl. Med. 2015, 7, 298ra118. [Google Scholar] [CrossRef] [Green Version]
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Guilliams, M.; Haskó, G. Rethinking Communication in the Immune System: The Quorum Sensing Concept. Trends Immunol. 2019, 40, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Verschuur, C.; Agyemang-Prempeh, A.; Newman, T.A. Inflammation is associated with a worsening of presbycusis: Evidence from the MRC national study of hearing. Int. J. Audiol. 2014, 53, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Mukherjea, D.; Sheehan, K.; Jajoo, S.; Rybak, L.P.; Ramkumar, V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011, 2, e180. [Google Scholar] [CrossRef] [Green Version]
- So, H.; Kim, H.; Lee, J.-H.; Park, C.; Kim, Y.; Kim, E.; Kim, J.-K.; Yun, K.-J.; Lee, K.-M.; Lee, H.-Y.; et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J. Assoc. Res. Otolaryngol. 2007, 8, 338–355. [Google Scholar] [CrossRef] [Green Version]
- Hirose, K.; Sato, E. Comparative analysis of combination kanamycin-furosemide versus kanamycin alone in the mouse cochlea. Hear. Res. 2011, 272, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Kaur, T.; Ohlemiller, K.K.; Warchol, M.E. Genetic disruption of fractalkine signaling leads to enhanced loss of cochlear afferents following ototoxic or acoustic injury. J. Comp. Neurol. 2018, 526, 824–835. [Google Scholar] [CrossRef]
- Ladrech, S.; Wang, J.; Simonneau, L.; Puel, J.-L.; Lenoir, M. Macrophage contribution to the response of the rat organ of Corti to amikacin. J. Neurosci. Res. 2007, 85, 1970–1979. [Google Scholar] [CrossRef]
- Sato, E.; Shick, H.E.; Ransohoff, R.M.; Hirose, K. Expression of Fractalkine Receptor CX3CR1 on Cochlear Macrophages Influences Survival of Hair Cells Following Ototoxic Injury. J. Assoc. Res. Otolaryngol. 2010, 11, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.J.; Sha, S.H.; McLaren, J.D.; Kawamoto, K.; Raphael, Y.; Schacht, J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear. Res. 2001, 158, 165–178. [Google Scholar] [CrossRef]
- Cianfrone, G.; Pentangelo, D.; Cianfrone, F.; Mazzei, F.; Turchetta, R.; Orlando, M.P.; Altissimi, G. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: A reasoned and updated guide. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 601–636. [Google Scholar]
- Oh, G.-S.; Kim, H.-J.; Choi, J.-H.; Shen, A.; Kim, C.-H.; Kim, S.-J.; Shin, S.-R.; Hong, S.-H.; Kim, Y.; Park, C.; et al. Activation of Lipopolysaccharide-TLR4 Signaling Accelerates the Ototoxic Potential of Cisplatin in Mice. J. Immunol. 2011, 186, 1140–1150. [Google Scholar] [CrossRef]
- Guthrie, O.W. Aminoglycoside induced ototoxicity. Toxicology 2008, 249, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Spielbauer, K.; Cunningham, L.; Schmitt, N. PD-1 Inhibition Minimally Affects Cisplatin-Induced Toxicities in a Murine Model. Otolaryngol. Head Neck Surg. 2018, 159, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Kuzucu, İ.; Baklacı, D.; Guler, İ.; Uçaryılmaz, E.Ö.; Kum, R.O.; Özcan, M. Investigation of the Ototoxic Effect of Pembrolizumab Using a Rat Model. Cureus 2019, 11, e6057. [Google Scholar] [CrossRef] [PubMed]
- Viberg, A.; Canlon, B. The guide to plotting a cochleogram. Hear. Res. 2004, 197, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bohne, B.A.; Harding, G.W. Combined organ of Corti/modiolus technique for preparing mammalian cochleas for quantitative microscopy. Hear. Res. 1993, 71, 114–124. [Google Scholar] [CrossRef]
- Raphael, Y.; Altschuler, R.A. Scar formation after drug-induced cochlear insult. Hear. Res. 1991, 51, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Kaur, T.; Zamani, D.; Tong, L.; Rubel, E.W.; Ohlemiller, K.K.; Hirose, K.; Warchol, M.E. Fractalkine Signaling Regulates Macrophage Recruitment into the Cochlea and Promotes the Survival of Spiral Ganglion Neurons after Selective Hair Cell Lesion. J. Neurosci. 2015, 35, 15050–15061. [Google Scholar] [CrossRef] [Green Version]
