Abstract
IKKγ/NEMO is the regulatory subunit of the IκB kinase (IKK) complex, which regulates the NF-κB signaling pathway. Within the IKK complex, IKKγ/NEMO is the non-catalytic subunit, whereas IKKα and IKKβ are the structurally related catalytic subunits. In this study, TmIKKγ was screened from the Tenebrio molitor RNA-Seq database and functionally characterized using RNAi screening for its role in regulating T. molitor antimicrobial peptide (AMP) genes after microbial challenges. The TmIKKγ transcript is 1521 bp that putatively encodes a polypeptide of 506 amino acid residues. TmIKKγ contains a NF-κB essential modulator (NEMO) and a leucine zipper domain of coiled coil region 2 (LZCC2). A phylogenetic analysis confirmed its homology to the red flour beetle, Tribolium castaneum IKKγ (TcIKKγ). The expression of TmIKKγ mRNA showed that it might function in diverse tissues of the insect, with a higher expression in the hemocytes and the fat body of the late-instar larvae. TmIKKγ mRNA expression was induced by Escherichia coli, Staphylococcus aureus, and Candida albicans challenges in the whole larvae and in tissues such as the hemocytes, gut and fat body. The knockdown of TmIKKγ mRNA significantly reduced the survival of the larvae after microbial challenges. Furthermore, we investigated the tissue-specific induction patterns of fourteen T. molitor AMP genes in TmIKKγ mRNA-silenced individuals after microbial challenges. In general, the mRNA expression of TmTenecin1, -2, and -4; TmDefensin1 and -2; TmColeoptericin1 and 2; and TmAttacin1a, 1b, and 2 were found to be downregulated in the hemocytes, gut, and fat body tissues in the TmIKKγ-silenced individuals after microbial challenges. Under similar conditions, TmRelish (NF-κB transcription factor) mRNA was also found to be downregulated. Thus, TmIKKγ is an important factor in the antimicrobial innate immune response of T. molitor.
1. Introduction
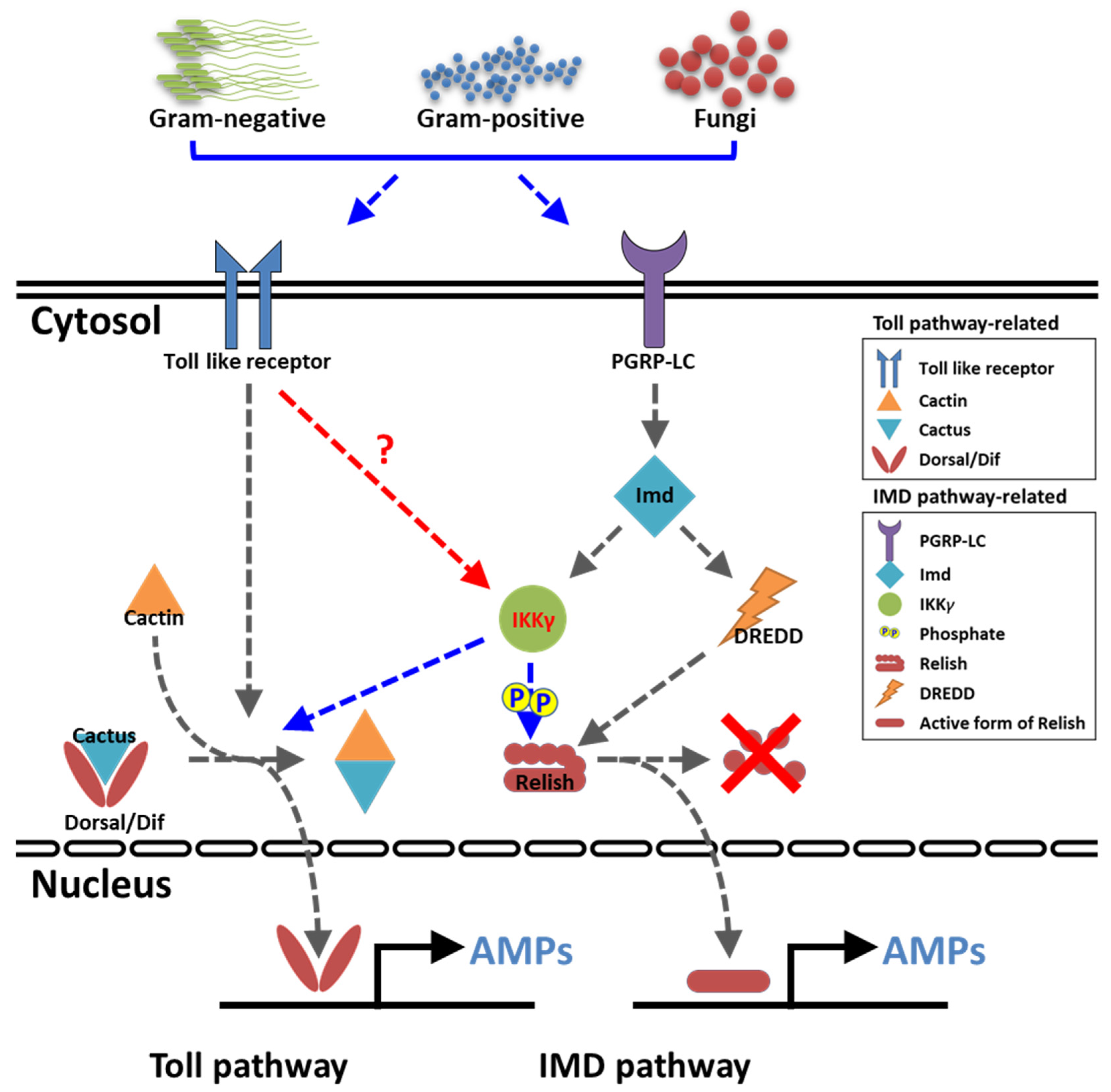
Unlike vertebrates, insects rely on their innate defense mechanisms for pathogen surveillance and immunity. The production of antimicrobial peptides (AMPs) is the most important mechanism of innate immunity in insects. AMPs are induced through the activation of two key signaling pathways, namely the toll and immune deficiency (IMD) pathways. In humans and most other vertebrates, the mode of action of the toll and IMD pathways are clearly identified. In humans and mice, ten (TLR1–10) and twelve (TLR1–9 and 11–13) functional toll-like receptors (TLRs) are known, respectively. TLRs provide protection against a wide variety of pathogens by regulating signaling through adaptor proteins, including the myeloid differentiation factor 88 (MyD88), TIR-domain-containing adapter-inducing interferon-β (TRIF), TRIF-related adaptor molecule (TRAM), and toll-interleukin 1 receptor domain containing adaptor protein (TIRAP). The antiviral [1,2,3], antibacterial [4,5], antifungal [6], and antiparasitic [7,8] roles of TLRs have been characterized in vertebrates. However, the IMD pathway has been partially characterized in anticancer [9] and anti-inflammatory properties [10,11,12] and implicated in neuronal development [13]. Further, the toll and IMD pathways do not appear to share any intermediate components and regulate differential expression of AMP-encoding genes via distinct NF-κB-like transcription factors [14,15].
In the context of the humoral immune response, especially in reference to AMP induction against invading pathogens, the toll and IMD pathways have been partially characterized in insects. In Drosophila, the requirement of toll and IMD pathway-related genes against septic injury have been monitored using an oligonucleotide microarray [16]. Further, while peptidoglycan recognition protein (PGRP)-SC1 and -SC2 are required for recognizing Gram-positive bacteria to initiate signaling through the toll pathway, PGRP-LB is required for recognizing Gram-negative bacteria and initiates the IMD pathway [17]. Furthermore, PGRP-SD (similar to mammalian CD14) acts as an extracellular receptor for Gram-negative bacteria and recognizes meso-diaminopimelic acid-type peptidoglycan [18] In addition, two miRNAs, miR-9a and miR-981, negatively regulate the IMD pathway, thereby downregulating the expression of the antibacterial peptide diptericin [19]. In Bombyx mori, the expression patterns of 11 putative toll-related receptors and two toll analogs were analyzed using microarrays, which showed that BmToll and BmToll-2 are related to embryogenesis; BmToll-2 and -4 may have a function in gut immunity; and BmToll-10, -11, and BmToLK-2 may be involved in sex-specific biological functions [20]. Further, while the toll pathway is triggered by the invasive fungal pathogen Beauveria bassiana, the IMD pathway is activated by the Gram-negative bacteria Escherichia coli and Serratia marcescens [21]. Most significantly, the extracellular serine protease cascade involved in the toll signaling pathway has been described in great detail, wherein the processing of active spätzle (toll ligand) from pro-spätzle is manifested by the spätzle-processing enzyme and negatively regulated by serpin-48 [22,23]. The downstream regulators of the toll signaling cascade, including the adaptor protein MyD88/Tube, the kinase Pelle, IκB-like protein Cactus, the NF-κB-like transcription factor dorsal-related immunity factor (Dif), and dorsal, have been known in Drosophila and Tribolium [24,25] but partially characterized in other insects. The information provides compelling evidence that the toll pathway is activated by soluble recognition molecules that trigger distinct proteolytic cascades converging on spätzle.
The inhibitor of nuclear factor kappa B kinase regulatory subunit gamma (IKKγ) encodes the regulatory subunit of the inhibitor of the kappa B kinase (IKK) complex that activates inflammation, immunity, cell survival, and other pathway genes. Together with IKKα and IKKβ, IKKγ is required for cytokine-mediated activation of the NF-κB pathway. IKKγ also transcriptionally represses the NF-κB pathway by competitively binding with the nuclear coactivator cAMP-responsive element binding protein [26]. Further, IKKγ responds to various stimuli by phosphorylation, sumoylation, and ubiquitination, which also positively or negatively regulate NF-κB pathway activation [27]. The knockdown of IKKγ in mouse embryonic fibroblasts leads to inactivation of the NF-κB pathway in response to tumor necrosis factor-α and lipopolysaccharide [28]. The Drosophila IKK complex (signaling activated by lipopolysaccharides and mediated by IMD) phosphorylates the downstream NF-κB factor, Relish, and transcriptionally initiates the expression of the AMP diptericin. Thus, the Drosophila IKKγ-dependent signaling complex is exclusively IMD-dependent and toll-independent [29].
The yellow mealworm beetle Tenebrio molitor has emerged as a convenient model for studying insect innate immunity in the last decade. Previously, we have described a three-step proteolytic cascade of the extracellular toll signaling pathway that is elicited against Gram-positive bacteria and fungi [22,30]. In the present study, we have discovered the downstream regulatory components of the toll signaling pathway and their function in AMP induction using RNA interference (RNAi) screens [31,32,33]. TmToll-7 RNAi showed the downregulation of five AMP genes in T. molitor when challenged with E. coli [34]. Moreover, the T. molitor IMD, in the IMD signaling pathway, was found to confer resistance against the Gram-negative bacteria E. coli by regulating the expression of nine AMP genes [35]. Furthermore, the silencing of TmRelish, the downstream NF-κB gene of the IMD pathway, showed the pivotal functional regulation in the immune response against Gram-negative and Gram-positive bacteria [36,37]. In T. molitor, regulatory components of the pathway downstream of IMD are yet to be elucidated. In this study, we have functionally characterized T. molitor IKKγ (TmIKKγ) using an RNAi screen by elucidating its role in regulating T. molitor AMP genes after microbial challenges. Moreover, we have studied the mRNA expression of TmRelish (NF-κB factor in the IMD pathway), TmDorsal isoform X1 (TmDorX1), and TmDorX2 (NF-κB factor in the toll pathway) in TmIKKγ knockdown individuals to understand the crosstalk with NF-κB signaling in the T. molitor toll and IMD pathways.
