Age and Sex-Dependent ADNP Regulation of Muscle Gene Expression Is Correlated with Motor Behavior: Possible Feedback Mechanism with PACAP
Abstract
1. Introduction
2. Results
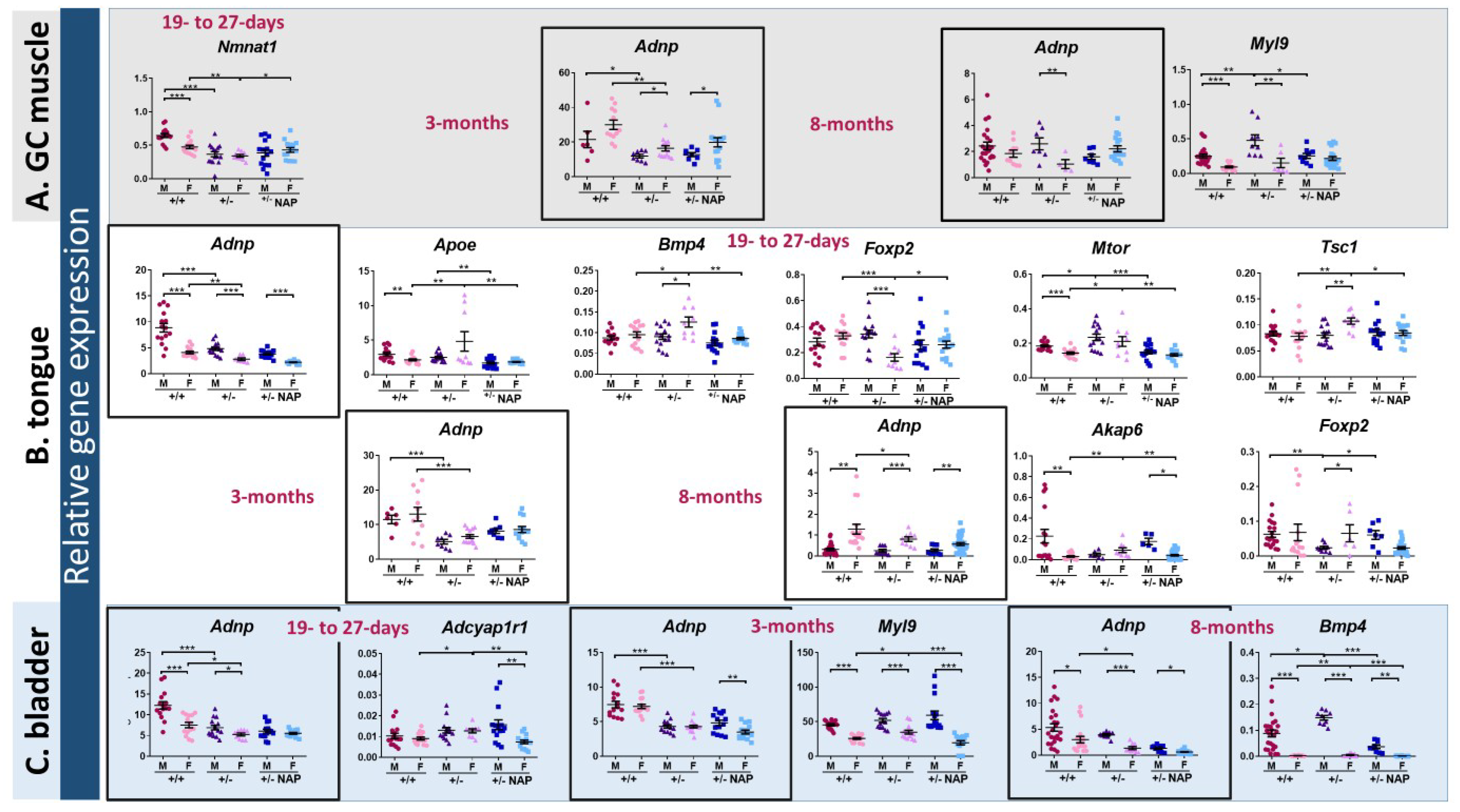
2.1. Adnp+/– Mice Display Age-Dependent Aberrant Gene Expression in Skeletal Muscle, Tongue, and Bladder, Compared with Adnp+/+ Mice, Corrected by NAP Treatment
2.2. Muscle Aberrant Gene Expression Is Correlated with Adnp Deficiency in a Sex and Age-Dependent Manner
2.3. Adnp+/– Mice Display of Age-Dependent Aberrant Gene Expression in Skeletal Muscle is Correlated with Behavior
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Peptide Synthesis and Formulations
4.3. NAP Age Treatment Groups
4.4. Gait Analysis
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantitative Real-Time PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADNP | Activity-dependent neuroprotective protein |
| ALS | Amyotrophic lateral sclerosis |
| BMD | Becker muscular dystrophy |
| DMD | Duchenne muscular dystrophy |
| EB’s | Microtubule end-binding proteins |
| GC | Gastrocnemius |
| LC3B | Microtubule associated protein 1 light chain 3B |
| mRNA | Messenger ribonucleic acid |
| NAP | NAPVSIPQ |
| NMNAT1 | Nicotinamide nucleotide adenylyl (NAD) transferase 1 |
| PACAP | Pituitary adenylate cyclase activating polypeptide |
| PAC1 | Pituitary adenylate cyclase-activating polypeptide type I receptor |
| qPCR | Quantitative real time PCR |
| SBMA | Spinobulbar muscular atrophy |
| VIP | Vasoactive intestinal peptide |
References
- Bassan, M.; Zamostiano, R.; Davidson, A.; Pinhasov, A.; Giladi, E.; Perl, O.; Bassan, H.; Blat, C.; Gibney, G.; Glazner, G.; et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999, 72, 1283–1293. [Google Scholar] [CrossRef]
- Zamostiano, R.; Pinhasov, A.; Gelber, E.; Steingart, R.A.; Seroussi, E.; Giladi, E.; Bassan, M.; Wollman, Y.; Eyre, H.J.; Mulley, J.C.; et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001, 276, 708–714. [Google Scholar] [CrossRef]
- Sragovich, S.; Ziv, Y.; Vaisvaser, S.; Shomron, N.; Hendler, T.; Gozes, I. The autism-mutated ADNP plays a key role in stress response. Transl. Psychiatry 2019, 9, 235. [Google Scholar] [CrossRef]
- Nakamachi, T.; Tsuchida, M.; Kagami, N.; Yofu, S.; Wada, Y.; Hori, M.; Tsuchikawa, D.; Yoshikawa, A.; Imai, N.; Nakamura, K.; et al. IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J. Mol. Neurosci. 2012, 48, 518–525. [Google Scholar] [CrossRef]
