Cellular and Molecular Aspects of Anti-Melanoma Effect of Minocycline—A Study of Cytotoxicity and Apoptosis on Human Melanotic Melanoma Cells
Abstract
1. Introduction
2. Results
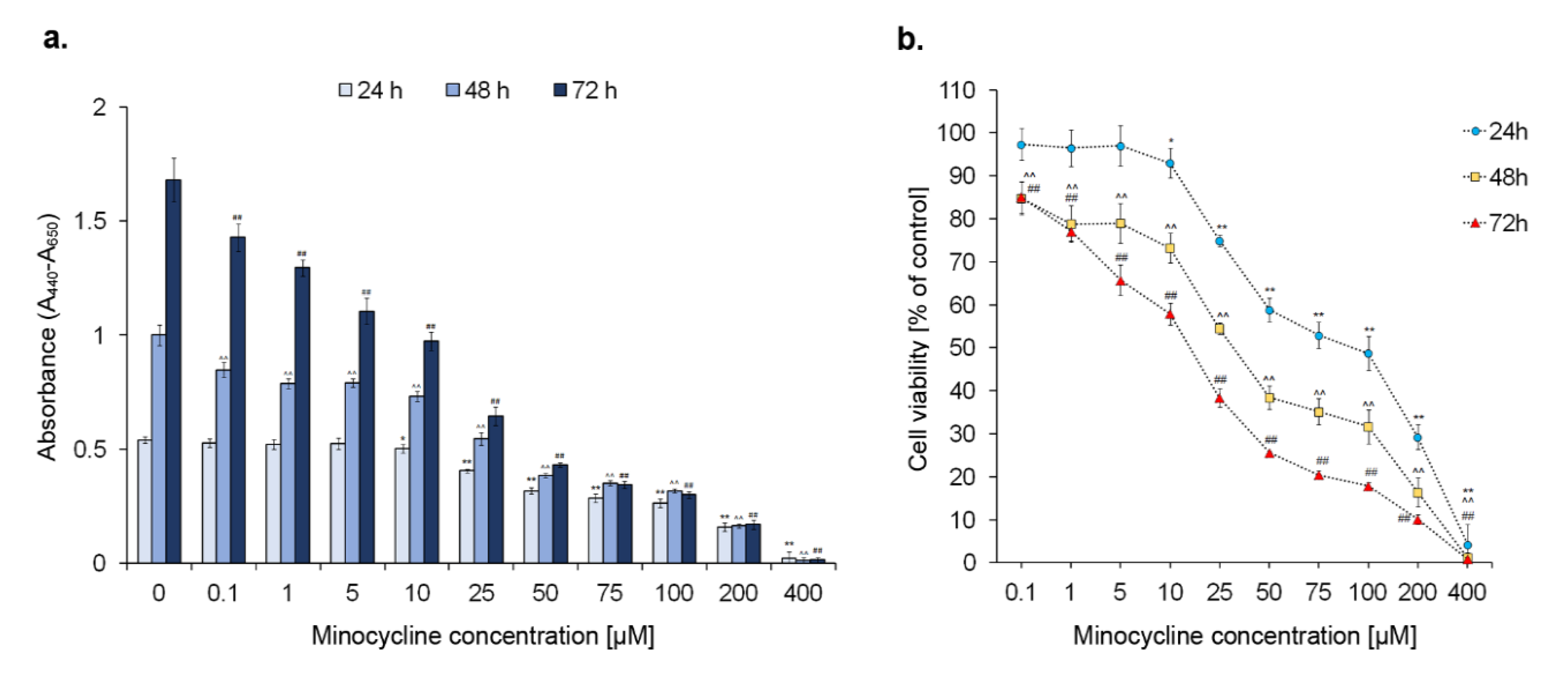
2.1. The Viability of Human Melanoma Cells Exposed to Minocycline
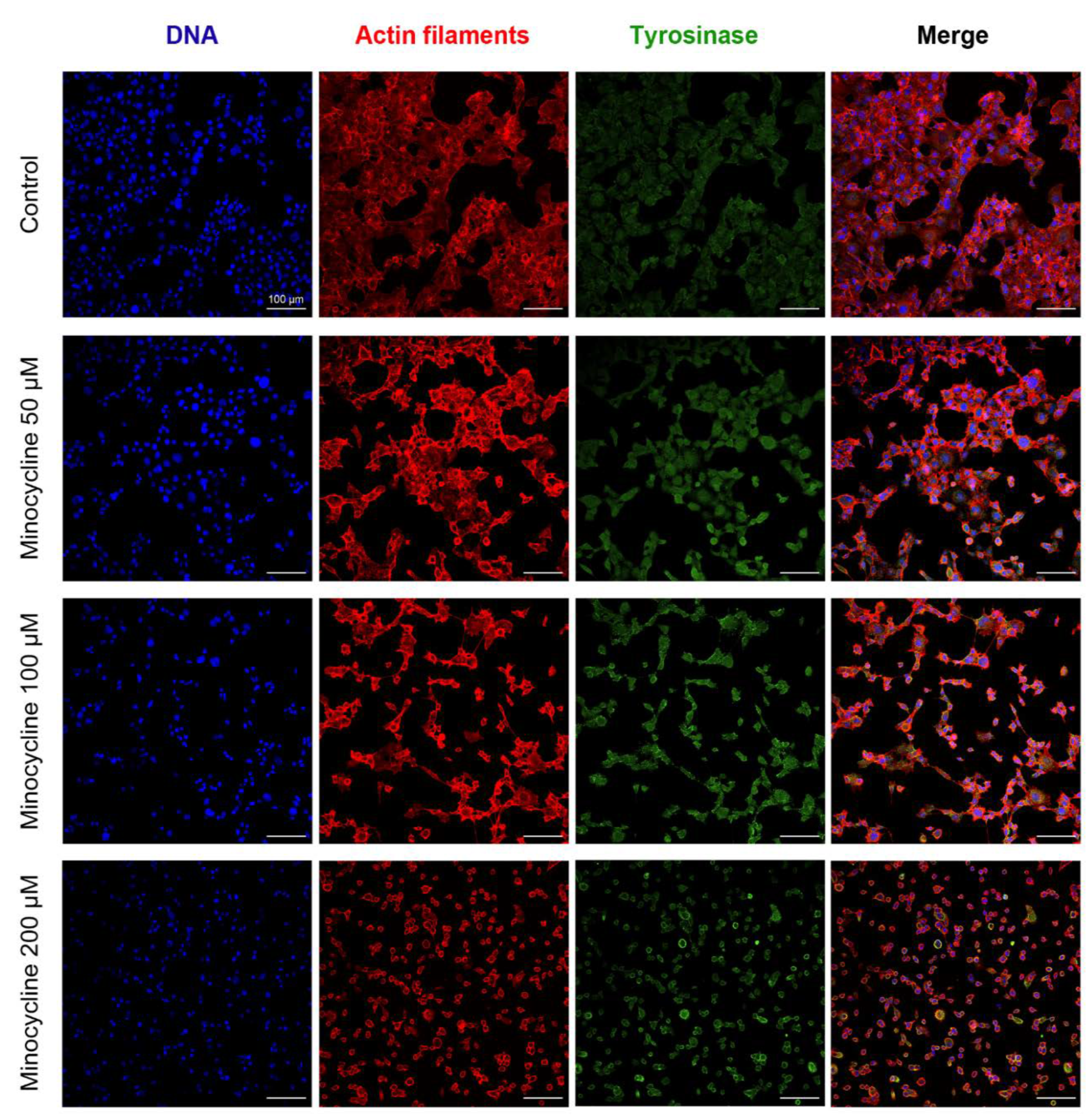
2.2. The General Evaluation of Human Melanoma Cell Culture Treated with Minocycline
2.3. The Assessment of Human Melanocyte Viability after the Treatment with Minocycline
2.4. Minocycline-Induced Alterations of Human Melanoma Cell Cycle
2.5. Minocycline Triggers Melanoma Cell Apoptosis (Annexin V Assay Confirmation)
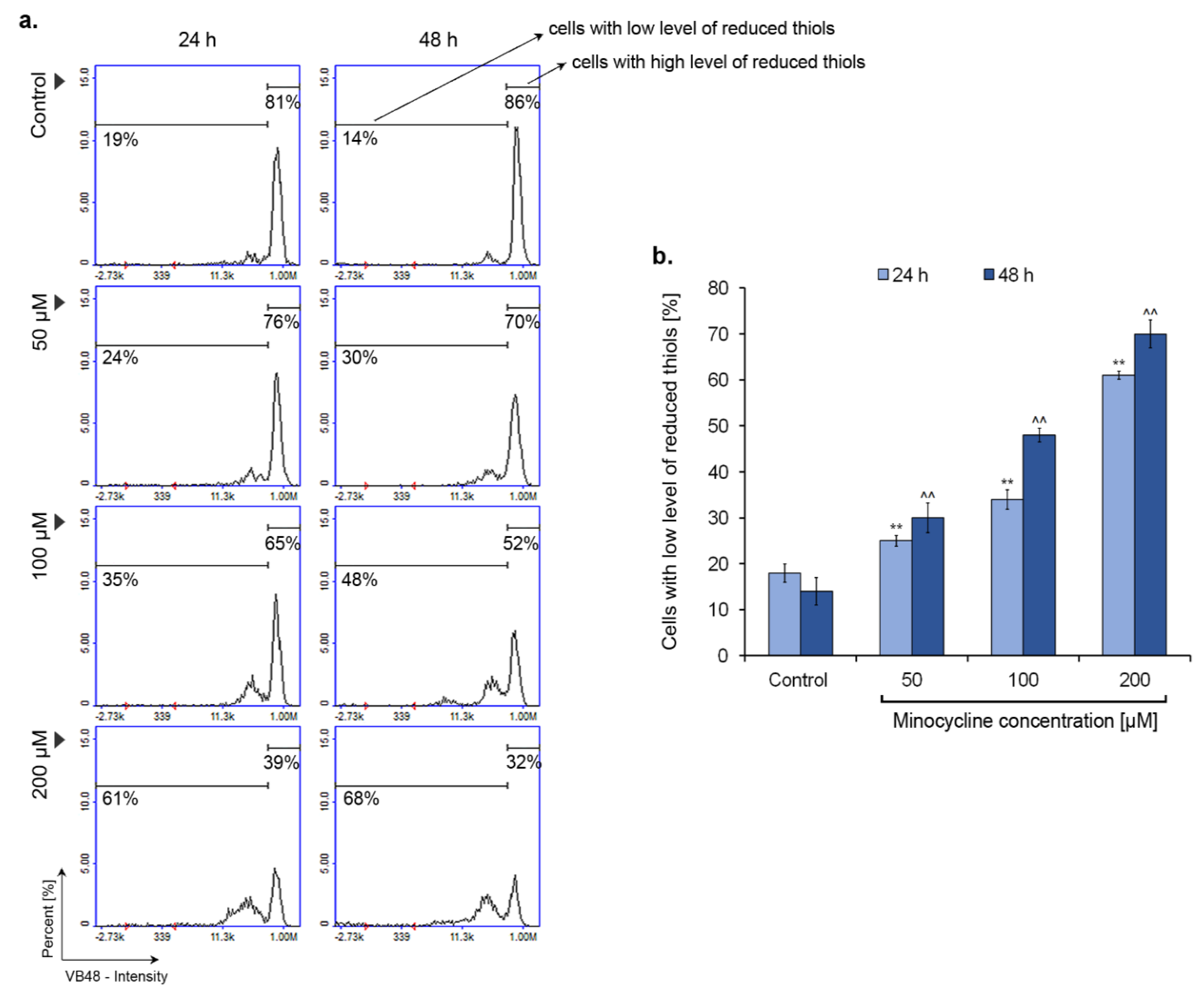
2.6. Minocycline Decreases the Intracellular Level of Reduced Thiols
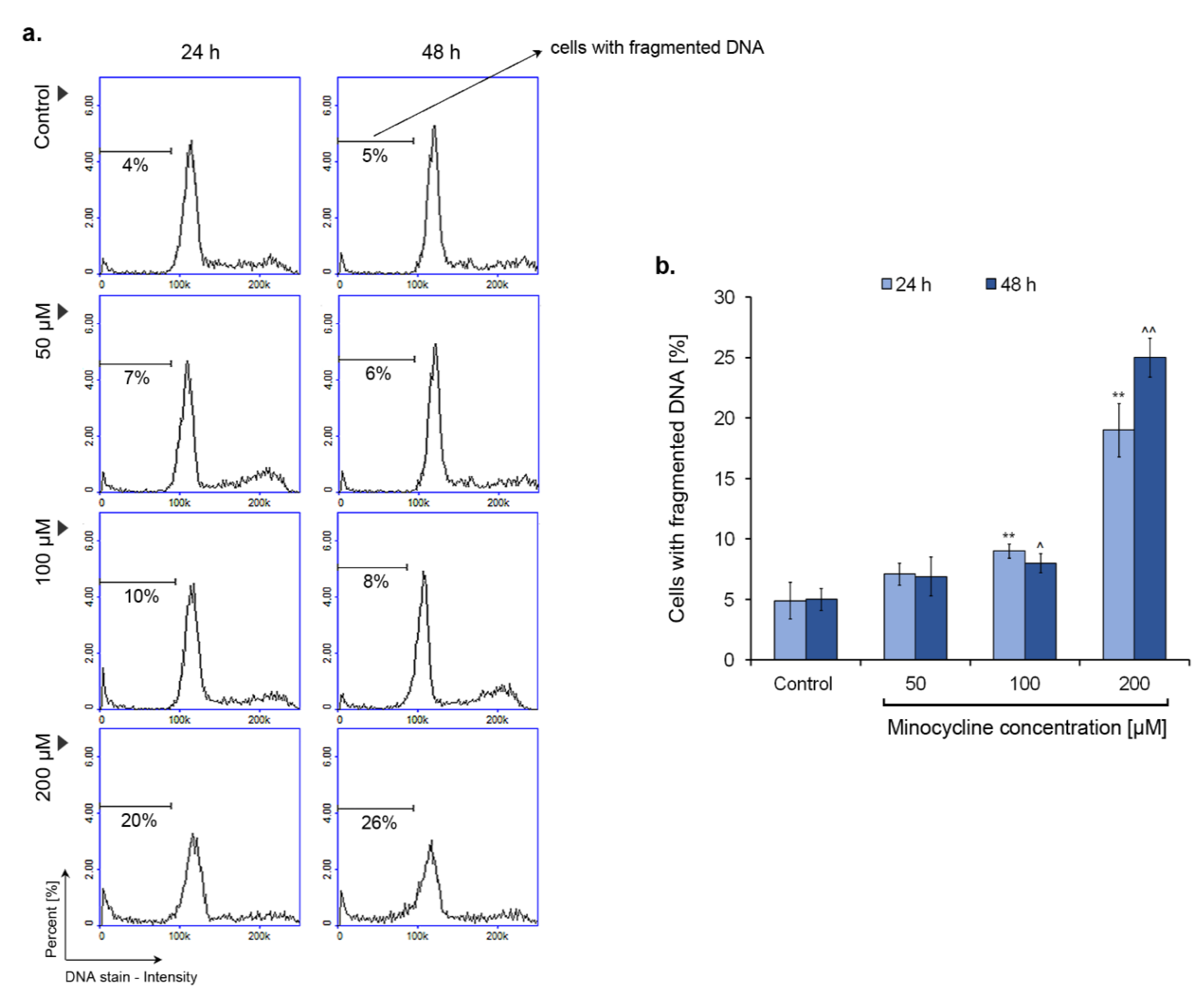
2.7. Minocycline Induces DNA Fragmentation in Human Melanoma Cells
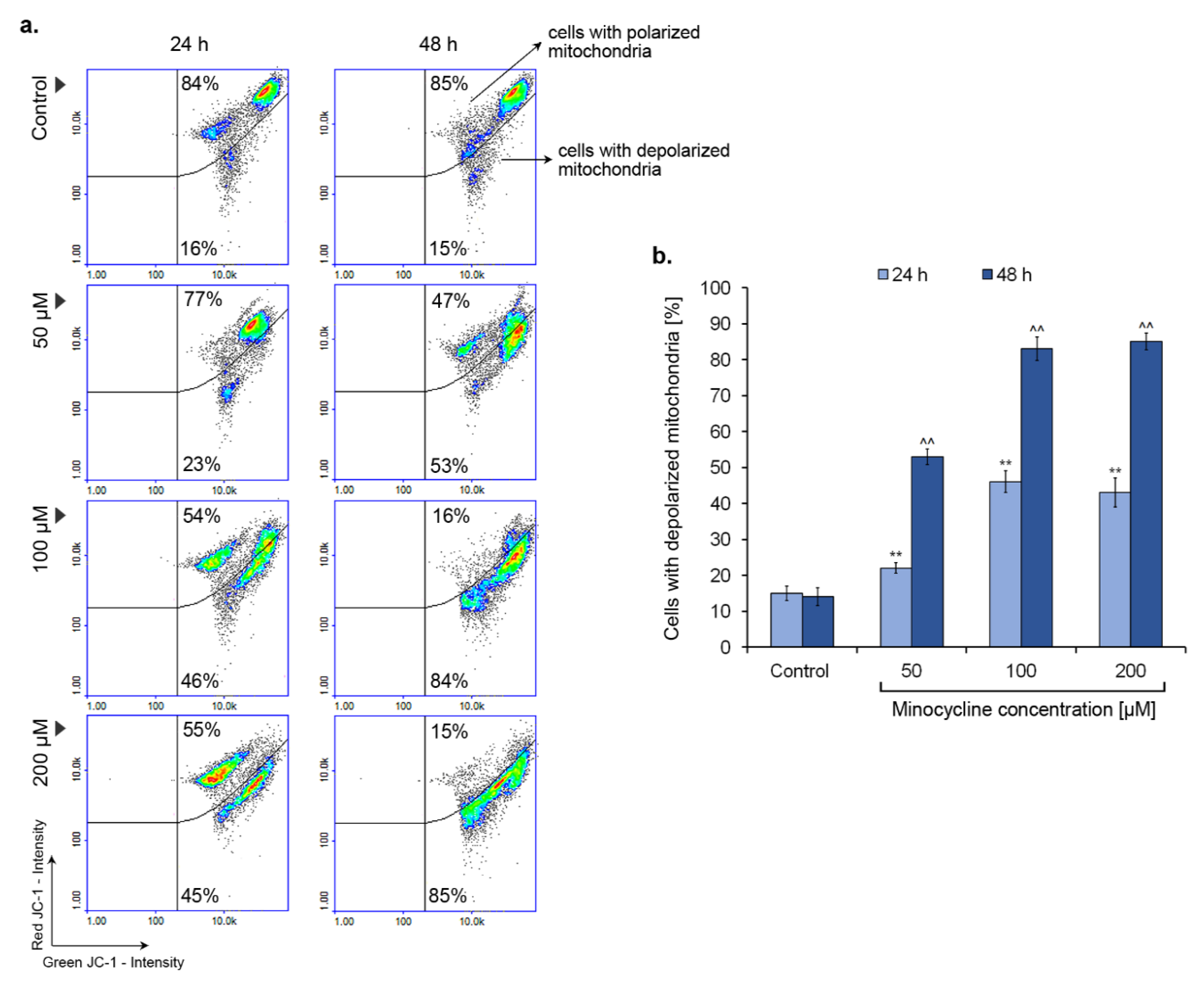
2.8. Minocycline Triggers a Decrease in Mitochondrial Membrane Potential
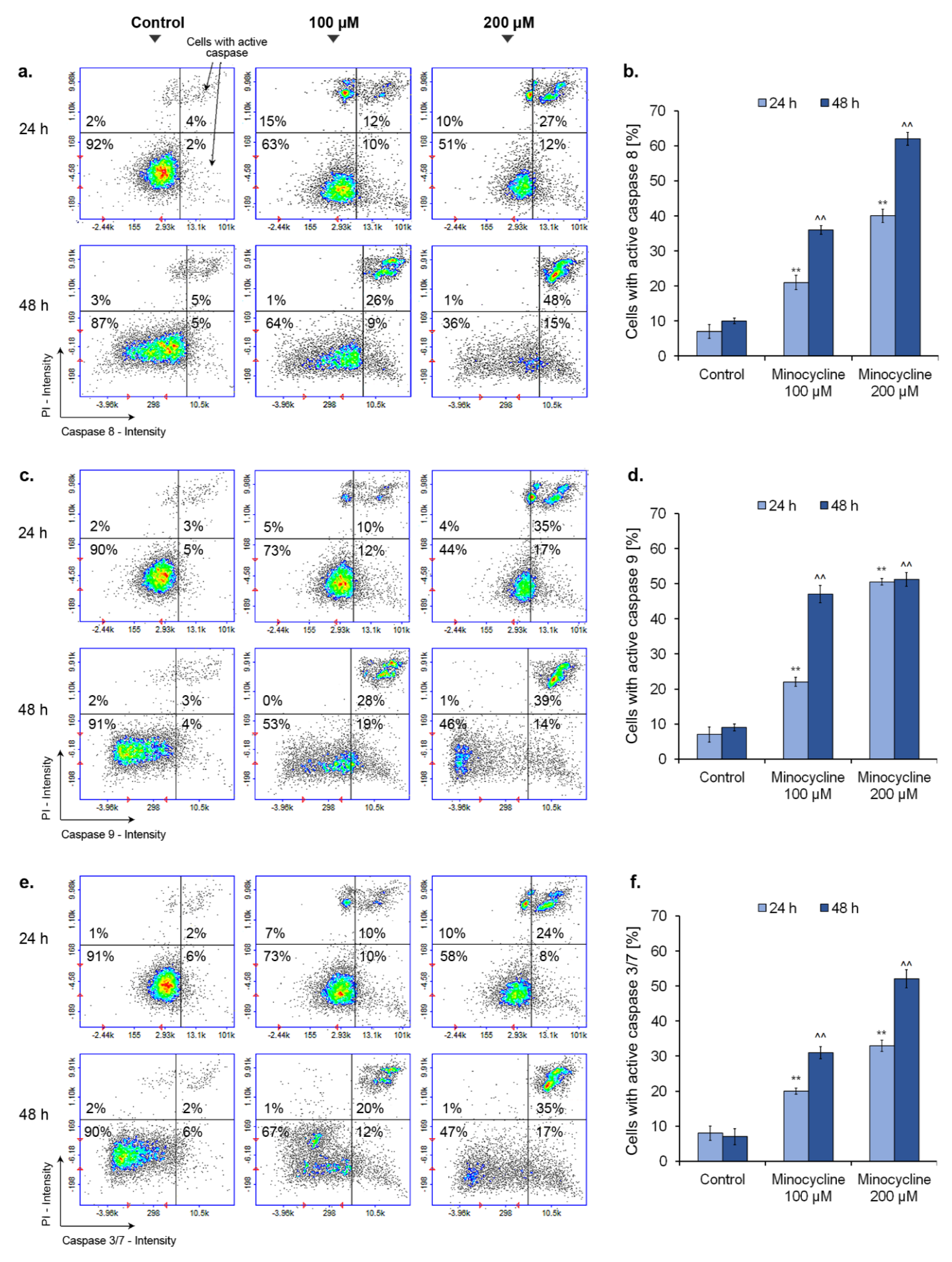
2.9. Minocycline Activates Caspase 8, 9 and 3/7 in Human Melanoma Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cell Cultures
5.3. Screening Test of Cells Viability
5.4. Cell Treatment
5.5. The Evaluation of Cell Number and Cell Viability
5.6. Cell Morphology Assessment
5.7. Cell Cycle Assay
5.8. Annexin V Assay
5.9. Analysis of Intracellular Thiol Level
5.10. DNA Fragmentation Assay
5.11. Mitochondrial Potential Assay
5.12. Caspase Activity Assay
5.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rebecca, V.W.; Sondak, V.K.; Smalley, K.S. A brief history of melanoma: From mummies to mutations. Melanoma Res. 2012, 22, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U.; ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and