2. Results
2.1. Gene Organization and cDNA Structure of TmIKKγ
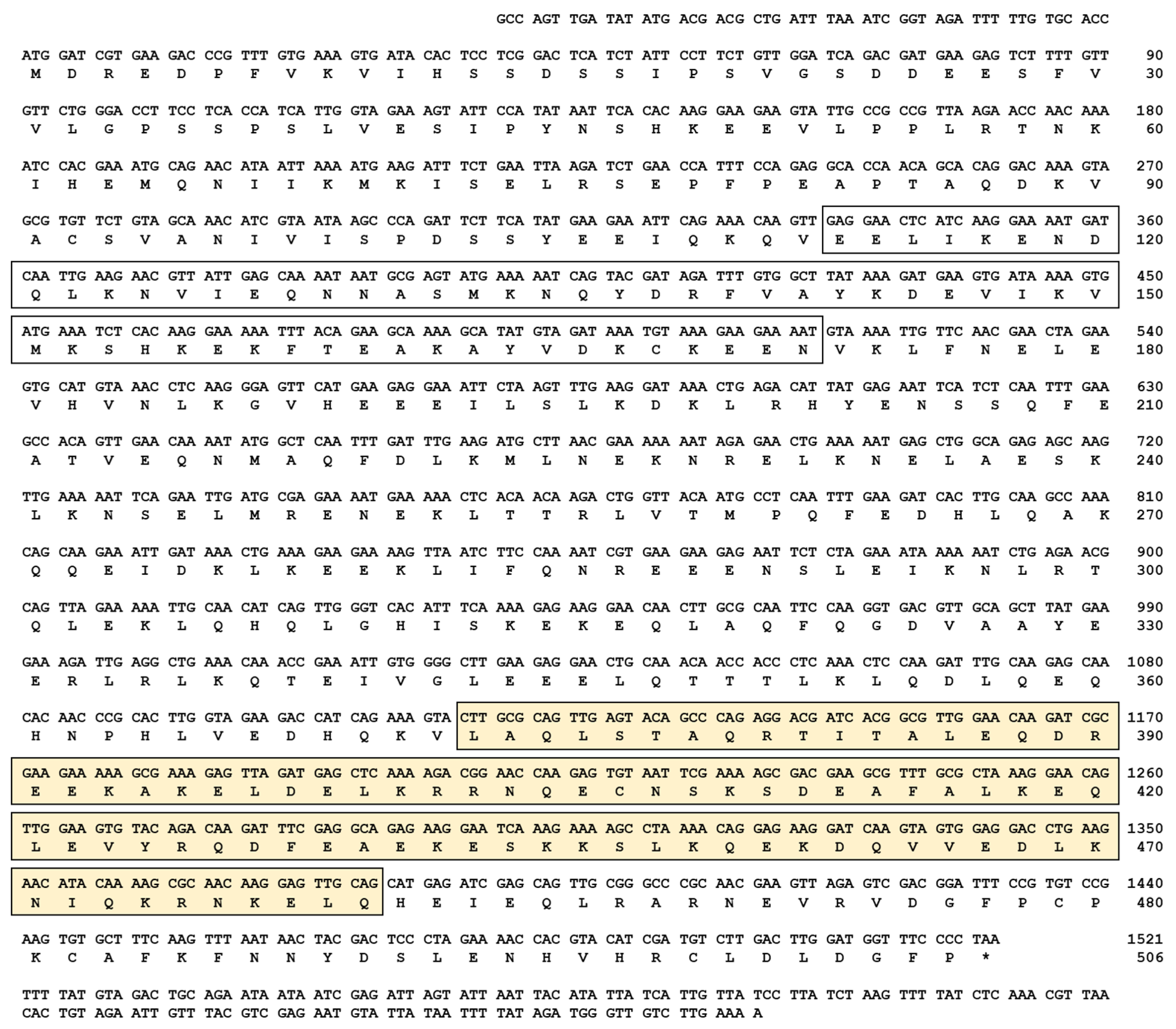
The cDNA sequence of TmIKKγ was determined by a local tblastn search against the T. molitor genome and the expressed sequence tag database using the T. castanuem IKKγ (TcIKKγ) protein sequence (EEZ99267.2) as a query. Homology mapping of TmIKKγ against the T. molitor DNA-Seq database (unpublished) revealed a single exon of 1521 bp, beginning with the translation start codon “ATG” and ending with the stop codon “TAA” (Figure S1A,B). The coding region of TmIKKγ translated to a polypeptide of 506 amino acid residues (Figure 1).
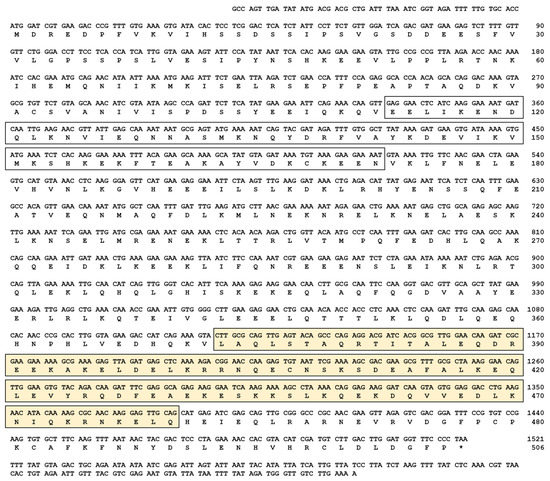
Figure 1.
The complete nucleotide and deduced amino acid sequence of Tenebrio molitor (Tm)IKKγ. The nucleotides are numbered from the first base of the initiation codon to the stop codon. This represents the open reading frame region. The asterisk indicates the stop codon. The characteristic NF-κB essential modulator (NEMO) domain is enclosed by an open box, and the leucine zipper domain of coiled coil region 2 (LZCC2) of NEMO is enclosed by a yellow box.
2.2. Domain Architecture and Phylogenetics of TmIKKγ
Domain analysis using InterProScan and a conserved domain search in the NCBI database revealed two conserved domains for TmIKKγ: the NF-κB essential modulator (NEMO) domain (between amino acids 106 and 172) and the leucine zipper domain of coiled coil region 2 (LZCC2) of NEMO (between amino acids 373 and 460) (Figure 1). A multiple sequence alignment of the TmIKKγ amino acid sequence with the IKKγ of insect orthologs showed conservation at the level of the NEMO domain and LZ of domain CC2 of NEMO (Figure S2A). To explore the evolutionary position of TmIKKγ proteins, we constructed a phylogenetic tree using the IKKγ homologs from different insect species. TmIKKγ was clustered with TcIKKγ (Figure S2B). The full-length amino acid sequences of TmIKKγ and TcIKKγ showed a high degree of conservation with amino acid sequences from the Asian longhorned beetle Anoplophora glabripennis NEMO isoform X2 (AgIKKγ) and the Colorado potato beetle Leptinotarsa decemlineata NEMO isoform X2 (LdIKKγ). The beetle cluster of IKKγ proteins was related with the lepidopteran IKKγ cluster, which included IKKγ amino acid sequences from Bombyx mori, Spodoptera litura, and Plutella xylostella. The IKKγ sequences from dipterans (including Drosophila melanogaster Kenny; DmKenny) and hymenopterans formed separate clusters. This represents a valid evolutionary lineage for TmIKKγ, clustering within the coleopteran IKKγ amino acid sequences.
2.3. Developmental and Tissue-Specific Expression Patterns of TmIKKγ mRNA
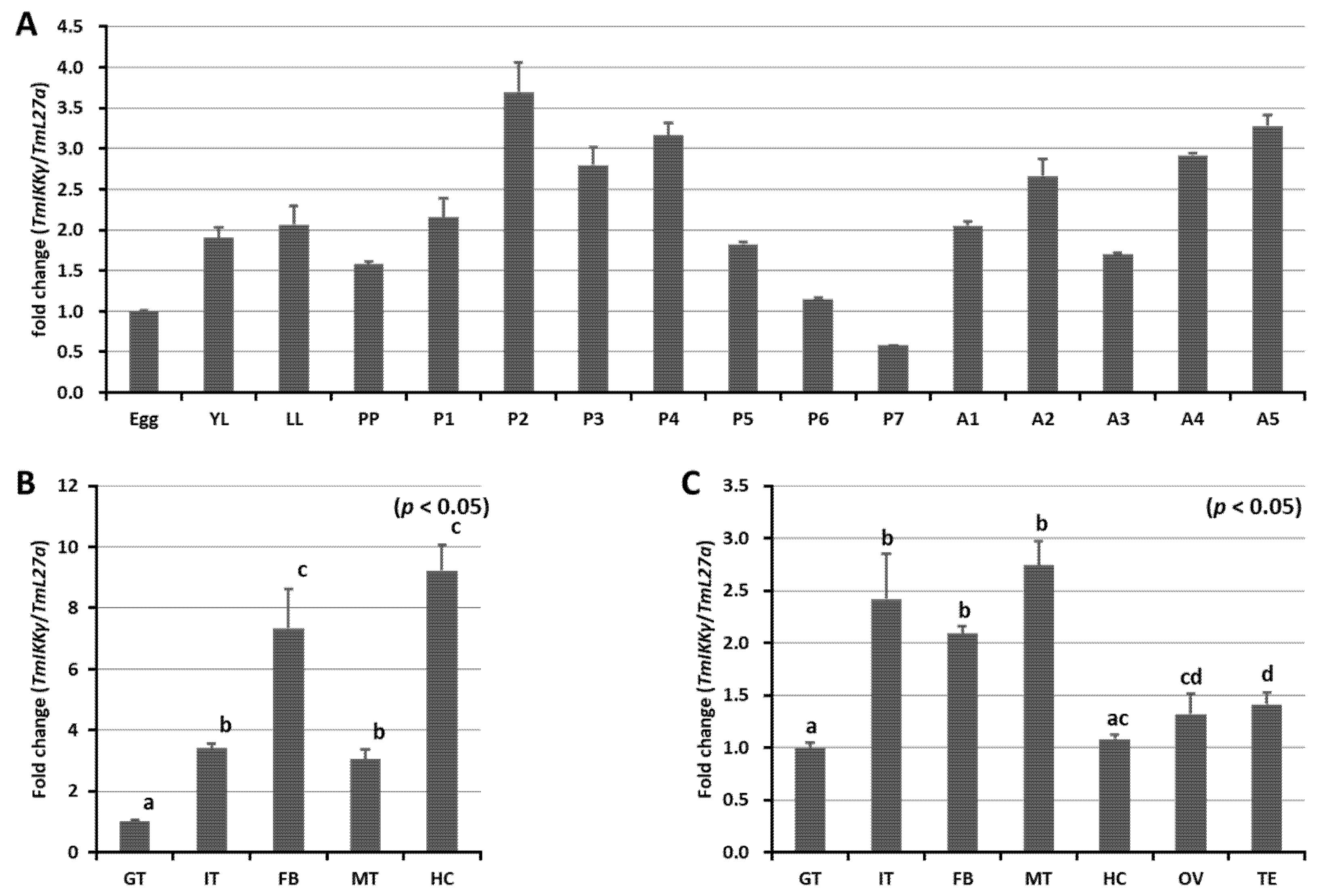
We measured the mRNA expression levels of TmIKKγ at five different developmental stages (egg, larva (early larva [YL] and late larva [LL]), prepupa (PP), pupa (days 1–7 [P1–P7]), and adult (days 1–5 [A1–A5])) using qRT-PCR (Figure 2A). Our results showed that TmIKKγ mRNA expression levels were consistent in all the developmental stages, with higher expressions in the pupal and adult stages. A slight decline in expression was noticed in the late-larval, prepupal, and late-pupal (days 5–7) stages. Further, we examined the TmIKKγ mRNA levels in the larval and adult tissues of T. molitor. The results indicated that TmIKKγ mRNA was expressed in all the larval tissues examined, with higher expressions in the hemocytes and fat body (Figure 2B). In T. molitor adults (five days old), the expression levels of TmIKKγ mRNA were greater in the Malpighian tubules, integument, and fat body (Figure 2C).
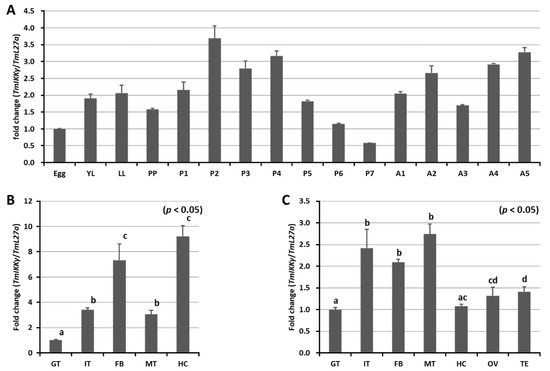
Figure 2.