- Castorina, A.; Giunta, S.; Scuderi, S.; D’Agata, V. Involvement of PACAP/ADNP signaling in the resistance to cell death in malignant peripheral nerve sheath tumor (MPNST) cells. J. Mol. Neurosci. 2012, 48, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Ohtaki, H.; Yofu, S.; Dohi, K.; Watanabe, J.; Hayashi, D.; Matsuno, R.; Nonaka, N.; Itabashi, K.; Shioda, S. Pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) co-localizes with activity-dependent neuroprotective protein (ADNP) in the mouse brains. Regul. Pept. 2008, 145, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Li, M.; Shioda, S.; Arimura, A. Signaling involved in pituitary adenylate cyclase-activating polypeptide-stimulated ADNP expression. Peptides 2006, 27, 1859–1894. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; David, C.; Kikuta, T.; Somogyvari-Vigh, A.; Arimura, A. Signaling cascades involved in neuroprotection by subpicomolar pituitary adenylate cyclase-activating polypeptide 38. J. Mol. Neurosci. 2005, 27, 91–105. [Google Scholar] [CrossRef]
- Zusev, M.; Gozes, I. Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul. Pept. 2004, 123, 33–41. [Google Scholar] [CrossRef]
- Kaaij, L.J.T.; Mohn, F.; Van der Weide, R.H.; De Wit, E.; Buhler, M. The ChAHP Complex Counteracts Chromatin Looping at CTCF Sites that Emerged from SINE Expansions in Mouse. Cell 2019, 178, 1437–1451. [Google Scholar] [CrossRef]
- Mandel, S.; Gozes, I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J. Biol. Chem. 2007, 282, 34448–34456.e14. [Google Scholar] [CrossRef] [PubMed]
- Amram, N.; Hacohen-Kleiman, G.; Sragovich, S.; Malishkevich, A.; Katz, J.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.C.; Giladi, E.; Yeheskel, A.; et al. Sexual divergence in microtubule function: The novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol. Psychiatry 2016, 21, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Hacohen-Kleiman, G.; Sragovich, S.; Karmon, G.; Gao, A.Y.L.; Grigg, I.; Pasmanik-Chor, M.; Le, A.; Korenkova, V.; McKinney, R.A.; Gozes, I. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J. Clin. Investig. 2018, 128, 4956–4969. [Google Scholar] [CrossRef] [PubMed]
- Gozes, I.; Van Dijck, A.; Hacohen-Kleiman, G.; Grigg, I.; Karmon, G.; Giladi, E.; Eger, M.; Gabet, Y.; Pasmanik-Chor, M.; Cappuyns, E.; et al. Premature primary tooth eruption in cognitive/motor-delayed ADNP-mutated children. Transl. Psychiatry 2017, 7, e1043. [Google Scholar] [CrossRef]
- Grigg, I.; Ivashko-Pachima, Y.; Hait, T.A.; Korenkova, V.; Touloumi, O.; Lagoudaki, R.; Van Dijck, A.; Marusic, Z.; Anicic, M.; Vukovic, J.; et al. Tauopathy in the young autistic brain: Novel biomarker and therapeutic target. Transl. Psychiatry 2020, 10, 228. [Google Scholar] [CrossRef]
- Vulih-Shultzman, I.; Pinhasov, A.; Mandel, S.; Grigoriadis, N.; Touloumi, O.; Pittel, Z.; Gozes, I. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J. Pharmacol. Exp. Ther. 2007, 323, 438–449. [Google Scholar] [CrossRef]
- Van Dijck, A.; Vulto-van Silfhout, A.T.; Cappuyns, E.; Van der Werf, I.M.; Mancini, G.M.; Tzschach, A.; Bernier, R.; Gozes, I.; Eichler, E.E.; Romano, C.; et al. Clinical Presentation of a Complex Neurodevelopmental Disorder Caused by Mutations in ADNP. Biol. Psychiatry 2019, 85, 287–297. [Google Scholar] [CrossRef]
