A Rare Natural Benzo[k,l]xanthene as a Turn-Off Fluorescent Sensor for Cu2+ Ion
Abstract
:1. Introduction
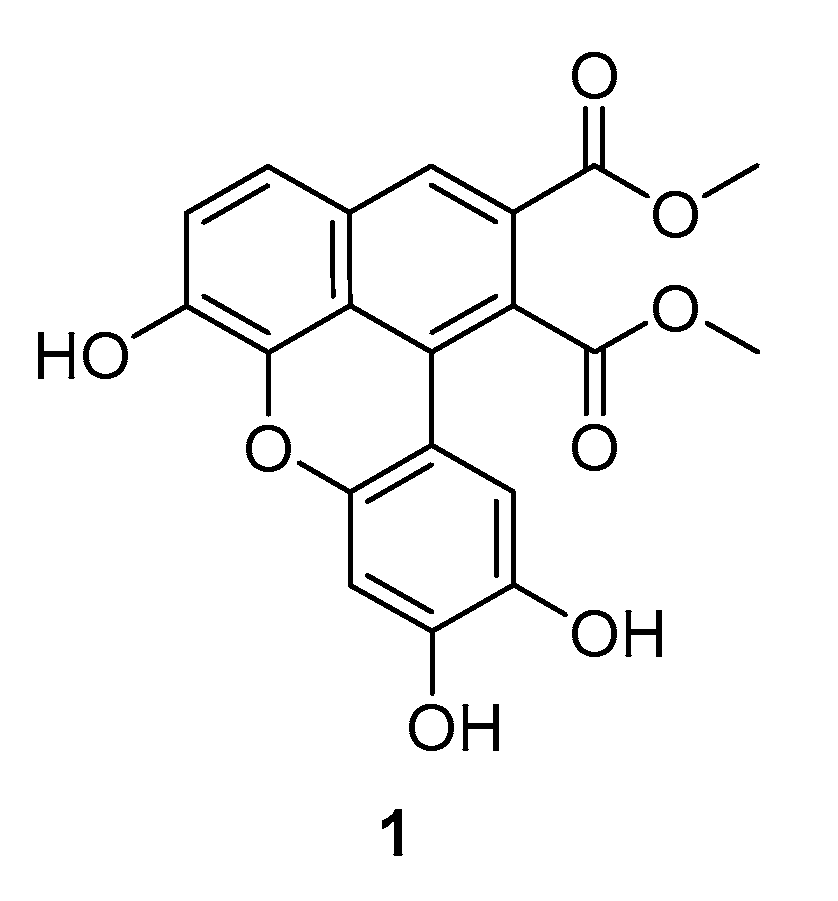
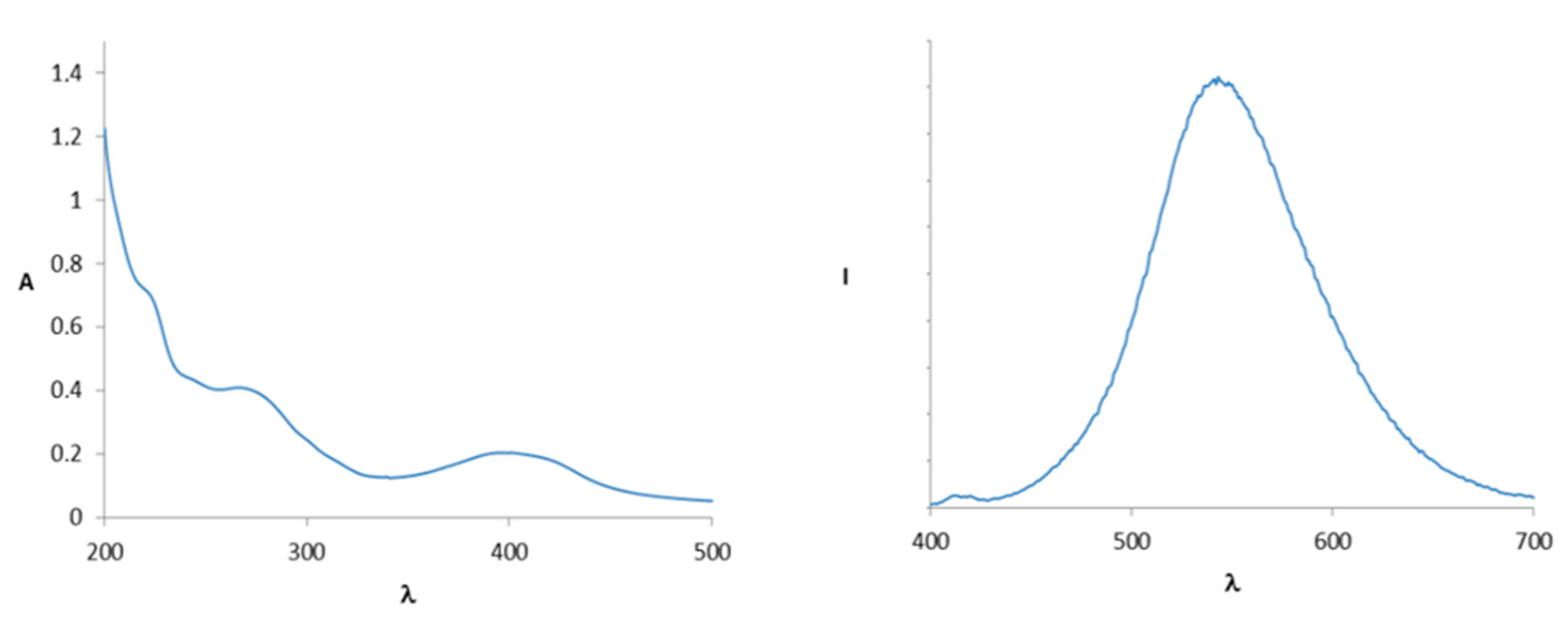
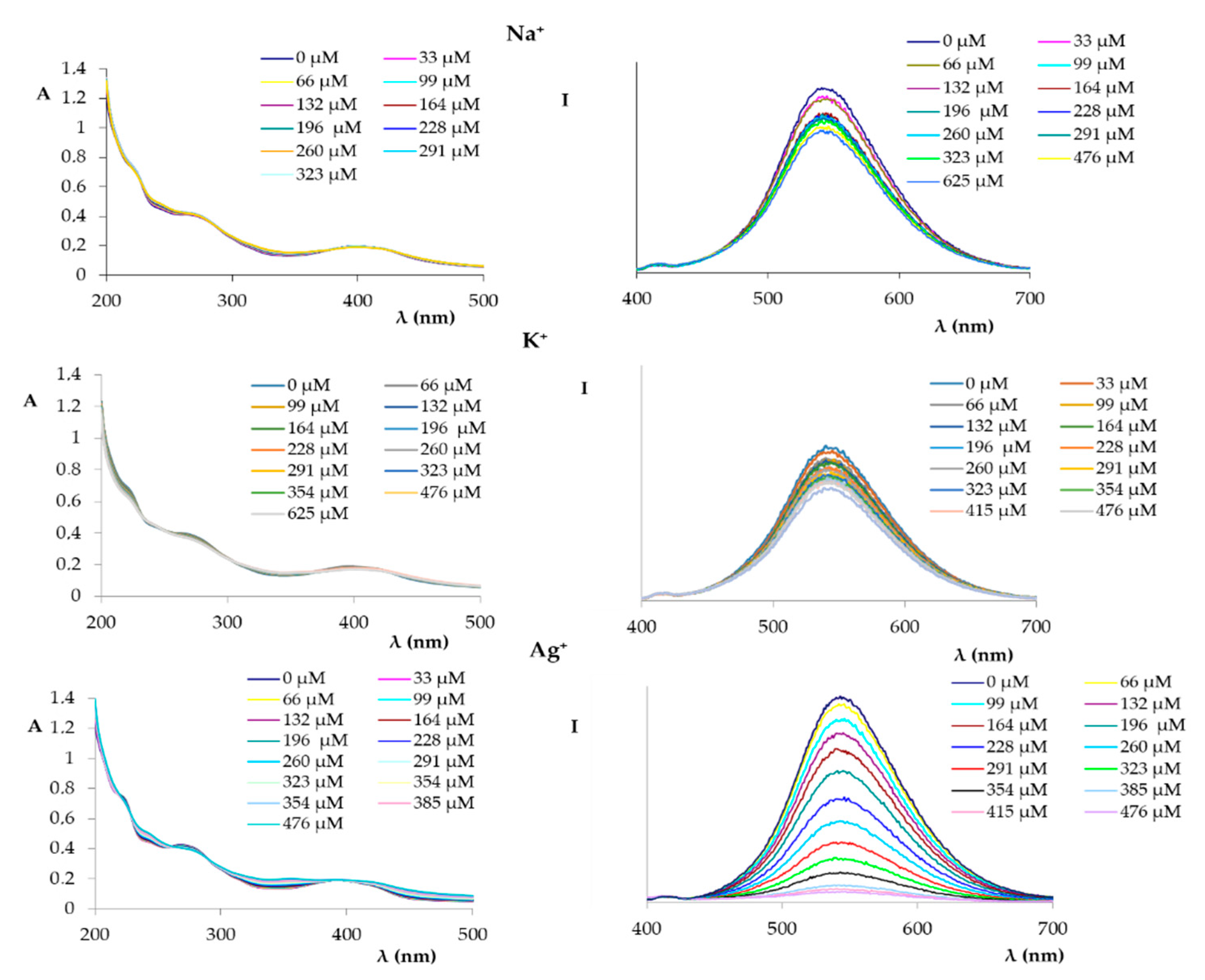
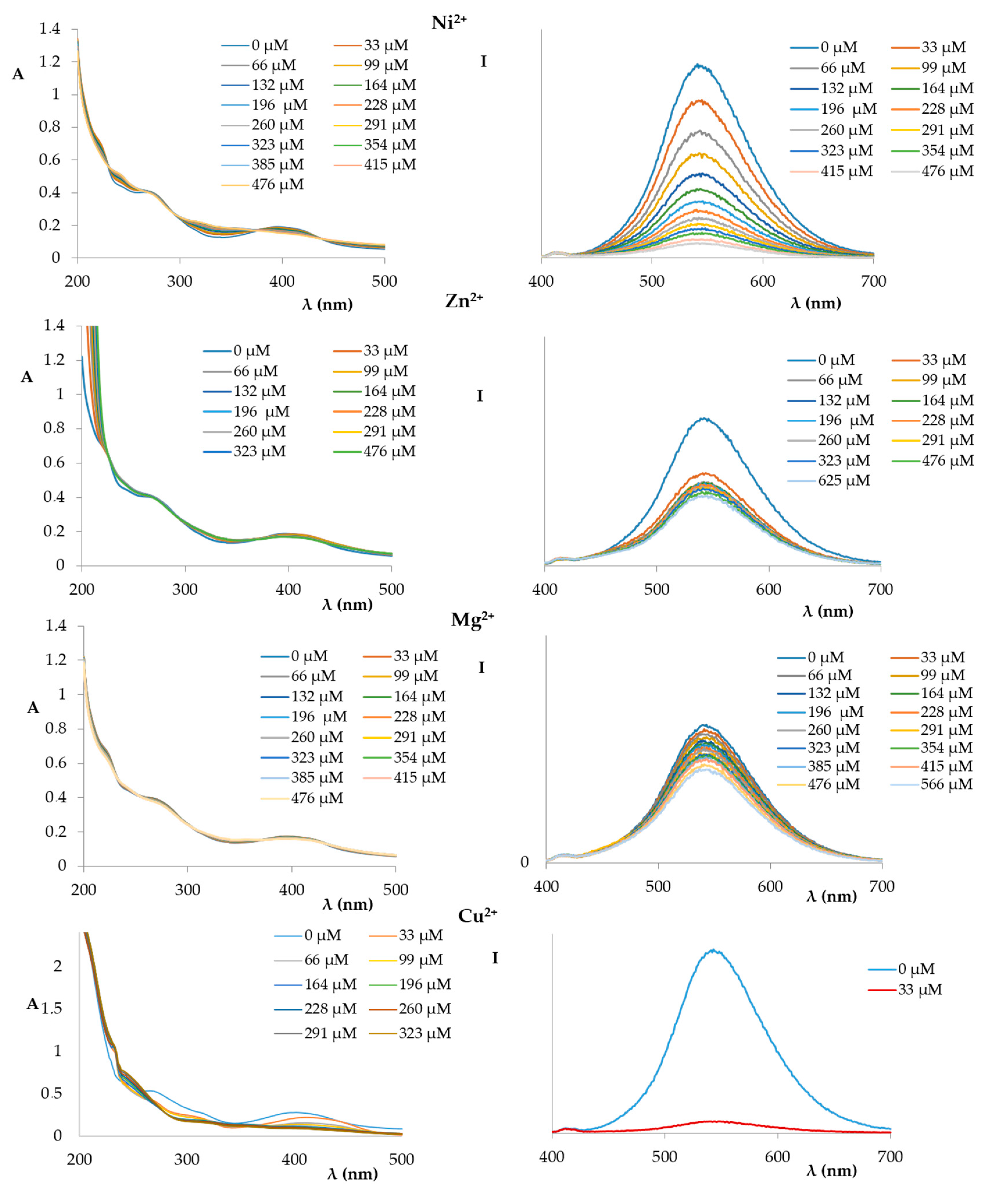
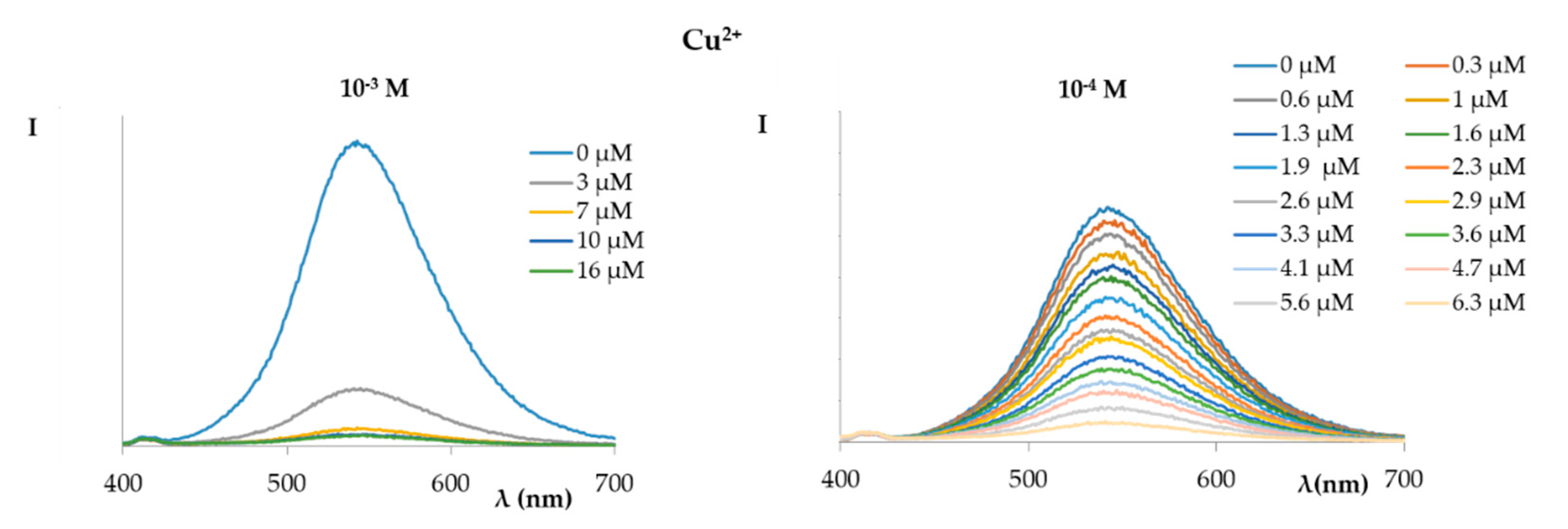
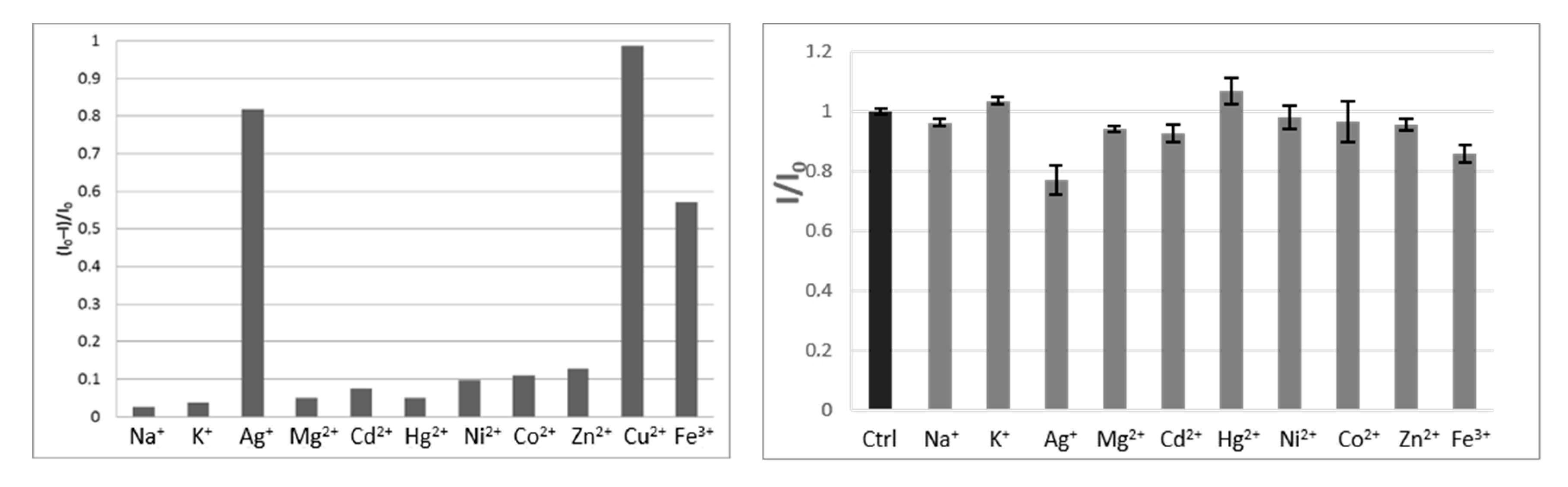
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Methyl Ester of Caffeic Acid
3.2. Preparation of Dimethyl 6,9,10-Trihydroxybenzo[k,l]Xanthene-1,2-Dicarboxylate (1)
3.3. Preparation of UV-Vis and Fluorometric Titration Solutions
3.4. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [Green Version]
- Multhaup, G.; Schlicksupp, A.; Hesse, L.; Beher, D.; Ruppert, T.; Masters, C.L.; Beyreuther, K. The amyloid precursor protein of Alzheimer’s disease in the reduction of copper(II) to copper(I). Science 1996, 271, 1406–1409. [Google Scholar] [CrossRef]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef]
- Zong, L.Y.; Song, Y.C.; Li, Q.Q.; Li, Z. A “turn-on” fluorescence probe towards copper ions based on core-substitued naphthalene diimide. Sens. Actuators B-Chem. 2016, 226, 239–244. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.W.; Qian, S.Y.; Lu, G.; Chen, J.L. A selective fluorescent chemosensor with 1,2,4-triazole as subunit for Cu (II) and its application in imaging Cu (II) in living cells. Dyes Pigments 2012, 92, 1370–1375. [Google Scholar] [CrossRef]