risk factors of melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Cust, A.E.; Mishra, K.; Berwick, M. Melanoma-role of the environment and genetics. Photochem. Photobiol. Sci. 2018, 17, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, C.Y.; Xu, Y.Y.; Deng, X.Y.; Wang, Q.; Ying, J.H.; Zhang, S.M.; Yuan, X.; Xuan, T.F.; Pan, Y.Y.; et al. Prognostic genes of melanoma identified by weighted gene co-expression network analysis and drug repositioning using a network-based method. Oncol. Lett. 2019, 18, 6066–6078. [Google Scholar] [CrossRef]
- Trucco, L.D.; Mundra, P.A.; Hogan, K.; Garcia-Martinez, P.; Viros, A.; Mandal, A.K.; Macagno, N.; Gaudy-Marqueste, C.; Allan, D.; Baenke, F.; et al. Ultraviolet radiation-induced DNA damage is prognostic for outcome in melanoma. Nat. Med. 2019, 25, 221–224. [Google Scholar] [CrossRef]
- Mason, R.; Au, L.; Garces, A.I.; Larkin, J. Current and emerging systemic therapies for cutaneous metastatic melanoma. Expert Opin. Pharmacother. 2019, 20, 1135–1152. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Lim, J.; Cho, E.; Lee, K.; Choi, Y.; Seo, Y.; Jeon, H.; Choi, J. Current immunotherapy approaches for malignant melanoma. BioChip J. 2019, 13, 105–114. [Google Scholar] [CrossRef]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A.; et al. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef]
- Rayner, J.E.; McMeniman, E.K.; Duffy, D.L.; De’Ambrosis, B.; Smithers, B.M.; Jagirdar, K.; Lee, K.J.; Soyer, H.P.; Sturm, R.A. Phenotypic and genotypic analysis of amelanotic and hypomelanotic melanoma patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1076–1083. [Google Scholar] [CrossRef]
