Adenosine A2a Receptor Stimulation Attenuates Ischemia-Reperfusion Injury and Improves Survival in A Porcine Model of DCD Liver Transplantation
Abstract
1. Introduction
2. Results
2.1. Microcirculation
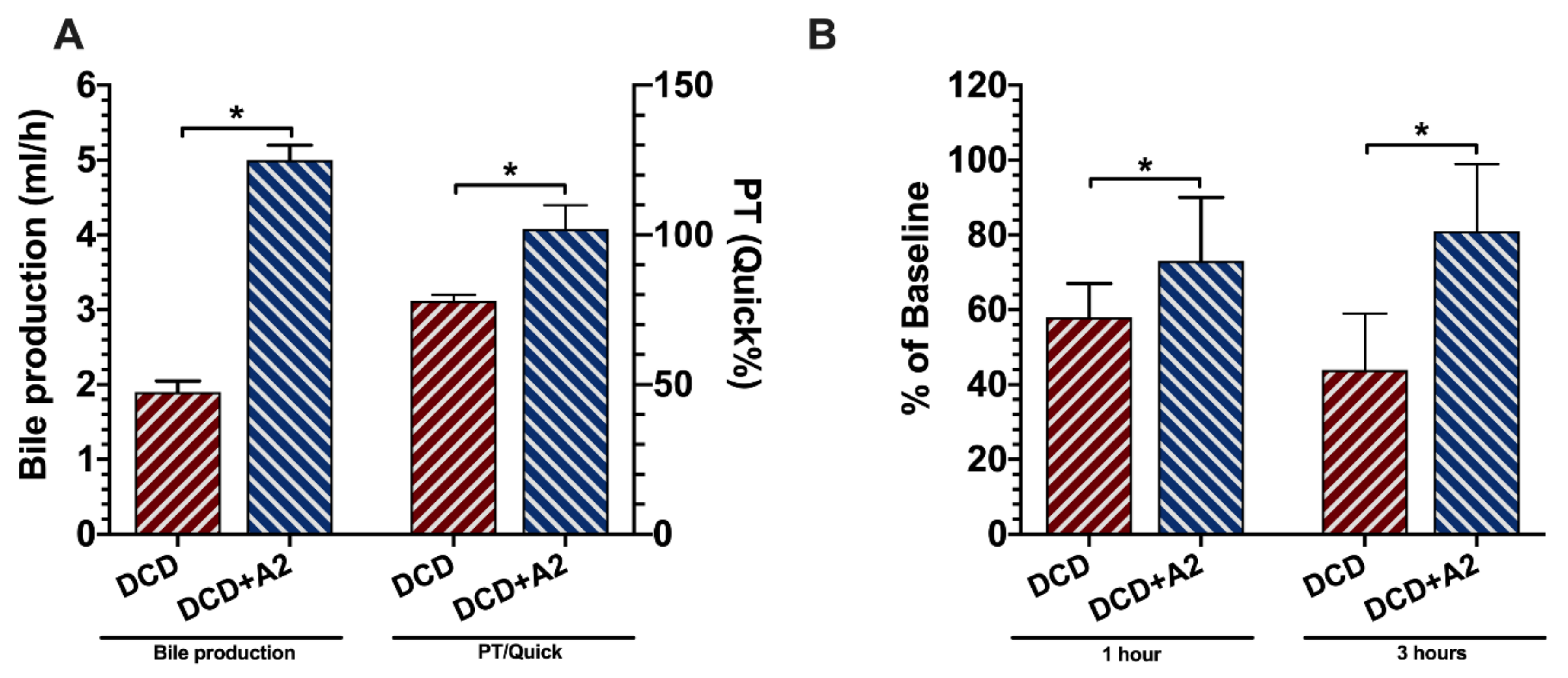
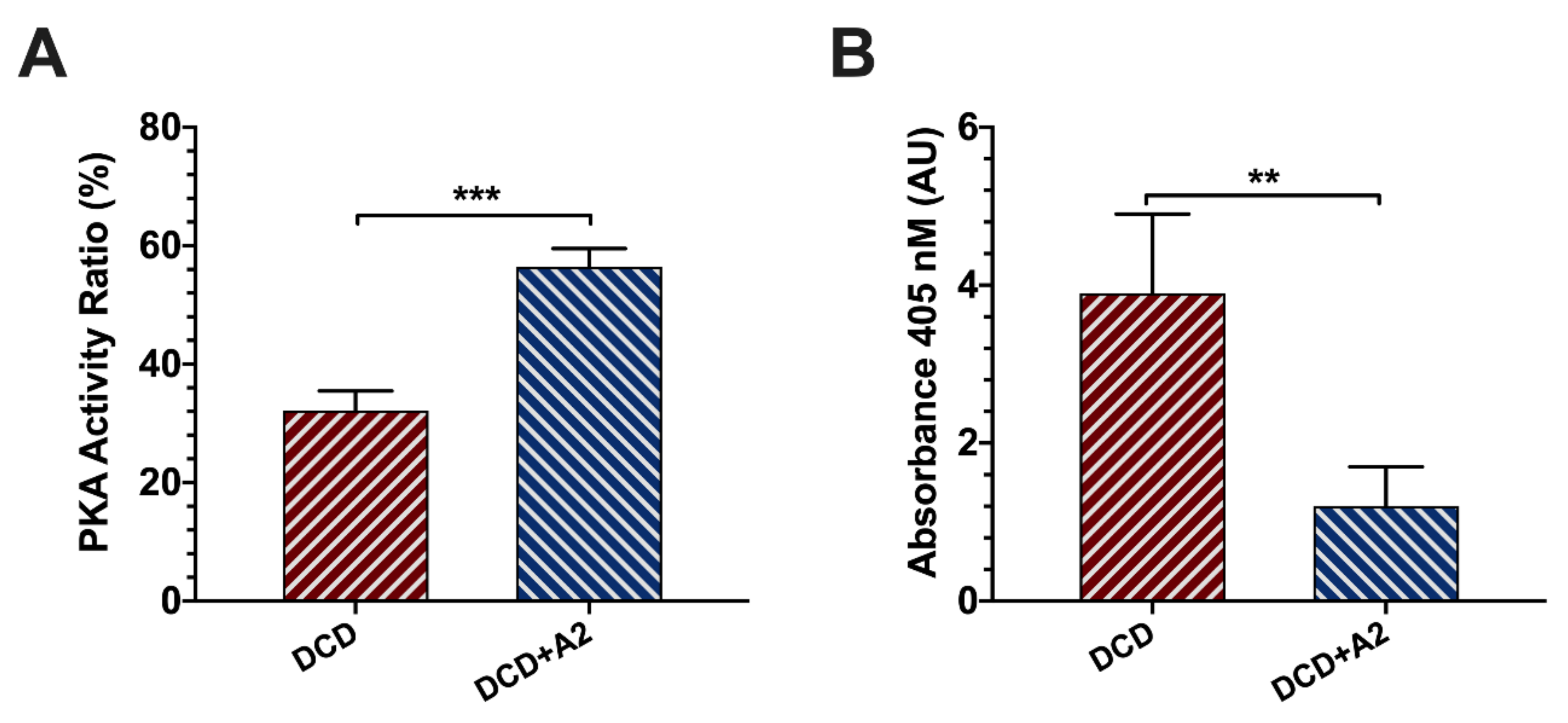
2.2. Allograft Functional Recovery
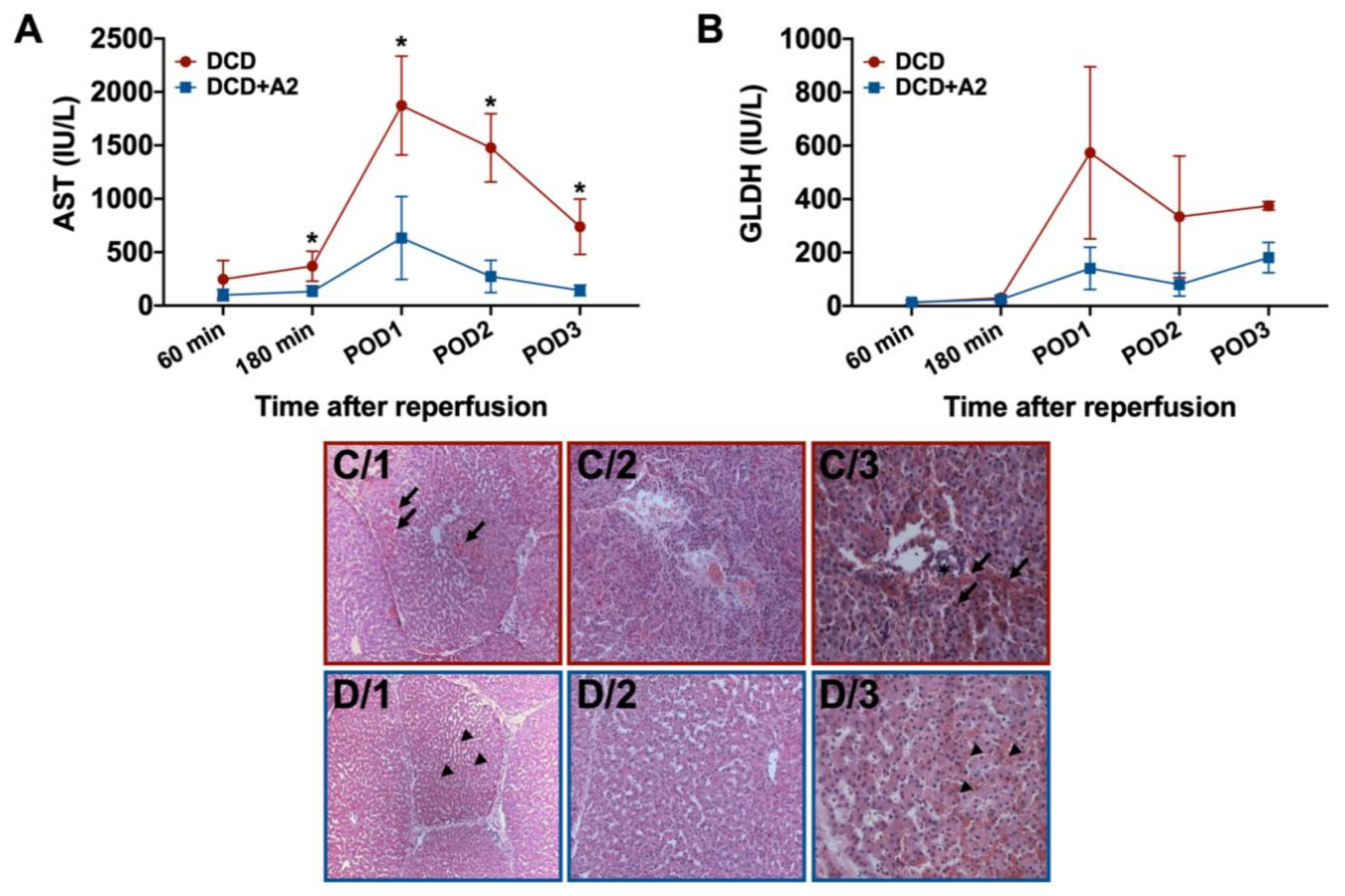
2.3. Hepatocellular Injury and Apoptosis
2.4. Survival
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics
4.2. Experimental Design
4.3. Anesthesia, Liver Retrieval, and Adenenosin A2a Receptor Stimulation
4.4. Bench Dissection and Cold Storage
4.5. Orthotopic Liver Transplantation and Postoperative Care
4.6. Enzyme Release and Prothrombine Time
4.7. Bile Production and ICG-Plasma Disappearance Rate
4.8. Hepatic Microcirculation
4.9. Serum PKA Activity Ratio
4.10. Histopathology and Apoptosis
4.11. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Czigany, Z.; Lurje, I.; Tolba, R.H.; Neumann, U.P.; Tacke, F.; Lurje, G. Machine perfusion for liver transplantation in the era of marginal organs-New kids on the block. Liver Int. Off. J. Int. Assoc. Study Liver 2018. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Fung, J.J. Themes of liver transplantation. Hepatology (Baltimore Md.) 2010, 51, 1869–1884. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Bleilevens, C.; Beckers, C.; Stoppe, C.; Mohring, M.; Fulop, A.; Szijarto, A.; Lurje, G.; Neumann, U.P.; Tolba, R.H. Limb remote ischemic conditioning of the recipient protects the liver in a rat model of arterialized orthotopic liver transplantation. PLoS ONE 2018, 13, e0195507. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Scherer, M.N.; Pratschke, J.; Guba, M.; Nadalin, S.; Mehrabi, A.; Berlakovich, G.; Rogiers, X.; Pirenne, J.; Lerut, J.; et al. Technical Aspects of Orthotopic Liver Transplantation-a Survey-Based Study Within the Eurotransplant, Swisstransplant, Scandiatransplant, and British Transplantation Society Networks. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2018. [Google Scholar] [CrossRef]
- Czigany, Z.; Schoning, W.; Ulmer, T.F.; Bednarsch, J.; Amygdalos, I.; Cramer, T.; Rogiers, X.; Popescu, I.; Botea, F.; Fronek, J.; et al. Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): A prospective multicentre randomised controlled trial (HOPE ECD-DBD). BMJ Open 2017, 7, e017558. [Google Scholar] [CrossRef]