- Wang, H.H.; Xue, L.; Fang, Z.J.; Li, G.P.; Jiang, H. A colorimetric and fluorescent chemosensor for copper ions in aqueous media and its application in living cells. New J. Chem. 2010, 34, 1239–1242. [Google Scholar] [CrossRef]
- Lu, D.L.; Lei, J.Y.; Tian, Z.D.; Wang, L.Z.; Zhang, J.L. Cu2+ fluorescent sensor based on mesoporous silica nanosphere. Dyes Pigments 2012, 94, 239–246. [Google Scholar] [CrossRef]
- Meng, Q.T.; Shi, Y.; Wang, C.P.; Jia, H.M.; Gao, X.; Zhang, R.; Wang, Y.F.; Zhang, Z.Q. NBD-based fluorescent chemosensor for the selective quantification of copper and sulfide in an aqueous solution and living cells. Org. Biomol. Chem. 2015, 13, 2918–2926. [Google Scholar] [CrossRef]
- Daquino, C.; Rescifina, A.; Spatafora, C.; Tringali, C. Biomimetic synthesis of natural and “unnatural” lignans by oxidative coupling of caffeic esters. Eur. J. Org. Chem. 2009, 2009, 6289–6300. [Google Scholar] [CrossRef]
- Bhusainahalli, V.M.; Spatafora, C.; Chalal, M.; Vervandier-Fasseur, D.; Meunier, P.; Latruffe, N.; Tringali, C. Resveratrol-related dehydrodimers: Laccase-mediated biomimetic synthesis and antiproliferative activity. Eur. J. Org. Chem. 2012, 2012, 5217–5224. [Google Scholar] [CrossRef]