- Grossman, D.; Kim, C.C.; Hartman, R.I.; Berry, E.; Nelson, K.C.; Okwundu, N.; Curiel-Lewandrowski, C.; Leachman, S.A.; Swetter, S.M. Prognostic gene expression profiling in melanoma: Necessary steps to incorporate into clinical practice. Melanoma Manag. 2019, 6, MMT32. [Google Scholar] [CrossRef]
- Doma, V.; Barbai, T.; Beleaua, M.A.; Kovalszky, I.; Rásó, E.; Tímár, J. KIT mutation incidence and pattern of melanoma in Central Europe. Pathol. Oncol. Res. 2020, 26, 17–22. [Google Scholar] [CrossRef]
- Amaral, T.; Hoffmann, M.C.; Sinnberg, T.; Niessner, H.; Sülberg, H.; Eigentler, T.K.; Garbe, C. Clinical validation of a prognostic 11-gene expression profiling score in prospectively collected FFPE tissue of patients with AJCC v8 stage II cutaneous melanoma. Eur. J. Cancer 2020, 125, 38–45. [Google Scholar] [CrossRef]
- Ciołczyk-Wierzbicka, D.; Zarzycka, M.; Gil, D.; Laidler, P. mTOR inhibitor Everolimus-induced apoptosis in melanoma cells. J. Cell Commun. Signal. 2019, 13, 357–368. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Z.; Su, J.; Li, J.; Zhao, S.; Wu, L.; Zhang, J.; He, Y.; Zhang, G.; Tao, J.; et al. Benserazide is a novel inhibitor targeting PKM2 for melanoma treatment. Int. J. Cancer. 2020, 147, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Delgado-Hernández, R.; Hernández-Balmaseda, I.; Rodeiro-Guerra, I.; Gonzalez, J.C.R.; De Wever, O.; Logie, E.; Declerck, K.; Pérez-Novo, C.; Berghe, W.V. Anti-angiogenic effects of mangiferin and mechanism of action in metastatic melanoma. Melanoma Res. 2020, 30, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. What is behind the non-antibiotic properties of minocycline? Pharmacol. Res. 2013, 67, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Heydari-Kamjani, M.; Beckler, M.D.; Kesselman, M.M. Reconsidering the use of minocycline in the preliminary treatment regime of rheumatoid arthritis. Cureus 2019, 11, e5351. [Google Scholar] [CrossRef]