Developmental and tissue-specific expression of TmIKKγ mRNA using qRT-PCR. (A) Relative expression of TmIKKγ mRNA in the egg (Egg), early larva (YL), late larva (LL), prepupa (PP), pupa days 1–7 (P1–P7), and adult days 1–5 (A1–A5). (B) TmIKKγ mRNA expression in last-instar larvae. (C) Distribution of TmIKKγ mRNA in adult tissues. IT, integument; GT, gut; FB, fat body; HC, hemocytes; MT, Malpighian tubules; OV, ovary, and TE, testis. L27a from T. molitor was included as an internal control to normalize RNA levels between samples. Vertical bars represent standard errors (n = 3). Different letters above bars represent significant differences between groups.
2.4. Temporal Expression of TmIKKγ mRNA after Microbial Infection
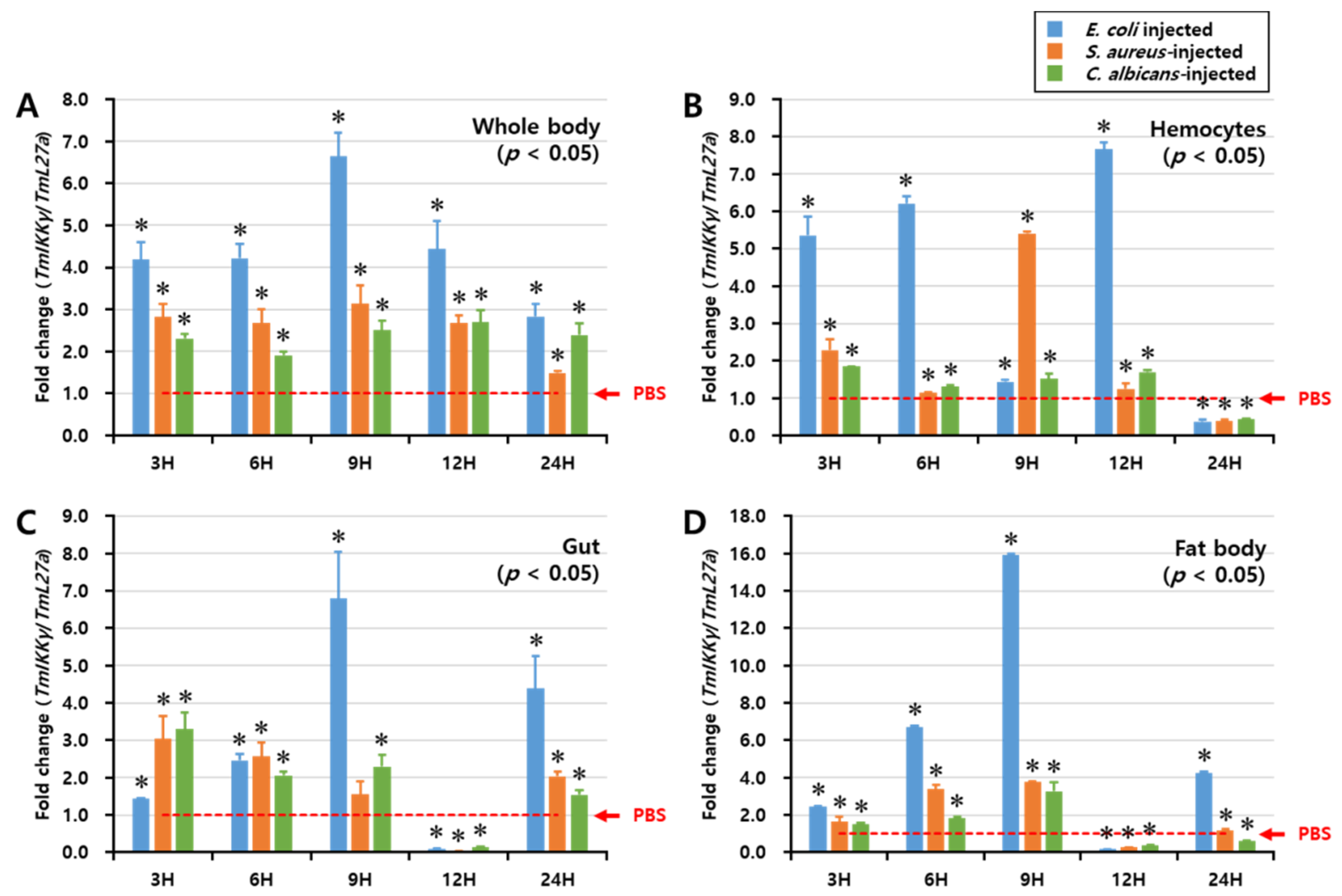
We investigated the regulation of TmIKKγ mRNA expression after fungal (C. albicans) and bacterial (E. coli and S. aureus) infections by performing a time-course study, conducted over a period of 48 h. TmIKKγ expression was analyzed by qRT-PCR in the whole body, hemocytes, gut, and fat body tissues of T. molitor at intervals of 3, 6, 9, 12, and 24 h post-microbial infection (Figure 3). In the whole body, E. coli was found to be the strongest elicitor of TmIKKγ mRNA at 9 h post-infection (Figure 3A). Furthermore, E. coli was the strongest elicitor of TmIKKγ mRNA in the hemocytes (Figure 3B), gut (Figure 3C), and fat body tissue (Figure 3D) of T. molitor larvae, in comparison to S. aureus and C. albicans. Maximum induction of the transcript was recorded at 9 h (16-fold) in the fat body tissue, following E. coli infection.
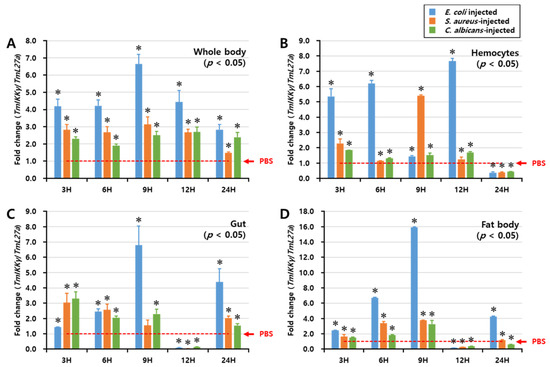
Figure 3.
Temporal expression patterns of TmIKKγ mRNA in the whole body (A), hemocytes (B), gut (C), and fat body (D) of T. molitor post-challenge with Escherichia coli, Staphylococcus aureus, or Candida albicans. The expression was analyzed by qRT-PCR using L27a (T. molitor) as the internal control. For each time point, the expression level in the phosphate-buffered saline (PBS)-injected control (mock control) was set to 1; this is represented by a dotted line. Values represent the mean of triplicate experiments, mean ± SE (n = 20). Asterisk denotes significant differences from the mock control at 95% confidence level (Student’s t-test).
2.5. TmIKKγ RNAi and Larval Mortality Assay
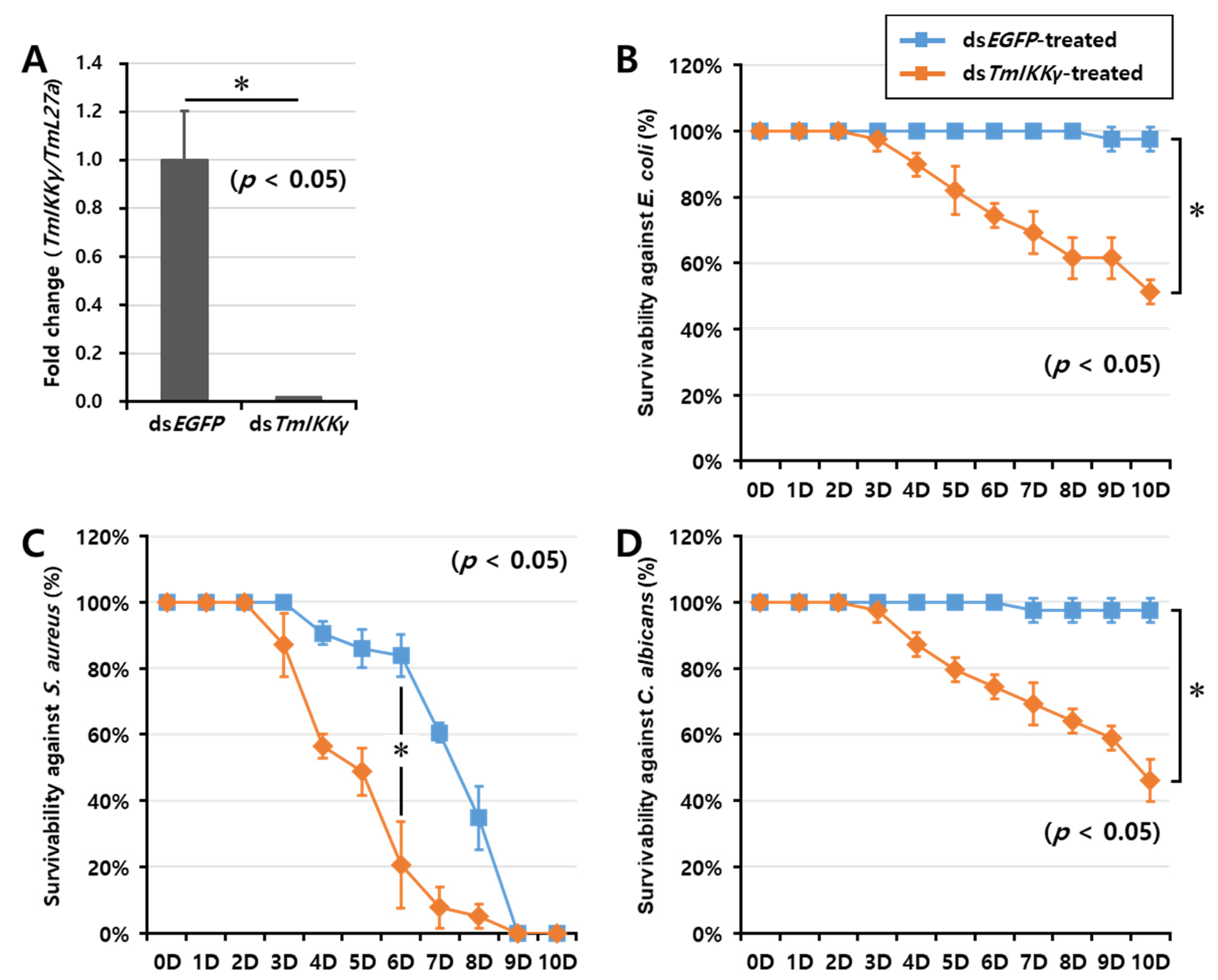
As TmIKKγ mRNA expression was induced after infection with E. coli, S. aureus, and C. albicans, we hypothesized that TmIKKγ could be a candidate for conferring resistance against bacterial and fungal infections. To test this hypothesis, we investigated the survival of T. molitor larvae after silencing TmIKKγ mRNA, followed by a microbial challenge (Figure 4). The RNAi efficiency of TmIKKγ mRNA was compared to that treated with the control double-stranded (ds)RNA (ds enhanced green fluorescent protein (EGFP)-treated group). We confirmed an efficient knockdown of TmIKKγ mRNA (about 99%) (Figure 4A). After confirming the knockdown of TmIKKγ mRNA, we challenged the dsTmIKKγ and dsEGFP-treated larvae with bacteria (E. coli and S. aureus) and fungi (C. albicans). The mortality of T. molitor larvae (dsTmIKKγ and dsEGFP-treated groups) in response to the microbial challenge was monitored for 10 days. We found that the dsTmIKKγ-treated larvae showed increased susceptibility to microbial infections. A clear trend was observed in E. coli-infected dsTmIKKγ-treated larvae, with 50% mortality compared with dsEGFP-treated larvae (Figure 4B). With the S. aureus infection, the mortality trend was unclear initially, but, at day 6 post-infection, there was a significant increase in mortality in dsTmIKKγ-treated larvae as compared with dsEGFP-treated larvae (Figure 4C). The mortality observed in TmIKKγ-silenced larvae with a S. aureus infection was considered significant, even though a high mortality rate in the dsEGFP control was perplexing and might be related to the overall health status of the larvae included in the group. Close to 50% mortality was observed in dsTmIKKγ-treated and C. albicans-infected larvae (Figure 4D). The results suggest the requirement of TmIKKγ mRNA against bacterial and fungal challenges in T. molitor larvae.