- Cardullo, N.; Pulvirenti, L.; Spatafora, C.; Musso, N.; Barresi, V.; Condorelli, D.F.; Tringalii, C. Dihydrobenzofuran neolignanamides: Lactase-mediated biomimetic synthesis and antiproliferative activity. J. Nat. Prod. 2016, 79, 2122–2134. [Google Scholar] [CrossRef]
- Di Micco, S.; Spatafora, C.; Cardullo, N.; Riccio, R.; Fischer, K.; Pergola, C.; Koeberle, A.; Werz, O.; Chalal, M.; Vervandier-Fasseur, D.; et al. 2,3-Dihydrobenzofuran privileged structures as new bioinspired lead compounds for the design of mPGES-1 inhibitors. Bioorg. Med. Chem. 2016, 24, 820–826. [Google Scholar] [CrossRef]
- Pulvirenti, L.; Muccilli, V.; Cardullo, N.; Spatafora, C.; Tringali, C. Chemoenzymatic synthesis and alpha-glucosidase inhibitory activity of dimeric neolignans inspired by magnolol. J. Nat. Prod. 2017, 80, 1648–1657. [Google Scholar] [CrossRef]
- Baschieri, A.; Pulvirenti, L.; Muccilli, V.; Amorati, R.; Tringali, C. Chain-breaking antioxidant activity of hydroxylated and methoxylated magnolol derivatives: The role of H-bonds. Org. Biomol. Chem. 2017, 15, 6177–6184. [Google Scholar] [CrossRef]
- Di Micco, S.; Pulvirenti, L.; Bruno, I.; Terracciano, S.; Russo, A.; Vaccaro, M.C.; Ruggiero, D.; Muccilli, V.; Cardullo, N.; Tringali, C.; et al. Identification by Inverse Virtual Screening of magnolol-based scaffold as new tankyrase-2 inhibitors. Bioorg. Med. Chem. 2018, 26, 3953–3957. [Google Scholar] [CrossRef]