- Schlegel, A.; de Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Durand, F.; Levitsky, J.; Cauchy, F.; Gilgenkrantz, H.; Soubrane, O.; Francoz, C. Age and liver transplantation. J. Hepatol. 2019, 70, 745–758. [Google Scholar] [CrossRef]
- Fondevila, C.; Hessheimer, A.J.; Maathuis, M.H.; Munoz, J.; Taura, P.; Calatayud, D.; Leuvenink, H.; Rimola, A.; Ploeg, R.J.; Garcia-Valdecasas, J.C. Superior preservation of DCD livers with continuous normothermic perfusion. Ann. Surg. 2011, 254, 1000–1007. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Baker, T.B. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2018, 24, 1470–1475. [Google Scholar] [CrossRef]
- Tacke, F.; Kroy, D.C.; Barreiros, A.P.; Neumann, U.P. Liver transplantation in Germany. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2016. [Google Scholar] [CrossRef]
- Czigany, Z.; Hata, K.; Lai, W.; Schwandt, T.; Yamamoto, Y.; Uemoto, S.; Tolba, R.H. A Dual Protective Effect of Intestinal Remote Ischemic Conditioning in a Rat Model of Total Hepatic Ischemia. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Emontzpohl, C.; Stoppe, C.; Theissen, A.; Beckers, C.; Neumann, U.P.; Lurje, G.; Ju, C.; Bernhagen, J.; Tolba, R.H.; Czigany, Z. The Role of Macrophage Migration Inhibitory Factor in Remote Ischemic Conditioning Induced Hepatoprotection in A Rodent Model of Liver Transplantation. Shock 2018. [Google Scholar] [CrossRef] [PubMed]
- Szijarto, A.; Czigany, Z.; Turoczi, Z.; Harsanyi, L. Remote ischemic perconditioning—A simple, low-risk method to decrease ischemic reperfusion injury: Models, protocols and mechanistic background. A review. J. Surg. Res. 2012, 178, 797–806. [Google Scholar] [CrossRef]
- Nagai, K.; Yagi, S.; Afify, M.; Bleilevens, C.; Uemoto, S.; Tolba, R.H. Impact of venous-systemic oxygen persufflation with nitric oxide gas on steatotic grafts after partial orthotopic liver transplantation in rats. Transplantation 2013, 95, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.K.; Yagi, S.; Doorschodt, B.; Nagai, K.; Afify, M.; Uemoto, S.; Tolba, R. Impact of venous systemic oxygen persufflation supplemented with nitric oxide gas on cold-stored, warm ischemia-damaged experimental liver grafts. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2012, 18, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Nagai, K.; Kadaba, P.; Afify, M.; Teramukai, S.; Uemoto, S.; Tolba, R.H. A novel organ preservation for small partial liver transplantations in rats: Venous systemic oxygen persuf fl ation with nitric oxide gas. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2013, 13, 222–228. [Google Scholar] [CrossRef]
- Minor, T.; Akbar, S.; Yamamoto, Y. Adenosine A2 receptor stimulation protects the predamaged liver from cold preservation through activation of cyclic adenosine monophosphate-protein kinase A pathway. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2000, 6, 196–200. [Google Scholar] [CrossRef]
- Yagi, S.; Doorschodt, B.M.; Afify, M.; Klinge, U.; Kobayashi, E.; Uemoto, S.; Tolba, R.H. Improved preservation and microcirculation with POLYSOL after partial liver transplantation in rats. J. Surg. Res. 2011, 167, e375–e383. [Google Scholar] [CrossRef]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Harada, N.; Okajima, K.; Murakami, K.; Usune, S.; Sato, C.; Ohshima, K.; Katsuragi, T. Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J. Pharmacol. Exp. Ther. 2000, 294, 1034–1042. [Google Scholar]
- Choukèr, A.; Ohta, A.; Martignoni, A.; Lukashev, D.; Zacharia, L.C.; Jackson, E.K.; Schnermann, J.; Ward, J.M.; Kaufmann, I.; Klaunberg, B.; et al. In vivo hypoxic preconditioning protects from warm liver ischemia-reperfusion injury through the adenosine A2B receptor. Transplantation 2012, 94, 894–902. [Google Scholar] [CrossRef]
- Chhabra, P.; Linden, J.; Lobo, P.; Okusa, M.D.; Brayman, K.L. The immunosuppressive role of adenosine A2A receptors in ischemia reperfusion injury and islet transplantation. Curr. Diabetes Rev. 2012, 8, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.M.; Trevethick, M.A. Suppression of inflammatory and immune responses by the A(2A) adenosine receptor: An introduction. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S27–S34. [Google Scholar] [CrossRef]