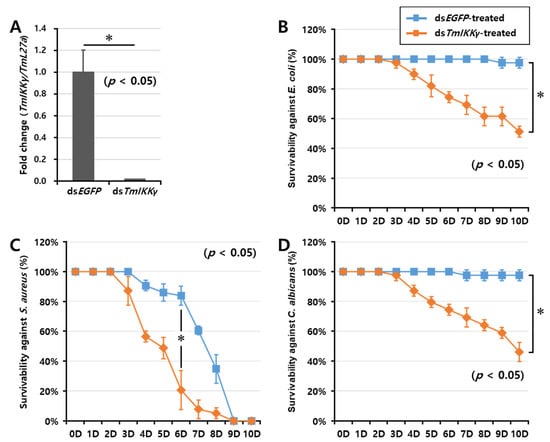
Figure 4.
RNA interference (RNAi)-based silencing assay for T. molitor larval survival analysis after E. coli, S. aureus, and C. albicans injections. (A) RNAi silencing efficiency of TmIKKγ was about 99%. Time-dependent survival of double-stranded (ds)TmIKKγ-injected T. molitor larvae after challenge with E. coli (B), S. aureus (C), and C. albicans (D). The survival was studied for 10 d after the microbial challenge, with dsEGFP)-treated larvae acting as negative controls. Results are shown as an average of three independent biological replicates with standard errors. Asterisks denote significant differences at 95% confidence level (Wilcoxon-Mann Whitney test).
2.6. Effects of TmIKKγ RNAi on the Expression of AMPs
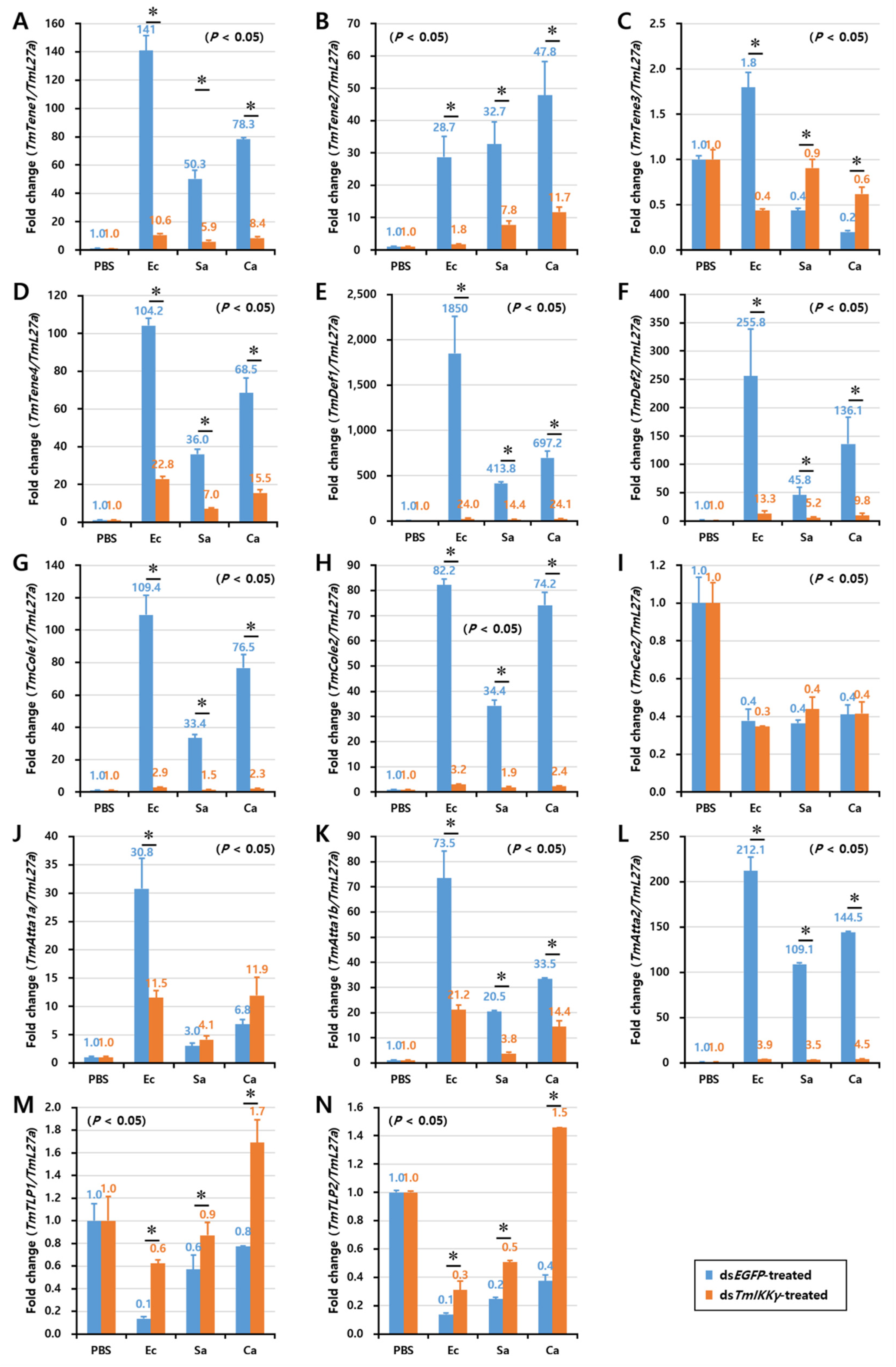
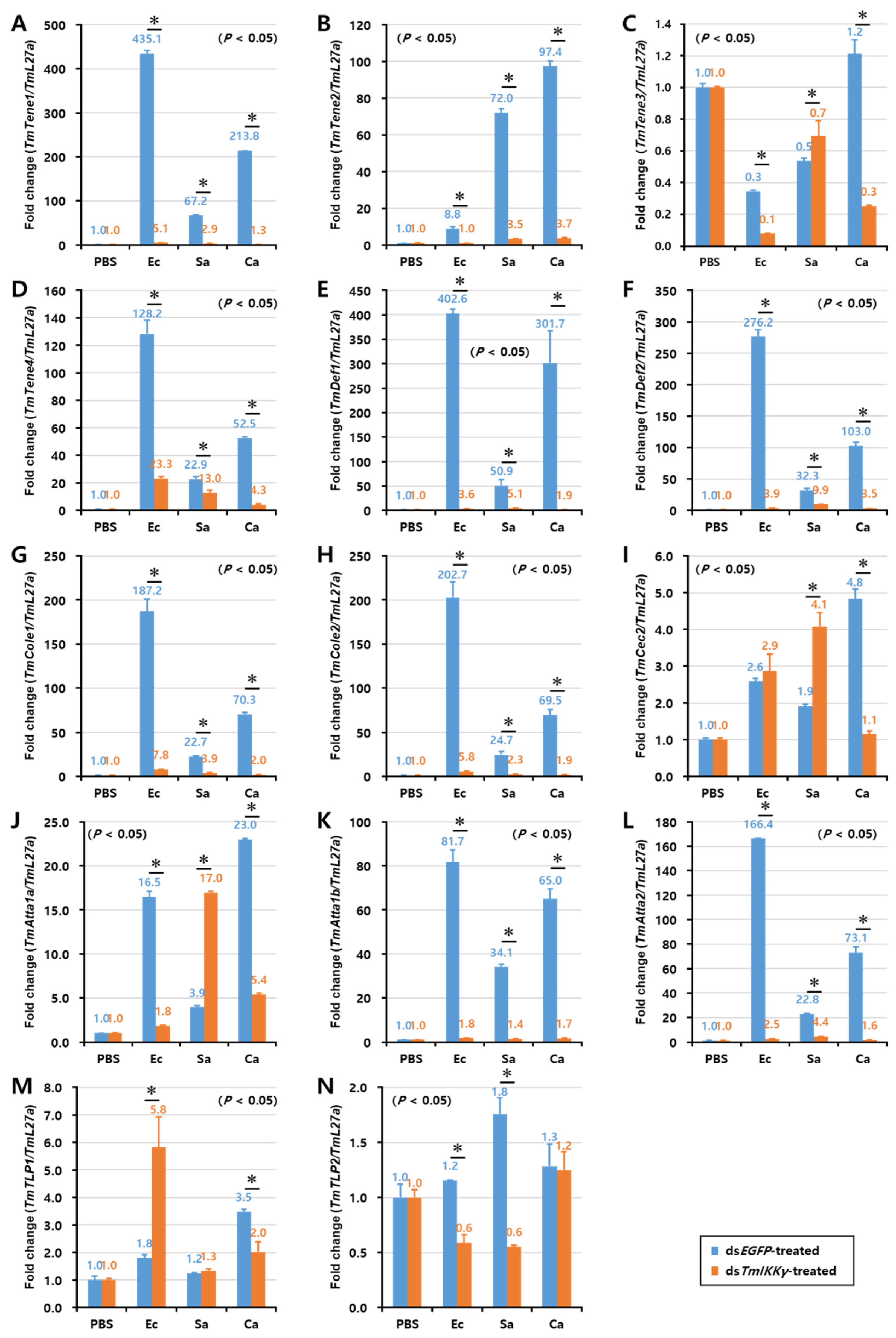
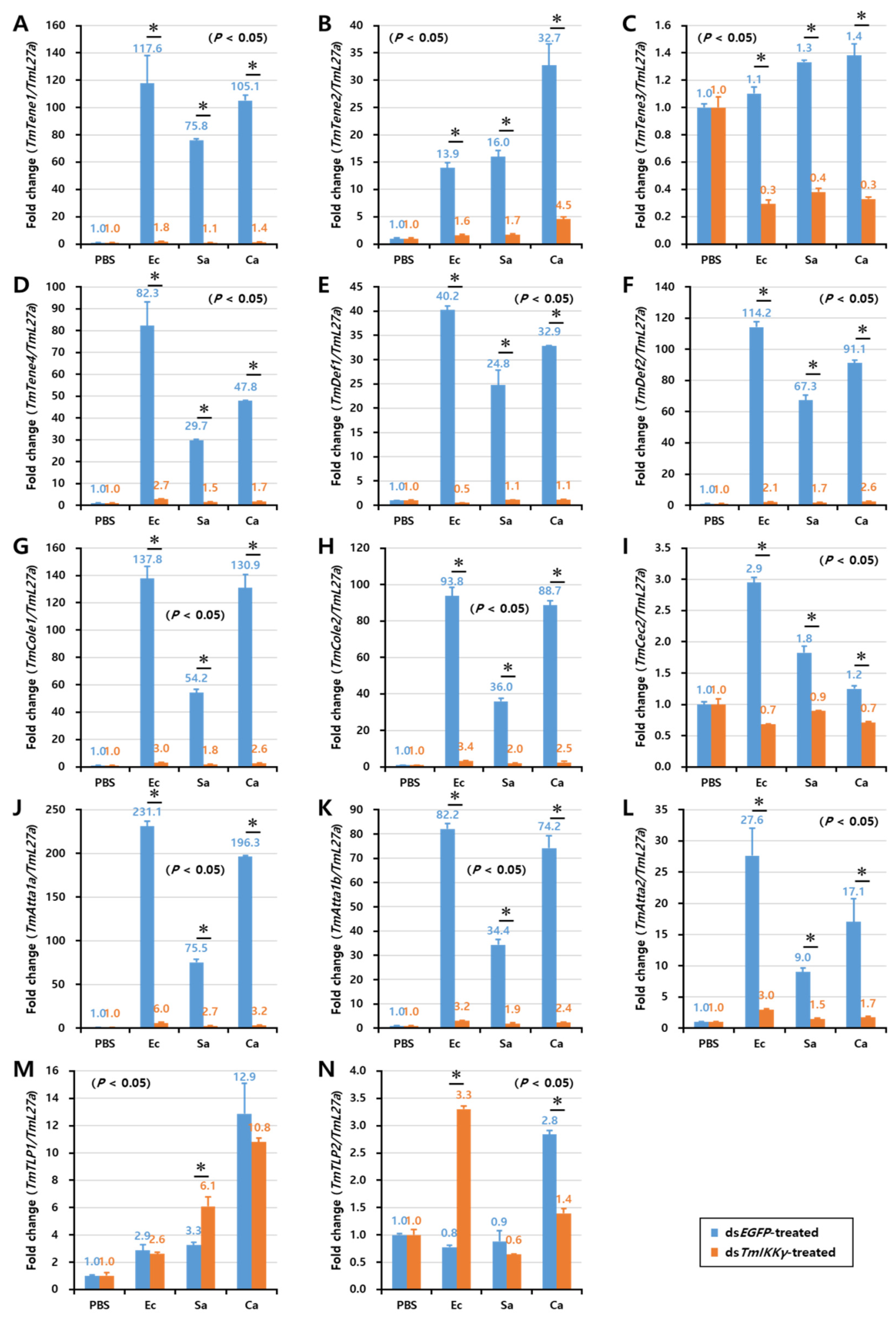
As TmIKKγ mRNA knockdown resulted in increased susceptibility of the larvae to bacterial and fungal infections, we wanted to understand if TmIKKγ is one of the important factors regulating AMP production. The transcriptional activation of 14 different AMP genes was studied in dsTmIKKγ-treated and microbial-challenged larvae compared to the control (dsEGFP-treated and microbial-challenged larvae). The results suggest that, of the 14 AMPs tested, the expression of ten AMP genes, including TmTene1, 2 and 4; TmDef1 and 2; TmCole1 and 2; and TmAtta1a, 1b, and 2, were significantly downregulated by TmIKKγ RNAi in hemocytes after being challenged with E. coli, S. aureus, and C. albicans (Figure 5A,B,D–H,J–L). This suggests a positive regulatory effect of TmIKKγ on the ten AMP genes. Similarly, in the gut tissue, the expression of ten AMP genes, including TmTene1, 2, and 4; TmDef1 and 2; TmCole1 and 2; and TmAtta1a, 1b, and 2, significantly decreased in dsTmIKKγ-treated larvae when compared to dsEGFP-treated larvae after the microbial challenge (Figure 6A,B,D–H,J–L). The same repertoire of AMP genes was also positively regulated in the fat body tissue of dsTmIKKγ-treated larvae after the challenge with E. coli, S. aureus, and C. albicans (Figure 7A,B,D–H,J–L). The transcriptional activation of TmTene3, TmCec2, TmTLP1, and TmTLP2 was not positively regulated by TmIKKγ RNAi.