- Cardullo, N.; Barresi, V.; Muccilli, V.; Spampinato, G.; D’Amico, M.; Condorelli, D.F.; Tringali, C. Synthesis of bisphenol neolignans inspired by honokiol as antiproliferative agents. Molecules 2020, 25, 733. [Google Scholar] [CrossRef] [Green Version]
- Spatafora, C.; Barresi, V.; Bhusainahalli, V.M.; Di Micco, S.; Musso, N.; Riccio, R.; Bifulco, G.; Condorelli, D.; Tringali, C. Bio-inspired benzo[k,l]xanthene lignans: Synthesis, DNA-interaction and antiproliferative properties. Org. Biomol. Chem. 2014, 12, 2686–2701. [Google Scholar] [CrossRef]
- Qu, Z.Y.; Zhang, Y.W.; Yao, C.L.; Jin, Y.P.; Zheng, P.H.; Sun, C.H.; Liu, J.X.; Wang, Y.S.; Wang, Y.P. Chemical constituents from Orobanche cernua Loefling. Biochem. Syst. Ecol. 2015, 60, 199–203. [Google Scholar] [CrossRef]
- Genovese, C.; Pulvirenti, L.; Cardullo, N.; Muccilli, V.; Tempera, G.; Nicolosi, D.; Tringali, C. Bioinspired benzoxanthene lignans as a new class of antimycotic agents: Synthesis and Candida spp. growth inhibition. Nat. Prod. Res. 2020, 34, 1653–1662. [Google Scholar] [CrossRef]
- Gerstmeier, J.; Kretzer, C.; Di Micco, S.; Miek, L.; Butschek, H.; Cantone, V.; Bilancia, R.; Rizza, R.; Troisi, F.; Cardullo, N.; et al. Novel benzoxanthene lignans that favorably modulate lipid mediator biosynthesis: A promising pharmacological strategy for anti-inflammatory therapy. Biochem. Pharmacol. 2019, 165, 263–274. [Google Scholar] [CrossRef]