- Zhu, X.; Shiba, H.; Fung, J.J.; Wang, L.F.; Arakawa, Y.; Irefin, S.; Demetris, A.J.; Kelly, D.M. The role of the A2a receptor agonist, regadenoson, in modulating hepatic artery flow in the porcine small-for-size liver graft. J. Surg. Res. 2012, 174, e37–e45. [Google Scholar] [CrossRef]
- Tang, L.M.; Wang, Y.P.; Wang, K.; Pu, L.Y.; Zhang, F.; Li, X.C.; Kong, L.B.; Sun, B.C.; Li, G.Q.; Wang, X.H. Protective effect of adenosine A2A receptor activation in small-for-size liver transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2007, 20, 93–101. [Google Scholar] [CrossRef]
- Net, M.; Valero, R.; Almenara, R.; Barros, P.; Capdevila, L.; Lopez-Boado, M.A.; Ruiz, A.; Sanchez-Crivaro, F.; Miquel, R.; Deulofeu, R.; et al. The effect of normothermic recirculation is mediated by ischemic preconditioning in NHBD liver transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2005, 5, 2385–2392. [Google Scholar] [CrossRef]
- Schlegel, A.; Kalisvaart, M.; Scalera, I.; Laing, R.W.; Mergental, H.; Mirza, D.F.; Perera, T.; Isaac, J.; Dutkowski, P.; Muiesan, P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J. Hepatol. 2018, 68, 456–464. [Google Scholar] [CrossRef]
- Fayek, S.A.; Quintini, C.; Chavin, K.D.; Marsh, C.L. The Current State of Liver Transplantation in the United States: Perspective from American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016, 16, 3093–3104. [Google Scholar] [CrossRef]
- de la Rosa, G.; Fondevila, C.; Navasa, M. Liver transplantation in Spain. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2016, 22, 1259–1264. [Google Scholar] [CrossRef]
- Orman, E.S.; Mayorga, M.E.; Wheeler, S.B.; Townsley, R.M.; Toro-Diaz, H.H.; Hayashi, P.H.; Barritt, A.S.t. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2015, 21, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, D.; Pirenne, J.; Talbot, D. Liver transplantation using Donation after Cardiac Death donors. J. Hepatol. 2012, 56, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Beller, J.P.; Boys, J.A.; Zhao, Y.; Phillips, J.; Cosner, M.; Conaway, M.R.; Petroni, G.; Charles, E.J.; Mehaffey, J.H.; et al. Adenosine A2A receptor agonist (regadenoson) in human lung transplantation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2020, 39, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Teoh, N.C.; Farrell, G.C. Hepatic ischemia reperfusion injury: Pathogenic mechanisms and basis for hepatoprotection. J. Gastroenterol. Hepatol. 2003, 18, 891–902. [Google Scholar] [CrossRef]