- Sasaki, S.; Kato, M.; Nakamura, K.; Namba, Y.; Nagashima, O.; Takahashi, K. Management of skin sarcoidosis with minocycline monotherapy. Respirol. Case Rep. 2019, 7, e00413. [Google Scholar] [CrossRef]
- Dabas, G.; Guliani, A.; Vinay, K.; Radotra, B.D. Nevoid hyperkeratosis of male breast: Successful treatment with minocycline. Dermatol. Ther. 2019, 32, e13019. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Jiang, Y.; Pi, R.; Liu, Y.; Ma, L. The prospects of minocycline in multiple sclerosis. J. Neuroimmunol. 2011, 235, 1–8. [Google Scholar] [CrossRef]
- Malhotra, K.; Chang, J.J.; Khunger, A.; Blacker, D.; Switzer, J.A.; Goyal, N.; Hernandez, A.V.; Pasupuleti, V.; Alexandrov, A.V.; Tsivgoulis, G. Minocycline for acute stroke treatment: A systematic review and meta-analysis of randomized clinical trials. J. Neurol. 2018, 265, 1871–1879. [Google Scholar] [CrossRef]
- Meythaler, J.; Fath, J.; Fuerst, D.; Zokary, H.; Freese, K.; Martin, H.B.; Reineke, J.; Peduzzi-Nelson, J.; Roskos, P.T. Safety and feasibility of minocycline in treatment of acute traumatic brain injury. Brain Inj. 2019, 33, 679–689. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Sun, J.; Wang, X.M.; Tian, Y.K.; Wu, W.; Ye, D.W. Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol. Res. 2018, 134, 305–310. [Google Scholar] [CrossRef]
- Cai, D.B.; Zheng, W.; Zhang, Q.E.; Ng, C.H.; Ungvari, G.S.; Huang, X.; Xiang, Y.T. Minocycline for depressive symptoms: A meta-analysis of randomized, double-blinded, placebo-controlled trials. Psychiatr. Q. 2020, 91, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, S.; Cankaya, B.; Kilic, U.; Kilic, E.; Yulug, B. The therapeutic role of minocycline in Parkinson’s disease. Drugs Context. 