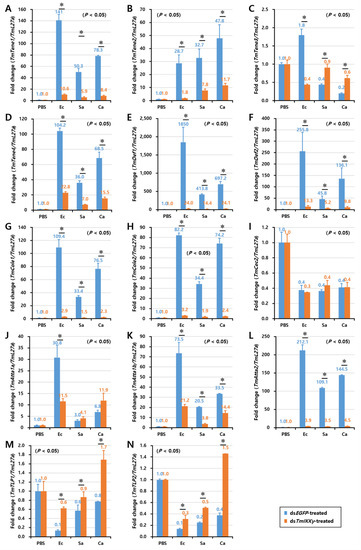
Figure 5.
Induction patterns of fourteen antimicrobial peptide (AMP) genes in TmIKKγ-silenced T. molitor larval hemocytes in response to E. coli, S. aureus, and C. albicans infections. This includes TmTene1 (A), TmTene2 (B), TmTene3 (C), TmTene4 (D), TmDef1 (E), TmDef2 (F), TmCole1 (G), TmCole2 (H), TmCec2 (I), TmAtta1a (J), TmAtta1b (K), TmAtta2 (L), TmTLP1 (M), and TmTLP2 (N). The experimental samples were divided into three groups (E. coli, S. aureus, or C. albicans-challenged groups) and a wounded control (PBS group). AMP expression was studied 24 h after microbial challenge. dsEGFP was used as a negative control, and TmL27a served as an internal control. The numbers above the bars represent the AMP transcription levels. All experiments were repeated three times, and similar results were obtained. Asterisks denote significance at 95% confidence levels.
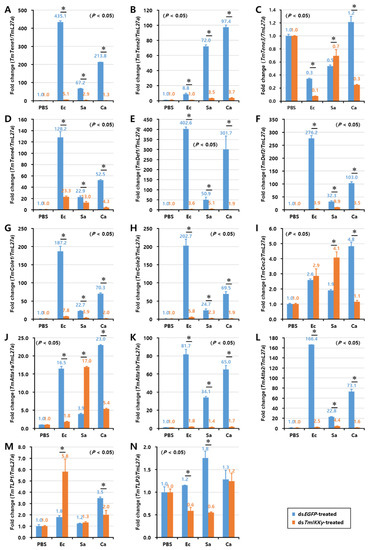
Figure 6.
Induction patterns of fourteen antimicrobial peptide (AMP) genes in the larval gut of TmIKKγ-silenced T. molitor, in response to E. coli, S. aureus, and C. albicans infections. This includes TmTene1 (A), TmTene2 (B), TmTene3 (C), TmTene4 (D), TmDef1 (E), TmDef2 (F), TmCole1 (G), TmCole2 (H), TmCec2 (I), TmAtta1a (J), TmAtta1b (K), TmAtta2 (L), TmTLP1 (M), and TmTLP2 (N). The experimental samples were divided into three groups (E. coli, S. aureus, or C. albicans-challenged groups) and a wounded control (PBS group). AMP expression was studied 24 h after microbial challenge. dsEGFP was used as a negative control, and TmL27a served as an internal control. The numbers above the bars represent the AMP transcription levels. All experiments were repeated three times, and similar results were obtained. Asterisks denote significance at 95% confidence levels.
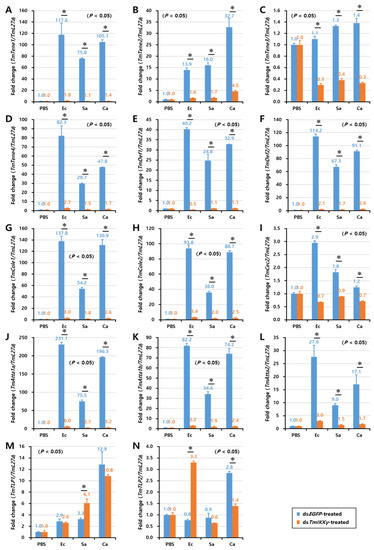
Figure 7.
Induction patterns of fourteen antimicrobial peptide (AMP) genes in the larval fat body of TmIKKγ-silenced T. molitor in response to E. coli, S. aureus, and C. albicans infections. This includes TmTene1 (A), TmTene2 (B), TmTene3 (C), TmTene4 (D), TmDef1 (E), TmDef2 (F), TmCole1 (G), TmCole2 (H), TmCec2 (I), TmAtta1a (J), TmAtta1b (K), TmAtta2 (L), TmTLP1 (M), and TmTLP2 (N). The experimental samples were divided into three groups (E. coli, S. aureus, or C. albicans-challenged groups) and a wounded control (PBS group). AMP expression was studied 24 h after the microbial challenge. dsEGFP was used as a negative control, and TmL27a served as an internal control. The numbers above the bars represent the AMP transcription levels. All experiments were repeated three times, and similar results were obtained. Asterisks denote significance at 95% confidence levels.
2.7. Effects of TmIKKγ RNAi on NF-κB Genes
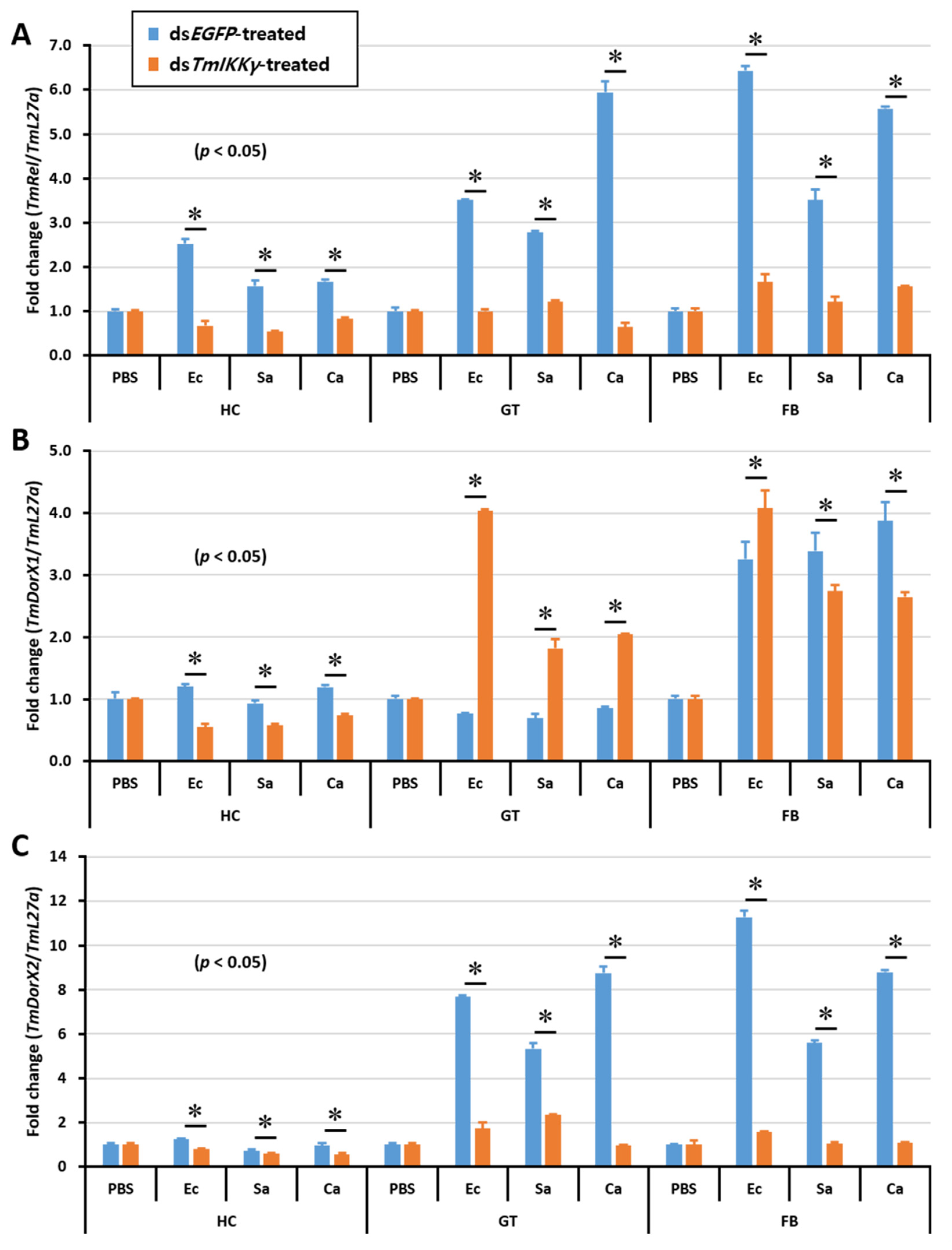
After discovering the regulatory effects of TmIKKγ RNAi on the activation of a specific set of Tenebrio AMP genes, we were interested in studying the expression of Tenebrio NF-κB genes in the toll (TmDorX1 and TmDorX2) and IMD (TmRelish) signaling pathways in dsTmIKKγ-treated and microbially challenged larvae. The results showed that the mRNA levels of TmRelish and TmDorX2 were dramatically decreased by TmIKKγ RNAi in the fat body tissue. The expression of TmRelish transcripts was significantly decreased by TmIKKγ RNAi in the hemocytes and gut. Further, TmDorX2 mRNA was decreased by TmIKKγ RNAi in the gut (Figure 8A–C).
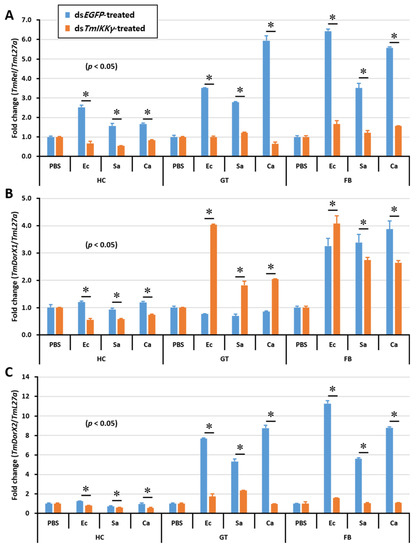
Figure 8.