- Vollmar, B.; Menger, M.D. The hepatic microcirculation: Mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 2009, 89, 1269–1339. [Google Scholar] [CrossRef]
- Niatsetskaya, Z.V.; Sosunov, S.A.; Matsiukevich, D.; Utkina-Sosunova, I.V.; Ratner, V.I.; Starkov, A.A.; Ten, V.S. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J. Neurosci. 2012, 32, 3235–3244. [Google Scholar] [CrossRef]
- van Golen, R.F.; Reiniers, M.J.; Olthof, P.B.; van Gulik, T.M.; Heger, M. Sterile inflammation in hepatic ischemia/reperfusion injury: Present concepts and potential therapeutics. J. Gastroenterol. Hepatol. 2013, 28, 394–400. [Google Scholar] [CrossRef]
- Schlegel, A.; Graf, R.; Clavien, P.A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J. Hepatol. 2013, 59, 984–991. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation—From bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Awad, A.S.; Rouse, M.; Huang, L.; Vergis, A.L.; Reutershan, J.; Cathro, H.P.; Linden, J.; Okusa, M.D. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009, 75, 689–698. [Google Scholar] [CrossRef]
- Le Dinh, H.; de Roover, A.; Kaba, A.; Lauwick, S.; Joris, J.; Delwaide, J.; Honoré, P.; Meurisse, M.; Detry, O. Donation after cardio-circulatory death liver transplantation. World J. Gastroenterol. 2012, 18, 4491–4506. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Maley, W.; Li, Z.; Thuluvath, P.J. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007, 13, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Vilca Melendez, H.; Rela, M.; Setchell, K.D.; Murphy, G.M.; Heaton, N.D. Bile acids analysis: A tool to assess graft function in human liver transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2004, 17, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, U.; Scholmerich, J.; Kremer, B.; Streckfuss, G.; Henne-Bruns, D.; Mergard, B.L.; Kraemer-Hansen, H.; Farthmann, E.H. Early detection of graft dysfunction after orthotopic liver transplantation in man by serum and biliary bile acid analysis. Hepatogastroenterology 1995, 42, 950–960. [Google Scholar] [PubMed]