- Gozes, I.; Patterson, M.C.; Van Dijck, A.; Kooy, R.F.; Peeden, J.N.; Eichenberger, J.A.; Zawacki-Downing, A.; Bedrosian-Sermone, S. The Eight and a Half Year Journey of Undiagnosed AD: Gene Sequencing and Funding of Advanced Genetic Testing Has Led to Hope and New Beginnings. Front. Endocrinol. 2017, 8, 107. [Google Scholar] [CrossRef]
- Kapitansky, O.; Gozes, I. ADNP differentially interact with genes/proteins in correlation with aging: A novel marker for muscle aging. Geroscience 2019, 41, 321–340. [Google Scholar] [CrossRef]
- De Guia, R.M.; Agerholm, M.; Nielsen, T.S.; Consitt, L.A.; Sogaard, D.; Helge, J.W.; Larsen, S.; Brandauer, J.; Houmard, J.A.; Treebak, J.T. Aerobic and resistance exercise training reverses age-dependent decline in NAD(+) salvage capacity in human skeletal muscle. Physiol. Rep. 2019, 7, e14139. [Google Scholar] [CrossRef]
- Mandel, S.; Spivak-Pohis, I.; Gozes, I. ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J. Mol. Neurosci. 2008, 35, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Oz, S.; Kapitansky, O.; Ivashco-Pachima, Y.; Malishkevich, A.; Giladi, E.; Skalka, N.; Rosin-Arbesfeld, R.; Mittelman, L.; Segev, O.; Hirsch, J.A. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol. Psychiatry 2014, 19, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Ivashko-Pachima, Y.; Sayas, C.L.; Malishkevich, A.; Gozes, I. ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: A novel avenue for protection against tauopathy. Mol. Psychiatry 2017, 22, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Ivashko-Pachima, Y.; Maor-Nof, M.; Gozes, I. NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies. PLoS ONE 2019, 14, e0213666. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.C.; Taylor, W.; Glucksberg, M.R.; Dean, D.A. Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther. 2006, 13, 725–731. [Google Scholar] [CrossRef]
- Esteves, A.R.; Gozes, I.; Cardoso, S.M. The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson’s disease. Biochim. Biophys. Acta 2014, 1842, 7–21. [Google Scholar] [CrossRef]
- Merenlender-Wagner, A.; Malishkevich, A.; Shemer, Z.; Udawela, M.; Gibbons, A.; Scarr, E.; Dean, B.; Levine, J.; Agam, G.; Gozes, I. Autophagy has a key role in the pathophysiology of schizophrenia. Mol. Psychiatry 2015, 20, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yiu, E.M.; Kornberg, A.J. Duchenne muscular dystrophy. J. Paediatr. Child Health 2015, 51, 759–764. [Google Scholar] [CrossRef]
- Belanto, J.J.; Mader, T.L.; Eckhoff, M.D.; Strandjord, D.M.; Banks, G.B.; Gardner, M.K.; Lowe, D.A.; Ervasti, J.M. Microtubule binding distinguishes dystrophin from utrophin. Proc. Natl. Acad. Sci. USA 2014, 111, 5723–5728. [Google Scholar] [CrossRef]