- Di Micco, S.; Mazue, F.; Daquino, C.; Spatafora, C.; Delmas, D.; Latruffe, N.; Tringali, C.; Riccio, R.; Bifulco, G. Structural basis for the potential antitumour activity of DNA-interacting benzo[kl]xanthene lignans. Org. Biomol. Chem. 2011, 9, 701–710. [Google Scholar] [CrossRef]
- Spatafora, C.; Daquino, C.; Tringali, C.; Amorati, R. Reaction of benzoxanthene lignans with peroxyl radicals in polar and non-polar media: Cooperative behaviour of OH groups. Org. Biomol. Chem. 2013, 11, 4291–4294. [Google Scholar] [CrossRef]
- Capolupo, A.; Tosco, A.; Mozzicafreddo, M.; Tringali, C.; Cardullo, N.; Monti, M.C.; Casapullo, A. Proteasome as a new target for bio-inspired benzo[k,l]xanthene lignans. Chem. Eur. J. 2017, 23, 8371–8374. [Google Scholar] [CrossRef]
- Truter, M.R. Recognition of metal cations by biological systems. Philos. Trans. R. Soc. B Biol. Sci. 1975, 272, 29–41. [Google Scholar]
- De Courcy, B.; Pedersen, L.G.; Parisel, O.; Gresh, N.; Silvi, B.; Pilme, J.; Piquemal, J.P. Understanding selectivity of hard and soft metal cations within biological systems using the subvalence concept. I. Application to blood coagulation: Direct cation-protein electronic effects vs. indirect interactions through water networks. J. Chem. Theory Comput. 2010, 6, 1048–1063. [Google Scholar] [CrossRef] [Green Version]