- Fulop, A.; Budai, A.; Czigany, Z.; Lotz, G.; Dezso, K.; Paku, S.; Harsanyi, L.; Szijarto, A. Alterations in hepatic lobar function in regenerating rat liver. J. Surg. Res. 2015, 197, 307–317. [Google Scholar] [CrossRef]
- Thomas, M.N.; Weninger, E.; Angele, M.; Bosch, F.; Pratschke, S.; Andrassy, J.; Rentsch, M.; Stangl, M.; Hartwig, W.; Werner, J.; et al. Intraoperative simulation of remnant liver function during anatomic liver resection with indocyanine green clearance (LiMON) measurements. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2015, 17, 471–476. [Google Scholar] [CrossRef]
- Levesque, E.; Saliba, F.; Benhamida, S.; Ichai, P.; Azoulay, D.; Adam, R.; Castaing, D.; Samuel, D. Plasma disappearance rate of indocyanine green: A tool to evaluate early graft outcome after liver transplantation. Liver Transpl. 2009, 15, 1358–1364. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Lee, C.; Noguchi, E.; Yersiz, H.; Agopian, V.G.; Kaldas, F.M.; Farmer, D.G.; Busuttil, R.W. A rapid, reproducible, noninvasive predictor of liver graft survival. J. Surg. Res. 2015, 197, 183–190. [Google Scholar] [CrossRef]
- Ben-Ari, Z.; Pappo, O.; Sulkes, J.; Cheporko, Y.; Vidne, B.A.; Hochhauser, E. Effect of adenosine A2A receptor agonist (CGS) on ischemia/reperfusion injury in isolated rat liver. Apoptosis Int. J. Program. Cell Death 2005, 10, 955–962. [Google Scholar] [CrossRef]
- Tang, L.M.; Zhu, J.F.; Wang, F.; Qian, J.; Zhu, J.; Mo, Q.; Lu, H.H.; Li, G.Q.; Wang, X.H. Activation of adenosine A2A receptor attenuates inflammatory response in a rat model of small-for-size liver transplantation. Transplant. Proc. 2010, 42, 1915–1920. [Google Scholar] [CrossRef]
- Cursio, R. Caspase inhibition in liver transplantation: From basic research to clinical studies. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2010, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, R.; Out, M.; Maas, W.J.; De Jong, J.W. Preconditioning of rat hearts by adenosine A1 or A3 receptor activation. Eur. J. Pharmacol. 2002, 441, 165–172. [Google Scholar] [CrossRef]
- Arasi, F.P.; Shahrestanaki, M.K.; Aghaei, M. A2a adenosine receptor agonist improves endoplasmic reticulum stress in MIN6 cell line through protein kinase A/protein kinase B/Cyclic adenosine monophosphate response element-binding protein/ and Growth Arrest And DNA-Damage-Inducible 34/ eukaryotic Initiation Factor 2α pathways. J. Cell Physiol. 2019, 234, 10500–10511. [Google Scholar] [CrossRef] [PubMed]