- Capitanio, D.; Moriggi, M.; Torretta, E.; Barbacini, P.; De Palma, S.; Vigano, A.; Lochmuller, H.; Muntoni, F.; Ferlini, A.; Mora, M.; et al. Comparative proteomic analyses of Duchenne muscular dystrophy and Becker muscular dystrophy muscles: Changes contributing to preserve muscle function in Becker muscular dystrophy patients. J. Cachexia Sarcopenia Muscle 2020, 11, 547–563. [Google Scholar] [CrossRef]
- Call, J.A.; Nichenko, A.S. Autophagy: An essential but limited cellular process for timely skeletal muscle recovery from injury. Autophagy 2020, 16, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.G.; Wahl, R.A. Duchenne and Becker muscular dystrophy in adolescents: Current perspectives. Adolesc. Health Med. Ther. 2018, 9, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Bongiorni, S.; Taddei, A.R.; Mezzetti, M.; Silvestri, F.; Coazzoli, M.; Zecchini, S.; Giovarelli, M.; Perrotta, C.; De Palma, C.; et al. Defects of full-length dystrophin trigger retinal neuron damage and synapse alterations by disrupting functional autophagy. Cell Mol. Life Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Savarese, M.; Sarparanta, J.; Vihola, A.; Udd, B.; Hackman, P. Increasing Role of Titin Mutations in Neuromuscular Disorders. J. Neuromuscul. Dis. 2016, 3, 293–308. [Google Scholar] [CrossRef]
- Polanco, M.J.; Parodi, S.; Piol, D.; Stack, C.; Chivet, M.; Contestabile, A.; Miranda, H.C.; Lievens, P.M.; Espinoza, S.; Jochum, T.; et al. Adenylyl cyclase activating polypeptide reduces phosphorylation and toxicity of the polyglutamine-expanded androgen receptor in spinobulbar muscular atrophy. Sci. Transl. Med. 2016, 8, 370ra181. [Google Scholar] [CrossRef]
- Jouroukhin, Y.; Ostritsky, R.; Assaf, Y.; Pelled, G.; Giladi, E.; Gozes, I. NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: Protection against impairments in axonal transport. Neurobiol. Dis. 2013, 56, 79–94. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Ni, J.; Pan, J.; Hua, H.; Wang, Y. Identification of suitable reference genes for gene expression studies in rat skeletal muscle following sciatic nerve crush injury. Mol. Med. Rep. 2019, 19, 4377–4387. [Google Scholar] [CrossRef]
- Caballero-Garrido, E.; Pena-Philippides, J.C.; Galochkina, Z.; Erhardt, E.; Roitbak, T. Characterization of long-term gait deficits in mouse dMCAO, using the CatWalk system. Behav. Brain Res. 2017, 331, 282–296. [Google Scholar] [CrossRef]
- Simoes, G.F.; Benitez, S.U.; Oliveira, A.L. Granulocyte colony-stimulating factor (G-CSF) positive effects on muscle fiber degeneration and gait recovery after nerve lesion in MDX mice. Brain Behav. 2014, 4, 738–753. [Google Scholar] [CrossRef]
- Hamers, F.P.; Koopmans, G.C.; Joosten, E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006, 23, 537–548. [Google Scholar] [CrossRef]
- Deumens, R.; Jaken, R.J.; Marcus, M.A.; Joosten, E.A. The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J. Neurosci. Methods 2007, 164, 120–130. [Google Scholar] [CrossRef]