Tissue-specific induction patterns of NF-κB genes in dsTmIKKγ-treated T. molitor larvae after injections of E. coli, S. aureus, and C. albicans. mRNA expression levels of TmRelish1 (A), TmDorX1 (B), and TmDorX2 (C). T. molitor larvae injected with PBS acted as the mock control group. dsEGFP was used as a negative control, and TmL27a served as an internal control. Asterisk denotes significant differences from the mock control at 95% confidence levels (Student’s t-test).
3. Discussion
The inhibitor of NF-κB (IκB) interacts with the Rel-homology domain of NF-κB and masks the nuclear localization signal of NF-κB. This prevents the translocation of NF-κB to the nucleus, retaining the inactive protein in the cytoplasm [38,39]. IκB kinases (IKKs) phosphorylate the IκB protein in response to various stimuli, leading to its ubiquitination and proteasomal degradation. This releases NF-κB factor to the nucleus, where it is needed for the transcriptional activation of AMPs [40]. IKKs are a part of the high molecular weight signalosome complex, where IKKα and IKKβ (also known as IKK1/CHUK and IKK2, respectively) are the two IκB kinases, and IKKγ (NEMO/NF-κB essential modulator/IKKAP-1/FIP-3) acts as the non-catalytic regulatory subunit [41]. In fact, IKKs serve as an adaptor between the receptor/associated protein complexes and the catalytic IKKα and IKKβ subunits [42].
In this study, we identified an IKKγ homolog in the yellow mealworm beetle T. molitor (TmIKKγ) and characterized it at the molecular level. The mammalian homologs of IKKγ have been known in D. melanogaster (DmKenny) and Crassostrea gigas (CgNEMO), among invertebrates [43,44]. The structural characteristics of TmIKKγ include the NEMO domain and the LZCC2 of NEMO. Similar to mammals, an IKK binding region, NEMO ubiquitin binding domain, and zinc finger domain have been reported in CgNEMO [38,45].
IKKγ/NEMO shows extensive phosphorylation regulating its function for the activation of the NF-κB signaling pathway. The phosphorylation of tyrosine 374 and serine 377 of IKKγ/NEMO suppresses NF-κB signaling in humans—mediated by tumor necrosis factor α and Kaposi’s sarcoma-associated herpesvirus (KSHV) FADD-like interleukin-1β converting enzyme (FLICE) inhibitory protein (KvFLIP), respectively [27].
Ubiquitination of IKKγ/NEMO at specific lysine residues is also found to be conserved, and site-directed mutagenesis studies have indicated their direct role in NF-κB activation [46]. DmKenny comprises the ubiquitin binding in the ABIN and NEMO domain (UBAN) and a LC3-interacting region (LIR) or Atg8-interacting protein (AIM) motif. The LIR motif is responsible for the interaction between Kenny and Atg8a and the selective degradation of Kenny by autophagy [47,48]. TmIKKγ also displayed the relaxed LIR motif (xLIR) from amino acid positions 27 to 32 (ESFVVL pattern) and from amino acid positions 103 to 108 (SSYEEI pattern). The iLIR server (http://ilir.warwick.ac.uk/) also predicted the conventional LIR motif (WxxL) in TmIKKγ from amino acid positions 5 to 10 (DPFVKV pattern), 174 to 179 (KLFNEL pattern), and 487 to 492 (NNYDSL pattern). This indicates that TmIKKγ could interact with the autophagosomal membrane protein microtubule-associated protein 1 light chain 3 (Atg8/LC3).
The alignment of TmIKKγ with IKKγ from orthologous species showed conservation at the domain level, indicating the presence of phosphorylation and ubiquitination sites, but the details need to be investigated in the future. Moreover, for the IKK complex, post-translational modifications at the level of catalytic subunits IKKα and IKKβ would also be crucial to understanding the positive or negative activation of the NF-κB signaling cascade in T. molitor. For the present study, we screened IKKα and IKKβ homologs from the T. molitor RNA-Seq database using T. castaneum sequences as the query. We were interested in understanding the involvement of TmIKKγ in the regulation of NF-κB genes, which are involved in the toll and IMD signaling pathways in Tenebrio, and the subsequent transcriptional control of AMP genes.
The induction of TmIKKγ mRNA expression in the whole body, hemocytes, gut, and fat body tissues of T. molitor after microbial challenges was an early indicator of the putative involvement of TmIKKγ in innate immunity. Further, RNAi experiments revealed that TmIKKγ is required for larval survival against the Gram-negative bacteria E. coli, the fungus C. albicans, and the Gram-positive bacteria S. aureus. This suggested that impairment in the larval survivability in the absence of TmIKKγ is possibly due to the susceptibility of the larvae to microbial infections. It is known that NF-κB activation downstream of IKKs is significant in mounting a humoral response against a plethora of biotic stimuli, including a diverse group of pathogens and parasites. In the Drosophila IMD pathway, TAK1 kinase complex phosphorylates the IKK complex that eventually phosphorylates Relish (NF-κB) to release the Rel homology domain to the nucleus and leads to the transcriptional activation of AMP genes [49,50].
In the present study, we tried to understand the transcriptional activation of fourteen Tenebrio AMP genes in the IKKγ-silenced T. molitor larval hemocytes, gut, and fat body tissues. In the hemocytes, gut, and fat body of TmIKKγ-silenced larvae the mRNA expression of ten, nine, and ten AMP genes were downregulated, respectively, when compared with dsEGFP injected larvae (negative control). AMP genes, such as TmTene1, TmTene2, TmTene4, TmDef1, TmDef2, TmCole1, TmCole2, TmAtta1a, TmAtta1b, and TmAtta2, were found to be downregulated in the hemocytes, gut, and fat body of TmIKKγ-silenced T. molitor larvae challenged with E. coli, S. aureus, and C. albicans. In a previous study, knockdown of the T. molitor IMD protein (central adaptor protein in the IMD pathway that receives signals through PGRP-LC and PGRP-LE), led to the downregulation of a similar set of AMPs (excepting TmDef1) after challenge with microbes. The suppression of AMP expression was more striking in the TmIMD-silenced larvae challenged with E. coli, suggesting the requirement of the transcript to mount a defense response against the pathogen [35]. Considering the IKK complex as a downstream protein complex in the IMD signaling pathway of T. molitor that transcribes AMP gene expression upon being microbially challenged, it was obvious to observe the regulation of the same set of AMPs in TmIMD and TmIKKγ knockdown models in separate studies.
Further, the increased susceptibility of T. molitor larvae to E. coli infection in a TmToll knockdown model was attributed to the downregulation of TmTene1, TmDef1, TmDef2, TmCole1, and TmAtta2 mRNA expression [34]. This highlights the diversity of the T. molitor innate immune system in recognizing the polymeric meso-diaminopimelic acid-type peptidoglycan of E. coli, signaling through the NF-κB protein sequences (Dif and Relish orthologs) for the transcriptional activation of a specific set of AMPs [51]. We therefore were interested to understand the NF-κB component that regulates the expression of the ten AMP genes in TmIKKγ knockdown T. molitor tissues. Interestingly, the expression of TmRelish was significantly decreased by TmIKKγ RNAi, and the expression of other NF-κB genes, including TmDorX1 and -X2, were slightly decreased, suggesting that these ten AMP genes were mainly regulated by the TmIKKγ-dependent IMD pathway. In contrast, mRNA levels for nine AMP genes and two NF-κB genes, including TmRelish and TmDorX2, were dramatically decreased by TmIKKγ RNAi in the gut, indicating that TmIKKγ activates both the TmRelish-dependent IMD pathway and TmDorX2-dependent toll pathway in the gut. Interestingly, the expression of TmDorX1 was drastically increased by TmIKKγ RNAi, suggesting that TmIKKγ positively and negatively regulates innate immune responses. In D. melanogaster, a synergistic immune response elicited by the toll and IMD pathways has been reported [14]. Another report stated that both the toll and IMD pathways could be activated by Gram-positive bacteria and fungi in D. melanogaster [52]. Further, AMPs have been clustered in three independent groups in T. castaneum: AMPs regulated by the toll pathway alone, by the IMD pathway alone, and by the toll and IMD pathways [15,53]. In the present study, we observed that TmIKKγ could activate both the TmRelish-dependent IMD pathway and TmDorX2-dependent toll pathway in T. molitor.
Given these observations, it is important to identify the α and β isoforms of IKK in T. molitor and to understand whether they work independently or in a complex to maintain the immune homeostasis. We also need to elucidate the putative role of TmIKKγ/NEMO in selective autophagic degradation of the IκB kinase. In the future, it will be interesting to visualize the crosstalk between the toll and IMD signaling pathways with respect to the immune response of T. molitor. Further, a categorization of Tenebrio AMPs regulated by the toll and IMD pathways independently and by the both toll and IMD pathways in response to various groups of microorganisms could be crucial towards understanding the humoral response.
4. Materials and Methods
4.1. Insect Rearing
T. molitor larvae were reared at 26 ± 1 °C and 60% ± 5% relative humidity in an environmental chamber established in the laboratory under dark conditions. The larvae were fed with an artificial diet (1.1 g of sorbic acid, 1.1 mL of propionic acid, 20 g of bean powder, 10 g of brewer’s yeast powder, and 200 g of wheat bran in 4400 mL of distilled water autoclaved at 121 °C for 15 min). Healthy 10th to 12th instar larvae were used for all experiments.
4.2. Preparation of Microorganisms
The Gram-negative bacterium E. coli (strain K12), Gram-positive bacterium Staphylococcus aureus (strain RN4220), and the fungus Candida albicans were used for the immune challenge experiments. While E. coli and S. aureus were cultured in Luria-Bertani broth (MB cell, Seoul, Korea) at 37 °C, C. albicans was cultured in Sabouraud dextrose broth (MB cell, Seoul, Korea) at 37 °C. The microorganisms were harvested, washed twice in 1X phosphate-buffered saline (PBS pH 7.0), centrifuged at 1146 × g for 10 min, and resuspended in 1X PBS. Thereafter, concentrations were measured at 600 nm (OD600) using a spectrophotometer (Eppendorf, Hamburg, Germany). Finally, 1 × 106 cells/μL of E. coli and S. aureus and 5 × 104 cells/μL of C. albicans were used for the immune challenge studies.
4.3. Identification and in Silico Analysis of TmIKKγ
TmIKKγ sequence was retrieved from the T. molitor RNA-Seq (unpublished) and expressed sequence tag database. Local tblastn analysis was conducted using Tribolium castanuem IKKγ amino acid sequence (EEZ99267.2) as a query. For the gene structure of TmIKKγ, a local tblastn analysis was performed with TmIKKγ as the query and the T. molitor DNA-Seq database (unpublished) as the subject. Full-length cDNA and deduced amino acid sequences of TmIKKγ were analyzed using the BLASTx and BLASTp algorithms at NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi), respectively. The full-length open reading frame (ORF) sequence was formatted using UltraEdit64-bit text-editor (http://www.ultraedit.com). The gene-finding software FGENESH was used for the prediction of the TmIKKγ open reading frame (http://www.softberry.com/berry.phtml?topic=fgenesh&group=programs&subgroup=gfind). The domain architecture of the protein sequences was retrieved using the InterProScan domain analysis program (https://www.ebi.ac.uk/interpro/search/sequence-search). Multiple sequence alignment, percentage identity, and percentage distance of the TmIKKγ amino acid sequence with representative IKKγ from other insects were analyzed by the ClustalX 2.1 program (www.clustal.org) [54]. Phylogenetic tree was constructed based on the amino acid sequence alignments using the neighbor-joining method (bootstrap trial set to 1000). The phylogram was analyzed using Tree Explorer view under the Molecular Evolutionary Genetics Analysis (MEGA) version 7.0 (https://www.megasoftware.net/) program [55].