- Fabbrizzi, L.; Licchelli, M.; Pallavicini, P.; Sacchi, D.; Taglietti, A. Sensing of transition metals through fluorescence quenching or enhancement—A review. Analyst 1996, 121, 1763–1768. [Google Scholar] [CrossRef]
- Li, L.N.; Shen, S.S.; Lin, R.Y.; Bai, Y.; Liu, H.W. Rapid and specific luminescence sensing of Cu(II) ions with a porphyrinic metal-organic framework. Chem. Commun. 2017, 53, 9986–9989. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Pulvirenti, L.; Cornu, A.; Pouysegu, L.; Deffieux, D.; Quideau, S.; Tringali, C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: A study of alpha-glucosidase and alpha-amylase inhibition. Food Chem. 2020, 313, 126099. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Quenching of fluorescence. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 277–328. [Google Scholar]
- Ghosh, K.S.; Sahoo, B.K.; Jana, D.; Dasgupta, S. Studies on the interaction of copper complexes of (-)-epicatechin gallate and (-)-epigallocatechin gallate with calf thymus DNA. J. Inorg. Biochem. 2008, 102, 1711–1718. [Google Scholar] [CrossRef]
- Liao, Q.; Kamerlin, S.C.; Strodel, B. Development and application of a nonbonded Cu(2+) model that Includes the Jahn-Teller effect. J. Phys. Chem. Lett. 2015, 6, 2657–2662. [Google Scholar] [CrossRef]