- Dresner, E.; Agam, G.; Gozes, I. Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: Deregulation in schizophrenia. Eur. Neuropsychopharmacol. 2011, 21, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Malishkevich, A.; Marshall, G.A.; Schultz, A.P.; Sperling, R.A.; Aharon-Peretz, J.; Gozes, I. Blood-Borne Activity-Dependent Neuroprotective Protein (ADNP) is Correlated with Premorbid Intelligence, Clinical Stage, and Alzheimer’s Disease Biomarkers. J. Alzheimers Dis. 2016, 50, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.; Dresner, E.; Mandel, S.; Gozes, I. Silencing of the ADNP-family member, ADNP2, results in changes in cellular viability under oxidative stress. J. Neurochem. 2008, 105, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Dresner, E.; Malishkevich, A.; Arviv, C.; Leibman, B.S.; Alon, S.; Ofir, R.; Gothilf, Y.; Gozes, I. Novel evolutionary-conserved role for the activity-dependent neuroprotective protein (ADNP) family that is important for erythropoiesis. J. Biol. Chem. 2012, 287, 40173–40185. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhao, P.; Huang, Y.; Cai, X.; Bi, B.; Lin, J. [Genome-wide copy number microarray analysis for a boy with autism]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2019, 36, 157–160. [Google Scholar] [PubMed]
- Cai, T.; Wu, B.; Tang, X.; Zhou, Z.; Yang, J.; Ke, R.; Mu, X. iTRAQ-Based Proteomic Analysis reveals possible target-related proteins and signal networks in human osteoblasts overexpressing FGFR2. Proteome Sci. 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Girard, B.M.; Campbell, S.E.; Beca, K.I.; Perkins, M.; Hsiang, H.; May, V.; Vizzard, M.A. Intrabladder PAC1 Receptor Antagonist, PACAP(6–38), Reduces Urinary Bladder Frequency and Pelvic Sensitivity in Mice Exposed to Repeated Variate Stress (RVS). J. Mol. Neurosci. 2020. [Google Scholar] [CrossRef]
- Arnett, A.B.; Rhoads, C.L.; Hoekzema, K.; Turner, T.N.; Gerdts, J.; Wallace, A.S.; Bedrosian-Sermone, S.; Eichler, E.E.; Bernier, R.A. The autism spectrum phenotype in ADNP syndrome. Autism Res. 2018, 11, 1300–1310. [Google Scholar] [CrossRef]
- Delwig, A.; Majumdar, S.; Ahern, K.; LaVail, M.M.; Edwards, R.; Hnasko, T.S.; Copenhagen, D.R. Glutamatergic neurotransmission from melanopsin retinal ganglion cells is required for neonatal photoaversion but not adult pupillary light reflex. PLoS ONE 2013, 8, e83974. [Google Scholar] [CrossRef]
- Manivannan, S.N.; Darouich, S.; Masmoudi, A.; Gordon, D.; Zender, G.; Han, Z.; Fitzgerald-Butt, S.; White, P.; McBride, K.L.; Kharrat, M. Novel frameshift variant in MYL2 reveals molecular differences between dominant and recessive forms of hypertrophic cardiomyopathy. PLoS Genet. 2020, 16, e1008639. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.A.; Sobreira, N.; Pugh, E.; Zhang, P.; Steel, G.; Torres, F.R.; Cavalcanti, D.P. Homozygous deletion in MYL9 expands the molecular basis of megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur. J. Hum. Genet. 2018, 26, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, S.; Qian, L.; Cai, C.; Bi, H.; Cui, W. Identification of genes related to skeletal muscle growth and development by integrated analysis of transcriptome and proteome in myostatin-edited Meishan pigs. J. Proteom. 2020, 213, 103628. [Google Scholar] [CrossRef]