- Carini, R.; Grazia De Cesaris, M.; Splendore, R.; Baldanzi, G.; Nitti, M.P.; Alchera, E.; Filigheddu, N.; Domenicotti, C.; Pronzato, M.A.; Graziani, A.; et al. Role of phosphatidylinositol 3-kinase in the development of hepatocyte preconditioning. Gastroenterology 2004, 127, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Tacke, F.; Lurje, G. Evolving Trends in Machine Liver Perfusion: Comments on Clinical End Points and Selection Criteria. Gastroenterology 2019, 157, 1166–1167. [Google Scholar] [CrossRef]
- Meister, F.A.; Czigany, Z.; Bednarsch, J.; Bocker, J.; Amygdalos, I.; Morales Santana, D.A.; Rietzler, K.; Moeller, M.; Tolba, R.; Boor, P.; et al. Hypothermic Oxygenated Machine Perfusion of Extended Criteria Kidney Allografts from Brain Dead Donors: Protocol for a Prospective Pilot Study. JMIR Res. Protoc. 2019, 8, e14622. [Google Scholar] [CrossRef] [PubMed]
- Meister, F.A.; Czigany, Z.; Bednarsch, J.; Boecker, J.; Wiltberger, G.; Rohlfs, W.; Neumann, U.P.; Lurje, G. Hypothermic oxygenated machine perfusion-Preliminary experience with end-ischemic reconditioning of marginal kidney allografts. Clin. Transplant. 2019, 33, e13673. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Vogel, T.; Brockmann, J.G.; Pigott, D.; Neil, D.A.H.; Muthusamy, A.S.R.; Coussios, C.C.; Friend, P.J. Successful transplantation of porcine liver grafts following 48-hour normothermic preservation. PLoS ONE 2017, 12, e0188494. [Google Scholar] [CrossRef]
- Iskandarov, E.; Kadaba Srinivasan, P.; Xin, W.; Bleilevens, C.; Afify, M.; Hamza, A.; Wei, L.; Hata, K.; Agayev, B.; Tolba, R. Protective Effects of Adenosine Receptor Agonist in a Cirrhotic Liver Resection Model. Hepat. Mon. 2016, 16, e36821. [Google Scholar] [CrossRef]
- Xanthos, T.; Lelovas, P.; Vlachos, I.; Tsirikos-Karapanos, N.; Kouskouni, E.; Perrea, D.; Dontas, I. Cardiopulmonary arrest and resuscitation in Landrace/Large White swine: A research model. Lab. Anim. 2007, 41, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, V.N.; Goldaracena, N.; Knaak, J.M.; Louis, K.S.; Selzner, N.; Selzner, M. Technique of porcine liver procurement and orthotopic transplantation using an active porto-caval shunt. J. Vis. Exp. JoVE 2015, e52055. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, S.; Rix, A.; Czigany, Z.; Tanaka, H.; Fukushima, K.; Kogel, B.; Pawlowsky, K.; Tolba, R.H. Recommendation for severity assessment following liver resection and liver transplantation in rats: Part I. Lab. Anim. 2016, 50, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Hagemeister, K.; Ernst, L.; Kadaba Srinivasan, P.; Tanaka, H.; Fukushima, K.; Tolba, R. Severity assessment in pigs after partial liver resection: Evaluation of a score sheet. Lab. Anim. 2019, 23677219871585. [Google Scholar] [CrossRef]
- Bleich, A.; Bankstahl, M.; Jirkof, P.; Prins, J.B.; Tolba, R.H. Severity Assessment in animal based research. Lab. Anim. 2020, 54, 16. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czigany, Z.; Craigie, E.C.; Lurje, G.; Song, S.; Yonezawa, K.; Yamamoto, Y.; Minor, T.; Tolba, R.H. Adenosine A2a Receptor Stimulation Attenuates Ischemia-Reperfusion Injury and Improves Survival in A Porcine Model of DCD Liver Transplantation. Int. J. Mol. Sci. 2020, 21, 6747. https://doi.org/10.3390/ijms21186747
Czigany Z, Craigie EC, Lurje G, Song S, Yonezawa K, Yamamoto Y, Minor T, Tolba RH. Adenosine A2a Receptor Stimulation Attenuates Ischemia-Reperfusion Injury and Improves Survival in A Porcine Model of DCD Liver Transplantation. International Journal of Molecular Sciences. 2020; 21(18):6747. https://doi.org/10.3390/ijms21186747
Chicago/Turabian StyleCzigany, Zoltan, Eve Christiana Craigie, Georg Lurje, Shaowei Song, Kei Yonezawa, Yuzo Yamamoto, Thomas Minor, and René Hany Tolba. 2020. "Adenosine A2a Receptor Stimulation Attenuates Ischemia-Reperfusion Injury and Improves Survival in A Porcine Model of DCD Liver Transplantation" International Journal of Molecular Sciences 21, no. 18: 6747. https://doi.org/10.3390/ijms21186747
APA StyleCzigany, Z., Craigie, E. C., Lurje, G., Song, S., Yonezawa, K., Yamamoto, Y., Minor, T., & Tolba, R. H. (2020). Adenosine A2a Receptor Stimulation Attenuates Ischemia-Reperfusion Injury and Improves Survival in A Porcine Model of DCD Liver Transplantation. International Journal of Molecular Sciences, 21(18), 6747. https://doi.org/10.3390/ijms21186747