2019, 8, 212553. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.A.; Dash, R.; Sarkar, S.; Azab, B.; Bhoopathi, P.; Das, S.K.; Emdad, L.; Wei, J.; Pellecchia, M.; Sarkar, D.; et al. Pancreatic cancer combination therapy using a BH3 mimetic and a synthetic tetracycline. Cancer Res. 2015, 75, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.; Dittmar, T. Minocycline impairs TNF-α-induced cell fusion of M13SV1-Cre cells with MDA-MB-435-pFDR1 cells by suppressing NF-κB transcriptional activity and its induction of target-gene expression of fusion-relevant factors. Cell Commun. Signal. 2019, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Shi, Q.; Mendoza, T.; Lin, S.; Chang, J.Y.; Bokhari, R.H.; Lin, H.K.; Garcia-Gonzalez, A.; Kamal, M.; Cleeland, C.S.; et al. Minocycline reduces chemoradiation-related symptom burden in patients with non-small cell lung cancer: A phase 2 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, A.; Ikeda, M.; Okuyama, H.; Kobayashi, M.; Funazaki, H.; Mitsunaga, S.; Shimizu, S.; Ohno, I.; Takahashi, H.; Ichida, Y.; et al. Efficacy of prophylactic minocycline treatment for skin toxicities induced by erlotinib plus gemcitabine in patients with advanced pancreatic cancer: A retrospective study. Am. J. Clin. Dermatol. 2015, 16, 221–229. [Google Scholar] [CrossRef]
- Huang, H.C.; Liu, J.; Baglo, Y.; Rizvi, I.; Anbil, S.; Pigula, M.; Hasan, T. Mechanism-informed repurposing of minocycline overcomes resistance to topoisomerase inhibition for peritoneal carcinomatosis. Mol. Cancer Ther. 2018, 17, 508–520. [Google Scholar] [CrossRef]
- Liu, F.Y.; Wu, Y.H.; Zhou, S.J.; Deng, Y.L.; Zhang, Z.Y.; Zhang, E.L.; Huang, Z.Y. Minocycline and cisplatin exert synergistic growth suppression on hepatocellular carcinoma by inducing S phase arrest and apoptosis. Oncol. Rep. 2014, 32, 835–844. [Google Scholar] [CrossRef]