- Gamboa, H.E.; Sood, M. Pediatric Intestinal Pseudo-obstruction in the Era of Genetic Sequencing. Curr. Gastroenterol. Rep. 2019, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Tayyeb, M.; Tadi, P. Neurogenic Bladder; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560617/ (accessed on 1 September 2020).
- Verstegen, A.M.J.; Vanderhorst, V.; Gray, P.A.; Zeidel, M.L.; Geerling, J.C. Barrington’s nucleus: Neuroanatomic landscape of the mouse “pontine micturition center”. J. Comp. Neurol. 2017, 525, 2287–2309. [Google Scholar] [CrossRef]
- Larsson, M.; Abbott, B.W. Is the Capacity for Vocal Learning in Vertebrates Rooted in Fish Schooling Behavior? Evol. Biol. 2018, 45, 359–373. [Google Scholar] [CrossRef]
- Frohlich, H.; Rafiullah, R.; Schmitt, N.; Abele, S.; Rappold, G.A. Foxp1 expression is essential for sex-specific murine neonatal ultrasonic vocalization. Hum. Mol. Genet. 2017, 26, 1511–1521. [Google Scholar] [CrossRef]
- Malishkevich, A.; Amram, N.; Hacohen-Kleiman, G.; Magen, I.; Giladi, E.; Gozes, I. Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer’s pathologies. Transl. Psychiatry 2015, 5, e501. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584 e23. [Google Scholar] [CrossRef]
- Lee, S.W.; Won, J.Y.; Yang, J.; Lee, J.; Kim, S.Y.; Lee, E.J.; Kim, H.S. AKAP6 inhibition impairs myoblast differentiation and muscle regeneration: Positive loop between AKAP6 and myogenin. Sci. Rep. 2015, 5, 16523. [Google Scholar] [CrossRef]
- Pinhasov, A.; Mandel, S.; Torchinsky, A.; Giladi, E.; Pittel, Z.; Goldsweig, A.M.; Servoss, S.J.; Brenneman, D.E.; Gozes, I. Activity-dependent neuroprotective protein: A novel gene essential for brain formation. Brain Res. Dev. Brain Res. 2003, 144, 83–90. [Google Scholar] [CrossRef]
- Mollinedo, P.; Kapitansky, O.; Gonzalez-Lamuno, D.; Zaslavsky, A.; Real, P.; Gozes, I.; Gandarillas, A.; Fernandez-Luna, J.L. Cellular and animal models of skin alterations in the autism-related ADNP syndrome. Sci. Rep. 2019, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Dangoor, D.; Biondi, B.; Gobbo, M.; Vachutinski, Y.; Fridkin, M.; Gozes, I.; Rocchi, R. Novel glycosylated VIP analogs: Synthesis, biological activity, and metabolic stability. J. Pept. Sci. 2008, 14, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, R.N.; Giladi, E.; Pick, C.G.; Gozes, I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci. Lett. 2004, 361, 128–131. [Google Scholar] [CrossRef]
- Morimoto, B.H.; Fox, A.W.; Stewart, A.J.; Gold, M. Davunetide: A review of safety and efficacy data with a focus on neurodegenerative diseases. Expert Rev. Clin. Pharmacol. 2013, 6, 483–502. [Google Scholar] [CrossRef]