- Hosain, M.A.; Le Floch, J.M.; Krupka, J.; Tobar, M.E. Aggregate frequency width, nuclear hyperfine coupling and Jahn-Teller effect of Cu(2+) impurity ion ESR in SrLaAlO4 dielectric resonator at 20 millikelvin. J. Phys. Condens. Matter 2018, 30, 015802. [Google Scholar] [CrossRef] [PubMed]
- Manch, W.; Fernelius, W.C. The structure and spectra of nickel(II) and copper(II) complexes. J. Chem. Educ. 1961, 38, 192. [Google Scholar] [CrossRef]
- Hwang, S.M.; Chae, J.B.; Kim, C. A phenanthroimidazole-based fluorescent turn-off chemosensor for the selective detection of Cu2+ in aqueous media. Bull. Korean Chem. Soc. 2018, 39, 925–930. [Google Scholar] [CrossRef]
- Arslan, F.N.; Geyik, G.A.; Koran, K.; Ozen, F.; Aydin, D.; Elmas, S.N.K.; Gorgulu, A.O.; Yilmaz, I. Fluorescence “turn on-off” sensing of copper (II) ions utilizing coumarin-based chemosensor: Experimental study, theoretical calculation, mineral and drinking water analysis. J. Fluoresc. 2020, 30, 317–327. [Google Scholar] [CrossRef]
- Cox, R.M.; Rivera, I.M. Predictability and reliability of hearing aid benefit measured using the PHAB. J. Am. Acad. Audiol. 1992, 3, 242–254. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods IV: Extension of MNDO, AM1, and PM3 to more main group elements. J. Mol. Model. 2004, 10, 155–164. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]




| Atom | O25 | O26 | O65 | O66 | O105 | O106 |