4.4. Developmental, Tissue-Specific, and Immune Induction of TmIKKγ mRNA
For the characterization of TmIKKγ mRNA in various developmental stages (egg, young-instar larva, late-instar larva, prepupa, 1–7-day-old pupa, and 1–2-day-old adult); larval tissues (integument, gut, fat body, Malpighian tubules, and hemocytes); and adult tissues (integument, gut, fat body, Malpighian tubules, hemocytes, ovary, and testes) of the insect were collected. Total RNAs were isolated by Clear-S Total RNA extraction kit (Invirustech Co., Gwangju, Korea). Briefly, samples were collected in 1-mL guanidine thiocyanate RNA lysis buffer (20-mM EDTA, 20-mM MES buffer, 3-M guanidine thiocyanate, 200-mM sodium chloride, 40-μM phenol red, 0.05% Tween-80, 0.5% acetic acid glacial (pH 5.5), and 1% isoamyl alcohol in 50-mL D.W.) and homogenized using a bead-based homogenizer (Bertin Technologies, Bretonneux, France) for 20 s. Subsequently, the extract was centrifuged at 21,000× g for 5 min at 4 °C. The supernatant fraction was mixed with 1 volume of 99% ethanol and incubated at room temperature (RT) for 1 min. The samples were transferred into a silica spin column (Bioneer, Daejeon, Korea, KA-0133-1) and centrifuged at 21,000× g for 30 s at 4 °C to remove debris. After eliminating the genomic DNA contamination (treatment with DNase at 1 U/µL and incubation at 37 °C for 15 min at RT), the samples were washed with 3-M sodium acetate, followed by 80% ethanol. After centrifugation at 21,000× g for 2 min at 4 °C, DNase and RNase-free water was used to elute the total RNA. cDNA was synthesized using total RNA (2 µg) as the template, Oligo (dT)12–18 primers, and AccuPower® RT PreMix (Bioneer, Daejeon, Korea), according to the manufacturer’s instructions.
For the immune induction of TmIKKγ mRNA, healthy T. molitor larvae were intra-abdominally injected with 1-μL suspension containing either 1 × 106 cells of E. coli or S. aureus or 5 × 104 cells of C. albicans. A similar volume of PBS was administered into a separate group of larvae, which served as the wounded control. Samples (whole body, hemocytes, gut, and fat body tissue) were collected at 3, 6, 9, 12, and 24 h post-injection. Total RNA isolation and cDNA synthesis were done as described before.
The expression of TmIKKγ mRNA was evaluated by performing relative quantitative PCR using AccuPower® 2X GreenStarTM qPCR Master Mix (Bioneer, Daejeon, Korea), synthesized cDNAs, and specific primers for TmIKKγ (Table 1). The cycling conditions included an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 30 s. Relative quantitative PCR assays were performed on an AriaMx Real-Time PCR System (Agilent Technologies, Santa Clara, CA, USA), and the results were analyzed using AriaMx Real-Time PCR software (www.agilent.com). Each analysis was measured independently at least three times. The 2−ΔΔCt method [56] was employed to analyze the TmIKKγ mRNA expression levels. The mRNA expression levels were normalized to T. molitor ribosomal protein L27a (TmL27a), which was used as an internal control. The results represent the mean ± SE of three biological replicates.

Table 1.
Primers used in this study.
4.5. TmIKKγ RNAi
For RNAi-based functional characterization, dsRNA fragments of TmIKKγ were synthesized—TmIKKγ DNA fragments were amplified by PCR using gene-specific primers tailed with a T7 promoter sequence (Table 1). The primers were designed using the SnapDragon software (http://www.flyrnai.org/cgi-bin/RNAi_find_primers.pl) to prevent any cross-silencing effects. The first PCR reaction for TmIKKγ was carried out using AccuPower Pfu-PCR PreMix (Bioneer, Daejeon, Korea) with cDNA and specific primers for TmIKKγ (Table 1). The second PCR reaction was conducted with 100-fold dilution of the first PCR product; the final PCR was performed with primers tailed with the T7 promoter sequence and 100-fold dilution of the second PCR product. The PCR reaction was conducted under the following conditions: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 53 °C for 1 min, and extension at 72 °C for 30 s on a MyGenie96 Thermal Block (Bioneer, Daejeon, Korea). The PCR products were purified using the AccuPrep® PCR Purification Kit (Bioneer, Daejeon, Korea), which were then used to synthesize double-stranded RNA (dsRNA) using the EZTM T7 High Yield in vitro transcription kit (Enzynomics, Daejeon, Korea). Thereafter, 1 μg of PCR purified product was used as template DNA and mixed with 4 μL of 5× transcription buffer, 2 μL of 10× MgCl2, 2 μL of 100-mM DTT, 1 μL of RNase Inhibitor (40 U/μL), 1 μL of 100-mM rATP, 1 μL of 100-mM rGTP, 1 μL of 100-mM rCTP, 1 μL of 100-mM rUTP, and 1 μL of RNA polymerase. Subsequently, the reaction mixture was incubated at 42 °C for 3 h. The dsRNA product was purified using the PCI (phenol:chloroform:isoamyl alcohol) method, precipitated with 5-M ammonium acetate, and washed with 70% and 90% ethanol. The synthesized dsRNA was stored at −20 °C until further use.
For the knockdown of TmIKKγ mRNA, 1 μg of synthesized dsRNA of enhanced green fluorescent protein (EGFP) and TmIKKγ were injected into the T. molitor 10th–12th instar larvae. EGFP dsRNA synthesized from pEGFP-C1 plasmid DNA was used as a negative control for RNAi.
4.6. Mortality Assay
To measure mortality, 1 × 106 cells/μL of E. coli and S. aureus and 5 × 104 cells/μL of C. albicans were injected into T. molitor larvae, which TmIKKγ had silenced. Dead larvae were counted daily up to 10 d post-infection; ten insect larvae were used for each set of the mortality assay, and the experiments were triplicated.
4.7. Effects of TmIKKγ RNAi on the Expression of AMP and NF-κB Genes
To further characterize the function of TmIKKγ in the humoral innate immune response, the effect of TmIKKγ silencing by RNAi on the expression levels of fourteen AMP genes in the presence of the microbial challenge were investigated. After treating the 10th to 12th instar T. molitor larvae with TmIKKγ dsRNA, E. coli (1 × 106 cells per larva), S. aureus (1 × 106 cells per larva), or C. albicans (5 × 104 cells per larva) were injected into the larvae, and the samples (from the hemocytes, gut, and fat body) were collected 24 h post-injection. As an injection control, 1× PBS was used. Expression levels of fourteen AMP genes, including TmTenecin 1, 2, 3, and 4 (TmTene1, 2, 3, and 4); TmDefensin 1 and 2 (TmDef1 and 2); TmColeoptericin 1 and 2 (TmCole1 and 2); TmAttacin 1a, 1b, and 2 (TmAtta1a, 1b, and 2); TmCecropin 2 (TmCec2); and TmThaumatin-like protein 1 and 2 (TmTLP1 and 2) were measured by qRT-PCR with AMP gene-specific primers (Table 1). In addition, the expression of Tenebrio NF-κB genes in the toll (TmDorX1 and TmDorX2) and IMD (TmRelish) signaling pathways were examined by qRT-PCR with the NF-κB-specific primers listed in Table 1. Relative quantitative PCR was performed, as mentioned above, with AMP-specific primers.
4.8. Statistical Analysis
All experiments were carried out in triplicate, and the data were subjected to one-way analysis of variance. Tukey’s multiple range test was used to evaluate the differences between groups (p < 0.05).
5. Conclusions
The study identified, for the first time, the IKKγ isoform in the beetle T. molitor. TmIKKγ transcript expression was induced in the whole larva and other tissues of T. molitor after E. coli, S. aureus, and C. albicans challenges. The RNAi experiments suggested that TmIKKγ might be required for larval survival against microbial challenges by regulating AMPs through the innate immune signaling cascade mechanisms. The NF-κB factors in the IMD pathway (Relish) and the toll pathway (Dorsal isoform 2) were involved, as their transcripts were downregulated in TmIKKγ-silenced larval tissues after the microbial challenge (Figure 9). This study advances our understanding of the beetles’ immunity in response to pathogenic challenges.
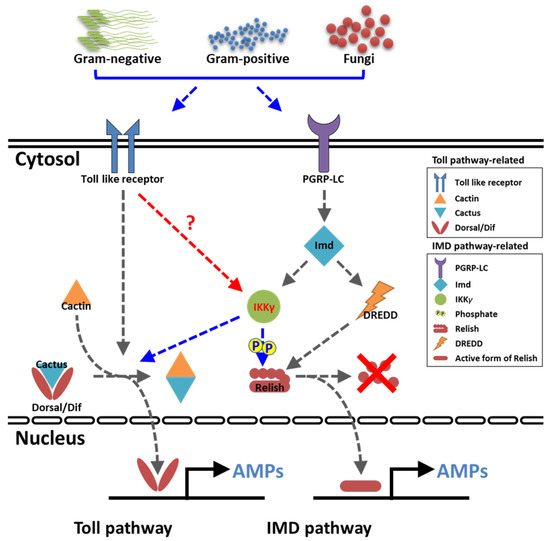
Figure 9.
Graphical summary of TmIKKγ on the AMP production in T. molitor. Our results suggested that TmIKKγ regulates the AMP gene expression in response to three pathogens, including E. coli, S. aureus, and C. albicans, through both NF-κB transcription factors, TmRelish and TmDorX2.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/18/6734/s1.