- Magen, I.; Ostritsky, R.; Richter, F.; Zhu, C.; Fleming, S.M.; Lemesre, V.; Stewart, A.J.; Morimoto, B.H.; Gozes, I.; Chesselet, M.F. Intranasal NAP (davunetide) decreases tau hyperphosphorylation and moderately improves behavioral deficits in mice overexpressing alpha-synuclein. Pharmacol. Res. Perspect. 2014, 2, e00065. [Google Scholar] [CrossRef]
- Sragovich, S.; Malishkevich, A.; Piontkewitz, Y.; Giladi, E.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.; Gozes, I. The autism/neuroprotection-linked ADNP/NAP regulate the excitatory glutamatergic synapse. Transl. Psychiatry 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, E.I.; Van der Kieft, J.G.; De Heer, R.C.; Spink, A.; Nguyen, H.P.; Homberg, J.R.; Van der Harst, J.E. Automated quantitative analysis to assess motor function in different rat models of impaired coordination and ataxia. J. Neurosci. Methods 2016, 268, 171–181. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, J.; Walczak, P.; Bulte, J.W.M. Quantification of motor neuron loss and muscular atrophy in ricin-induced focal nerve injury. J. Neurosci Methods 2018, 308, 142–150. [Google Scholar] [CrossRef]
- Hacohen, K.G.; Barnea, A.; Gozes, I. ADNP: A major autism mutated gene is differentially distributed (age and gender) in the songbird brain. Peptides 2015, 72, 75–79. [Google Scholar] [CrossRef]
- Idtdna. Available online: http://www.idtdna.com/SciTools/SciTools.aspx (accessed on 16 September 2016).
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapitansky, O.; Sragovich, S.; Jaljuli, I.; Hadar, A.; Giladi, E.; Gozes, I. Age and Sex-Dependent ADNP Regulation of Muscle Gene Expression Is Correlated with Motor Behavior: Possible Feedback Mechanism with PACAP. Int. J. Mol. Sci. 2020, 21, 6715. https://doi.org/10.3390/ijms21186715
Kapitansky O, Sragovich S, Jaljuli I, Hadar A, Giladi E, Gozes I. Age and Sex-Dependent ADNP Regulation of Muscle Gene Expression Is Correlated with Motor Behavior: Possible Feedback Mechanism with PACAP. International Journal of Molecular Sciences. 2020; 21(18):6715. https://doi.org/10.3390/ijms21186715
Chicago/Turabian StyleKapitansky, Oxana, Shlomo Sragovich, Iman Jaljuli, Adva Hadar, Eliezer Giladi, and Illana Gozes. 2020. "Age and Sex-Dependent ADNP Regulation of Muscle Gene Expression Is Correlated with Motor Behavior: Possible Feedback Mechanism with PACAP" International Journal of Molecular Sciences 21, no. 18: 6715. https://doi.org/10.3390/ijms21186715
APA StyleKapitansky, O., Sragovich, S., Jaljuli, I., Hadar, A., Giladi, E., & Gozes, I. (2020). Age and Sex-Dependent ADNP Regulation of Muscle Gene Expression Is Correlated with Motor Behavior: Possible Feedback Mechanism with PACAP. International Journal of Molecular Sciences, 21(18), 6715. https://doi.org/10.3390/ijms21186715