|---|---|---|---|---|---|---|
| Wiberg bond index | 0.1263 | 0.2850 | 0.2300 | 0.2717 | 0.1283 | 0.2850 |
| Donor NBO | Acceptor NBO | Energy (kcal/mol) |
|---|---|---|
| LP O25 | LP* Cu121 | 3.93 |
| LP O25 | LP* Cu121 | 3.81 |
| LP O26 | LP* Cu121 | 13.86 |
| LP O26 | LP* Cu121 | 12.08 |
| LP O65 | LP* Cu121 | 11.28 |
| LP O65 | LP* Cu121 | 10.14 |
| LP O66 | LP* Cu121 | 13.21 |
| LP O66 | LP* Cu121 | 9.71 |
| LP O105 | LP* Cu121 | 4.24 |
| LP O105 | LP* Cu121 | 3.89 |
| LP O106 | LP* Cu121 | 12.87 |
| LP O106 | LP* Cu121 | 12.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floresta, G.; Cardullo, N.; Spatafora, C.; Rescifina, A.; Tringali, C. A Rare Natural Benzo[k,l]xanthene as a Turn-Off Fluorescent Sensor for Cu2+ Ion. Int. J. Mol. Sci. 2020, 21, 6933. https://doi.org/10.3390/ijms21186933
Floresta G, Cardullo N, Spatafora C, Rescifina A, Tringali C. A Rare Natural Benzo[k,l]xanthene as a Turn-Off Fluorescent Sensor for Cu2+ Ion. International Journal of Molecular Sciences. 2020; 21(18):6933. https://doi.org/10.3390/ijms21186933
Chicago/Turabian StyleFloresta, Giuseppe, Nunzio Cardullo, Carmela Spatafora, Antonio Rescifina, and Corrado Tringali. 2020. "A Rare Natural Benzo[k,l]xanthene as a Turn-Off Fluorescent Sensor for Cu2+ Ion" International Journal of Molecular Sciences 21, no. 18: 6933. https://doi.org/10.3390/ijms21186933