Author Contributions
Conceptualization, Y.S.H. and Y.H.J.; methodology, H.J.K. and Y.H.J.; software, Y.S.H.; validation, Y.S.H.; formal analysis, H.J.K., K.B.P., and C.E.K.; investigation, H.J.K., K.B.P., C.E.K., and M.K.; resources, H.J.K. and Y.H.J.; data curation, Y.H.J., B.B.P., and H.J.K.; writing—original draft preparation, Y.S.H., J.H.K., B.B.P., and Y.H.J.; writing—review and editing, Y.S.L. and H.A.J.; visualization, Y.H.J., B.B.P., and H.J.K.; supervision, Y.S.H. and Y.H.J.; project administration, Y.H.J.; and funding acquisition, Y.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (Grant No. 2018R1A2A2A05023367).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zambon, R.A.; Nandakumar, M.; Vakharia, V.N.; Wu, L.P. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 7257–7262. [Google Scholar] [CrossRef]
- Vercammen, E.; Staal, J.; Beyaert, R. Sensing of Viral Infection and Activation of Innate Immunity by Toll-Like Receptor 3. Clin. Microbiol. Rev. 2008, 21, 13–25. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Hatch, S.; Marshall, W.L. Viral Infection: An Evolving Insight into the Signal Transduction Pathways Responsible for the Innate Immune Response. Adv. Virol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Matsuo, A.; Matsumoto, M.; Seya, T. Pan-Vertebrate Toll-Like Receptors During Evolution. Curr. Genom. 2008, 9, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Roy, D.; Patnaik, E.; Nongthomba, U. Muscles provide protection during microbial infection by activating innate immune response pathways in Drosophila and zebrafish. Dis. Model. Mech. 2016, 9, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Loures, F.V.; Pina, A.; Felonato, M.; Araújo, E.F.; Leite, K.M.; Calich, V.L.G. Toll-Like Receptor 4 Signaling Leads to Severe Fungal Infection Associated with Enhanced Proinflammatory Immunity and Impaired Expansion of Regulatory T Cells. Infect. Immun. 2009, 78, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, P.G.; Nutman, T.B.; Semnani, R.T. Activation and regulation of Toll-Like Receptors (TLRs) by helminth parasites. Immunol. Res. 2008, 43, 252–263. [Google Scholar] [CrossRef]
- Fouzder, C.; Mukhuty, A.; Das, S.; Chattopadhyay, D. TLR Signaling on Protozoan and Helminthic Parasite Infection. In Toll-Like Receptors; IntechOpen: London, UK, 2020. [Google Scholar]
- Leopizzi, M.; Cocchiola, R.; Milanetti, E.; Raimondo, D.; Politi, L.; Giordano, C.; Scandurra, R.; d’Abusco, A.S. IKKα inibition by a glucosamine derivative enhances maspin expression in osteosarcoma cell line. Chem. Biol. Interact. 2017, 262, 19–28. [Google Scholar] [CrossRef]
- Cao, C.C.; Li, L.P.; Chen, W.; Zhu, Y.F.; Qi, Y.C.; Wang, X.D.; Wan, X.; Chen, X. Deficiency of IKKε inhibits inflammation and induces cardiac protection in high-fat diet-induced obesity in mice. Int. J. Mol. Med. 2014, 34, 244–252. [Google Scholar] [CrossRef]
- De Falco, F.; Di Giovanni, C.; Cerchia, C.; De Stefano, D.; Capuozzo, A.; Irace, C.; Iuvone, T.; Santamaria, R.; Carnuccio, R.; Lavecchia, A. Novel non-peptide small molecules preventing IKKβ/NEMO association inhibit NF-κB activation in LPS-stimulated J774 macrophages. Biochem. Pharmacol. 2016, 104, 83–94. [Google Scholar] [CrossRef]
- Jiang, Y.; Tian, M.; Lin, W.; Wang, X.; Wang, X. Protein Kinase Serine/Threonine Kinase 24 Positively Regulates Interleukin 17-Induced Inflammation by Promoting IKK Complex Activation. Front. Immunol. 2018, 9, 921. [Google Scholar] [CrossRef]
- Mettang, M.; Reichel, S.N.; Lattke, M.; Palmer, A.; Abaei, A.; Rasche, V.; Huber-Lang, M.; Baumann, B.; Wirth, T. IKK2/NF-κB signaling protects neurons after traumatic brain injury. FASEB J. 2018, 32, 1916–1932. [Google Scholar] [CrossRef] [PubMed]
- Tanji, T.; Hu, X.; Weber, A.N.R.; Ip, Y.T. Toll and IMD Pathways Synergistically Activate an Innate Immune Response in Drosophila melanogaster. Mol. Cell. Boil. 2007, 27, 4578–4588. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Koyama, H.; Ito, W.; Minakuchi, C.; Tanaka, T.; Miura, K. Involvement of NF-κB transcription factors in antimicrobial peptide gene induction in the red flour beetle, Tribolium castaneum. Dev. Comp. Immunol. 2012, 38, 342–351. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Spellman, P.T.; Tzou, P.; Rubin, G.M.; Lemaitre, B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002, 21, 2568–2579. [Google Scholar] [CrossRef]
- Costechareyre, D.; Capo, F.; Fabre, A.; Chaduli, D.; Kellenberger, C.; Roussel, A.; Charroux, B.; Royet, J. Tissue-Specific Regulation of Drosophila NF-x03BA;B Pathway Activation by Peptidoglycan Recognition Protein SC. J. Innate Immun. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Iatsenko, I.; Kondo, S.; Mengin-Lecreulx, D.; Lemaitre, B. PGRP-SD, an Extracellular Pattern-Recognition Receptor, Enhances Peptidoglycan-Mediated Activation of the Drosophila Imd Pathway. Immunity 2016, 45, 1013–1023. [Google Scholar] [CrossRef]
- Li, S.; Shen, L.; Sun, L.; Xu, J.; Jin, P.; Chen, L.; Ma, F. Small RNA-Seq analysis reveals microRNA-regulation of the Imd pathway during Escherichia coli infection in Drosophila. Dev. Comp. Immunol. 2017, 70, 80–87. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.L.; Liu, C.; Xu, P.Z.; Gao, Z.H.; Xia, Q.; Xiang, Z.H. Identification and analysis of Toll-related genes in the domesticated silkworm, Bombyx mori. Dev. Comp. Immunol. 2008, 32, 464–475. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Lu, Y.; Gong, Y.; Zhu, M.; Chen, F.; Liang, Z.; Zhu, L.; Kuang, S.; Hu, X.; et al. Immune signaling pathways activated in response to different pathogenic micro-organisms in Bombyx mori. Mol. Immunol. 2015, 65, 391–397. [Google Scholar] [CrossRef]
- Roh, K.B.; Kim, C.H.; Lee, H.; Kwon, H.M.; Park, J.W.; Ryu, J.H.; Kurokawa, K.; Ha, N.C.; Lee, W.J.; Lemaitre, B.; et al. Proteolytic Cascade for the Activation of the Insect Toll Pathway Induced by the Fungal Cell Wall Component. J. Boil. Chem. 2009, 284, 19474–19481. [Google Scholar] [CrossRef] [PubMed]
- Dudzic, J.P.; Hanson, M.A.; Iatsenko, I.; Kondo, S.; Lemaitre, B. More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila. Cell Rep. 2019, 27, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Ha, E.M.; Lee, W.J. Innate immunity and gut–microbe mutualism in Drosophila. Dev. Comp. Immunol. 2010, 34, 369–376. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.H.; Rämet, M. The Drosophila Toll Signaling Pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Verma, U.N.; Yamamoto, Y.; Prajapati, S.; Gaynor, R.B. Nuclear role of IκB Kinase-γ/NF-κB Essential Modulator (IKKγ/NEMO) in NF-κB-dependent gene expression. J. Biol. Chem. 2004, 279, 3509–3515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Toth, Z.; Wong, L.Y.; Brulois, K.; Nguyen, J.; Lee, J.Y.; Zandi, E.; Jung, J.U. Novel phosphorylations of IKKγ/NEMO. MBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, D.; Yeh, W.C.; Wakeham, A.; Rudolph, B.; Nallainathan, D.; Potter, J.; Elia, A.J.; Mak, T.W. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ -deficient mice. Genes Dev. 2000, 14, 854–862. [Google Scholar] [PubMed]
- Rutschmann, S.; Jung, A.C.; Zhou, R.; Silverman, N.; Hoffmann, J.A.; Ferrandon, D. Role of Drosophila IKKγ in a Toll-independent antibacterial immune response. Nat. Immunol. 2000, 1, 342–347. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, S.J.; Kan, H.; Kwon, H.M.; Roh, K.B.; Jiang, R.; Yang, Y.; Park, J.W.; Lee, H.H.; Ha, N.C.; et al. A Three-step Proteolytic Cascade Mediates the Activation of the Peptidoglycan-induced Toll Pathway in an Insect. J. Boil. Chem. 2008, 283, 7599–7607. [Google Scholar] [CrossRef]
- Patnaik, B.B.; Patnaik, H.H.; Seo, G.W.; Jo, Y.H.; Lee, Y.S.; Lee, B.L.; Han, Y.S. Gene structure, cDNA characterization and RNAi-based functional analysis of a myeloid differentiation factor 88 homolog in Tenebrio molitor larvae exposed to Staphylococcus aureus infection. Dev. Comp. Immunol. 2014, 46, 208–221. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, Y.J.; Park, K.B.; Seong, J.H.; Kim, S.G.; Park, S.; Noh, M.Y.; Lee, Y.S.; Han, Y.S. TmCactin plays an important role in Gram-negative and -positive bacterial infection by regulating expression of 7 AMP genes in Tenebrio molitor. Sci. Rep. 2017, 7, 46459. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Jo, Y.H.; Park, K.B.; Ko, H.J.; Edosa, T.T.; Lee, Y.S.; Han, Y. TmDorX2 positively regulates antimicrobial peptides in Tenebrio molitor gut, fat body, and hemocytes in response to bacterial and fungal infection. Sci. Rep. 2019, 9, 16878. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jo, Y.H.; Park, K.B.; Ko, H.J.; Kim, C.E.; Bae, Y.M.; Kim, B.; Jun, S.A.; Bang, I.S.; Lee, Y.S.; et al. TmToll-7 Plays a Crucial Role in Innate Immune Responses Against Gram-Negative Bacteria by Regulating 5 AMP Genes in Tenebrio molitor. Front. Immunol. 2019, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Patnaik, B.B.; Hwang, J.; Park, K.B.; Ko, H.J.; Kim, C.E.; Bae, Y.M.; Jung, W.J.; Lee, Y.S.; Han, Y. Regulation of the expression of nine antimicrobial peptide genes by TmIMD confers resistance against Gram-negative bacteria. Sci. Rep. 2019, 9, 10138. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Jo, Y.; Edosa, T.; Han, Y. Two Roles for the Tenebrio molitor Relish in the Regulation of Antimicrobial Peptides and Autophagy-Related Genes in Response to Listeria monocytogenes. Insects 2020, 11, 188. [Google Scholar] [CrossRef]
- Keshavarz, M.; Jo, Y.H.; Patnaik, B.B.; Park, K.B.; Ko, H.J.; Kim, C.E.; Edosa, T.T.; Lee, Y.S.; Han, Y.S. Tmrelish is required for regulating the antimicrobial responses to Escherichia coli and Staphylococcus aureus in Tenebrio molitor. Sci. Rep. 2020, 10, 4258. [Google Scholar] [CrossRef]
- Ertürk-Hasdemir, D.; Broemer, M.; Leulier, F.; Lane, W.S.; Paquette, N.; Hwang, D.; Kim, C.-H.; Stöven, S.; Meier, P.; Silverman, N. Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proc. Natl. Acad. Sci. USA 2009, 106, 9779–9784. [Google Scholar] [CrossRef]
- Wang, P.H.; Gu, Z.H.; Wan, D.H.; Liu, B.D.; Huang, X.D.; Weng, S.P.; Yu, X.Q.; He, J.G. The shrimp IKK–NF-κB signaling pathway regulates antimicrobial peptide expression and may be subverted by white spot syndrome virus to facilitate viral gene expression. Cell Mol. Immunol. 2013, 10, 423–436. [Google Scholar] [CrossRef]
- Baldwin, A.S. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef]
- Li, X.; Massa, P.E.; Hanidu, A.; Peet, G.W.; Aro, P.; Savitt, A.; Mische, S.; Li, J.; Marcu, K.B. IKKα, IKKβ, and NEMO/IKKγ are each required for the NF-κB-mediated inflammatory response program. J. Biol. Chem. 2002, 277, 45129–45140. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Kovalenko, A.; Cantarella, G.; Wallach, D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 2000, 12, 301–311. [Google Scholar] [CrossRef]
- Tusco, R.; Jacomin, A.-C.; Jain, A.; Penman, B.; Larsen, K.B.; Johansen, T.; Nezis, I.P. Kenny mediates selective autophagic degradation of the IKK complex to control innate immune responses. Nat. Commun. 2017, 8, 1264. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, M.; Chen, H.; Zeng, M.; Sun, Y.; Huang, Q. Identification and functional characterization of nemo in Crassostrea gigas reveals its crucial role in the NF-κB activation. Fish Shellfish Immunol. 2018, 80, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wu, C.J.; Zhao, Y.; Ashwell, J.D. Optineurin negatively regulates TNFα- Induced NF-κB activation by competing with nemo for ubiquitinated rip. Curr. Biol. 2007, 17, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.D.; Wang, C.Y.; Xiong, Y.; Guan, K.L. A role for NF-κB Essential Modifier/IκB Kinase-γ (NEMO/IKKγ) IκB Kinase Complex by Tumor Necrosis Factor-α. J. Biol. Chem. 2003, 278, 37297–37305. [Google Scholar] [CrossRef]
- Kalvari, I.; Tsompanis, S.; Mulakkal, N.C.; Osgood, R.; Johansen, T.; Nezis, I.P.; Promponas, V.J. Ilir: A web resource for prediction of atg8-family interacting proteins. Autophagy 2014, 10, 913–925. [Google Scholar] [CrossRef]
- Jacomin, A.-C.; Samavedam, S.; Promponas, V.J.; Nezis, I.P. iLIR database: A web resource for LIR motif-containing proteins in eukaryotes. Autophagy 2016, 12, 1945–1953. [Google Scholar] [CrossRef]
- Ganesan, S.; Aggarwal, K.; Paquette, N.; Silverman, N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr. Top. Microbiol. Immunol. 2011, 349, 25–60. [Google Scholar] [PubMed]
- Kleino, A.; Silverman, N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2013, 42, 25–35. [Google Scholar] [CrossRef]
- Yu, Y.; Park, J.W.; Kwon, H.M.; Hwang, H.O.; Jang, I.H.; Masuda, A.; Kurokawa, K.; Nakayama, H.; Lee, W.J.; Dohmae, N.; et al. Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. J. Biol. Chem. 2010, 285, 32937–32945. [Google Scholar] [CrossRef]
- Hedengren-Olcott, M.; Olcott, M.C.; Mooney, D.T.; Ekengren, S.; Geller, B.L.; Taylor, B.J. Differential activation of the NF-κB-like factors relish and dif in Drosophila melanogaster by fungi and gram-positive bacteria. J. Biol. Chem. 2004, 279, 21121–21127. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Koyama, H.; Minakuchi, C.; Tanaka, T.; Miura, K. Antimicrobial peptide gene induction, involvement of Toll and IMD pathways and defense against bacteria in the red flour beetle, Tribolium castaneum. Results Immunol. 2012, 2, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.; McWilliam, H.; Valentin, F.; Wallace, I.; Wilm, A.; López, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).