- Sekiya, T.; Tanaka, M.; Shimamura, N.; Suzuki, S. Macrophage invasion into injured cochlear nerve and its modification by methylprednisolone. Brain Res. 2001, 905, 152–160. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Johnson, K.R.; Erway, L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999, 130, 94–107. [Google Scholar] [CrossRef] [Green Version]
- Spongr, V.P.; Flood, D.G.; Frisina, R.D.; Salvi, R.J. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J. Acoust. Soc. Am. 1997, 101, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; Wright, J.S.; Heidbreder, A.F. Vulnerability to noise-induced hearing loss in “middle-aged” and young adult mice: A dose-response approach in CBA, C57BL, and BALB inbred strains. Hear. Res. 2000, 149, 239–247. [Google Scholar] [CrossRef]
- Keithley, E.M.; Canto, C.; Zheng, Q.Y.; Fischel-Ghodsian, N.; Johnson, K.R. Age-related hearing loss and the ahl locus in mice. Hear. Res. 2004, 188, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Blasko, I.; Stampfer-Kountchev, M.; Robatscher, P.; Veerhuis, R.; Eikelenboom, P.; Grubeck-Loebenstein, B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: The role of microglia and astrocytes. Aging Cell 2004, 3, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M. Can immunotherapy treat neurodegeneration? Science 2017, 357, 254–255. [Google Scholar] [CrossRef]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef]
- Rosenzweig, N.; Dvir-Szternfeld, R.; Tsitsou-Kampeli, A.; Keren-Shaul, H.; Ben-Yehuda, H.; Weill-Raynal, P.; Cahalon, L.; Kertser, A.; Baruch, K.; Amit, I.; et al. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Martini, E.; Kunderfranco, P.; Peano, C.; Carullo, P.; Cremonesi, M.; Schorn, T.; Carriero, R.; Termanini, A.; Colombo, F.S.; Jachetti, E.; et al. Single-Cell Sequencing of Mouse Heart Immune Infiltrate in Pressure Overload-Driven Heart Failure Reveals Extent of Immune Activation. Circulation 2019, 140, 2089–2107. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Munn, D.H.; Wolchok, J.D.; Allison, J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013, 210, 1389–1402. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moynihan, K.D.; Opel, C.F.; Szeto, G.L.; Tzeng, A.; Zhu, E.F.; Engreitz, J.M.; Williams, R.T.; Rakhra, K.; Zhang, M.H.; Rothschilds, A.M.; et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 2016, 22, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Polony, G.; Humli, V.; Andó, R.; Aller, M.; Horváth, T.; Harnos, A.; Tamás, L.; Vizi, E.S.; Zelles, T. Protective effect of rasagiline in aminoglycoside ototoxicity. Neuroscience 2014, 265, 263–273. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szepesy, J.; Miklós, G.; Farkas, J.; Kucsera, D.; Giricz, Z.; Gáborján, A.; Polony, G.; Szirmai, Á.; Tamás, L.; Köles, L.; et al. Anti-PD-1 Therapy Does Not Influence Hearing Ability in the Most Sensitive Frequency Range, but Mitigates Outer Hair Cell Loss in the Basal Cochlear Region. Int. J. Mol. Sci. 2020, 21, 6701. https://doi.org/10.3390/ijms21186701
Szepesy J, Miklós G, Farkas J, Kucsera D, Giricz Z, Gáborján A, Polony G, Szirmai Á, Tamás L, Köles L, et al. Anti-PD-1 Therapy Does Not Influence Hearing Ability in the Most Sensitive Frequency Range, but Mitigates Outer Hair Cell Loss in the Basal Cochlear Region. International Journal of Molecular Sciences. 2020; 21(18):6701. https://doi.org/10.3390/ijms21186701
Chicago/Turabian StyleSzepesy, Judit, Gabriella Miklós, János Farkas, Dániel Kucsera, Zoltán Giricz, Anita Gáborján, Gábor Polony, Ágnes Szirmai, László Tamás, László Köles, and et al. 2020. "Anti-PD-1 Therapy Does Not Influence Hearing Ability in the Most Sensitive Frequency Range, but Mitigates Outer Hair Cell Loss in the Basal Cochlear Region" International Journal of Molecular Sciences 21, no. 18: 6701. https://doi.org/10.3390/ijms21186701
APA StyleSzepesy, J., Miklós, G., Farkas, J., Kucsera, D., Giricz, Z., Gáborján, A., Polony, G., Szirmai, Á., Tamás, L., Köles, L., Varga, Z. V., & Zelles, T. (2020). Anti-PD-1 Therapy Does Not Influence Hearing Ability in the Most Sensitive Frequency Range, but Mitigates Outer Hair Cell Loss in the Basal Cochlear Region. International Journal of Molecular Sciences, 21(18), 6701. https://doi.org/10.3390/ijms21186701