- Masumori, N.; Tsukamoto, T.; Miyao, N.; Kumamoto, Y.; Saiki, I.; Yoneda, J. Inhibitory effect of minocycline on in vitro invasion and experimental metastasis of mouse renal adenocarcinoma. J. Urol. 1994, 151, 1400–1404. [Google Scholar] [CrossRef]
- Niu, G.; Liao, Z.; Cai, L.; Wei, R.; Sun, L. The combined effects of celecoxib and minocycline hydrochloride on inhibiting the osseous metastasis of breast cancer in nude mice. Cancer Biother. Radiopharm. 2008, 23, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.S.; Vinnakota, K.; van Rooijen, N.; Kiwit, J.; Synowitz, M.; Glass, R.; Kettenmann, H. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav. Immun. 2011, 25, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson-Beadling, S.; Powers, E.A.; Stamp-Cole, M.; Scott, P.S.; Wallace, T.L.; Copeland, J.; Petzold, G.; Mitchell, M.; Ledbetter, S.; Poorman, R. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother. Pharmacol. 1995, 36, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Banning, T.P.; Heard, C.M. Binding of doxycycline to keratin, melanin and human epidermal tissue. Int. J. Pharm. 2002, 235, 219–227. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Respondek, M.; Beberok, A.; Wrześniok, D. Chlortetracycline and melanin biopolymer—The risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chem. Biol. Interact. 2019, 303, 27–34. [Google Scholar] [CrossRef]
- Miliński, M.; Delijewski, M.; Rok, J.; Wrześniok, D.; Beberok, A.; Chełmecka, E.; Buszman, E. The pivotal effect of suldinac on melanin-containing cancers. Acta Pol. Pharm. 2017, 74, 1681–1689. [Google Scholar]
- Pourgholami, M.H.; Mekkawy, A.H.; Badar, S.; Morris, D.L. Minocycline inhibits growth of epithelial ovarian cancer. Gynecol. Oncol. 2012, 125, 433–440. [Google Scholar] [CrossRef]
- Brüning, A.; Brem, G.J.; Vogel, M.; Mylonas, I. Tetracyclines cause cell stress-dependent atf4 activation and mtor inhibition. Exp. Cell Res. 2014, 320, 281–289. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Metabolic regulation of apoptosis in cancer. Int. Rev. Cell. Mol. Biol. 2016, 327, 43–87. [Google Scholar] [CrossRef] [PubMed]
- Letai, A. Apoptosis and cancer. Annu. Rev. Cancer Biol. 2017, 1, 275–294. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Hansen, J.M. Oxidative stress, thiols, and redox profiles. Methods Mol. Biol. 2012, 889, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Karasaki, M.; Ochiai, T.; Suzuki-Karasaki, Y. Crosstalk between mitochondrial ROS and depolarization in the potentiation of TRAIL-induced apoptosis in human tumor cells. Int. J. Oncol. 2014, 44, 616–628. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Liu, W.T.; Lin, C.H.; Hsiao, M.; Gean, P.W. Minocycline inhibits the growth of glioma by inducing autophagy. Autophagy 2011, 7, 166–175. [Google Scholar] [CrossRef]
- Song, H.; Fares, M.; Maguire, K.R.; Sidén, A.; Potácová, Z. Cytotoxic effects of tetracycline analogues (doxycycline, minocycline and COL-3) in acute myeloid leukemia HL-60 cells. PLoS ONE 2014, 9, e114457. [Google Scholar] [CrossRef]
- Scarabelli, T.M.; Stephanou, A.; Pasini, E.; Gitti, G.; Townsend, P.; Lawrence, K.; Chen-Scarabelli, C.; Saravolatz, L.; Latchman, D.; Knight, R.; et al. Minocycline inhibits caspase activation and reactivation, increases the ratio of XIAP to smac/DIABLO, and reduces the mitochondrial leakage of cytochrome C and smac/DIABLO. J. Am. Coll. Cardiol. 2004, 43, 865–874. [Google Scholar] [CrossRef]
- Chang, C.J.; Cherng, C.H.; Liou, W.S.; Liao, C.L. Minocycline partially inhibits caspase-3 activation and photoreceptor degeneration after photic injury. Ophthalmic Res. 2005, 37, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, S.; Drozda, M.; Zhang, W.; Stavrovskaya, I.G.; Cattaneo, E.; Ferrante, R.J.; Kristal, B.S.; Friedlander, R.M. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 10483–10487. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rok, J.; Rzepka, Z.; Beberok, A.; Pawlik, J.; Wrześniok, D. Cellular and Molecular Aspects of Anti-Melanoma Effect of Minocycline—A Study of Cytotoxicity and Apoptosis on Human Melanotic Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 6917. https://doi.org/10.3390/ijms21186917
Rok J, Rzepka Z, Beberok A, Pawlik J, Wrześniok D. Cellular and Molecular Aspects of Anti-Melanoma Effect of Minocycline—A Study of Cytotoxicity and Apoptosis on Human Melanotic Melanoma Cells. International Journal of Molecular Sciences. 2020; 21(18):6917. https://doi.org/10.3390/ijms21186917
Chicago/Turabian StyleRok, Jakub, Zuzanna Rzepka, Artur Beberok, Justyna Pawlik, and Dorota Wrześniok. 2020. "Cellular and Molecular Aspects of Anti-Melanoma Effect of Minocycline—A Study of Cytotoxicity and Apoptosis on Human Melanotic Melanoma Cells" International Journal of Molecular Sciences 21, no. 18: 6917. https://doi.org/10.3390/ijms21186917
APA StyleRok, J., Rzepka, Z., Beberok, A., Pawlik, J., & Wrześniok, D. (2020). Cellular and Molecular Aspects of Anti-Melanoma Effect of Minocycline—A Study of Cytotoxicity and Apoptosis on Human Melanotic Melanoma Cells. International Journal of Molecular Sciences, 21(18), 6917. https://doi.org/10.3390/ijms21186917





