HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription
Abstract
1. Introduction
2. Results
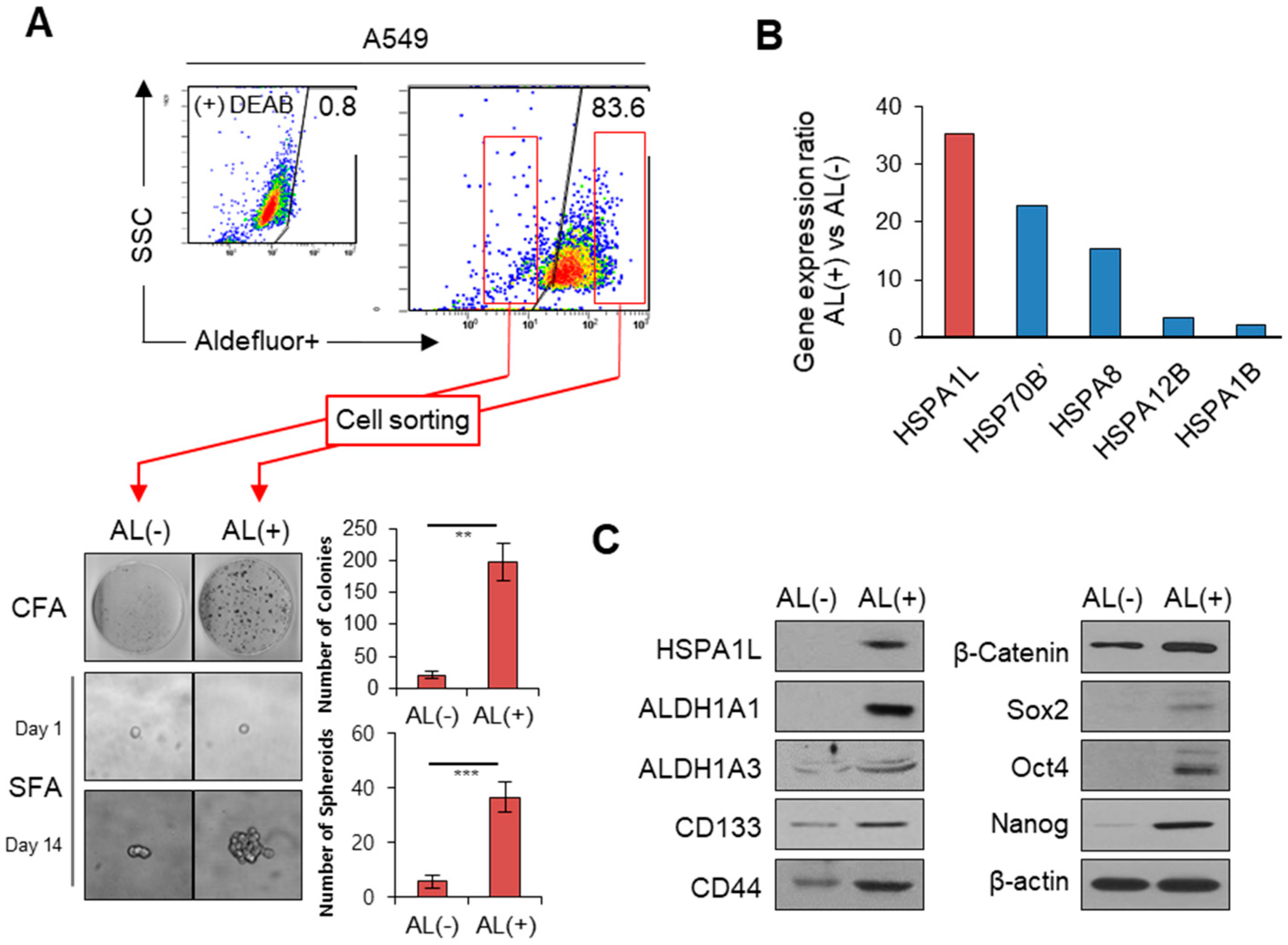
2.1. HSPA1L Was Upregulated in ALDH1high Cells Isolated from A549 Cells
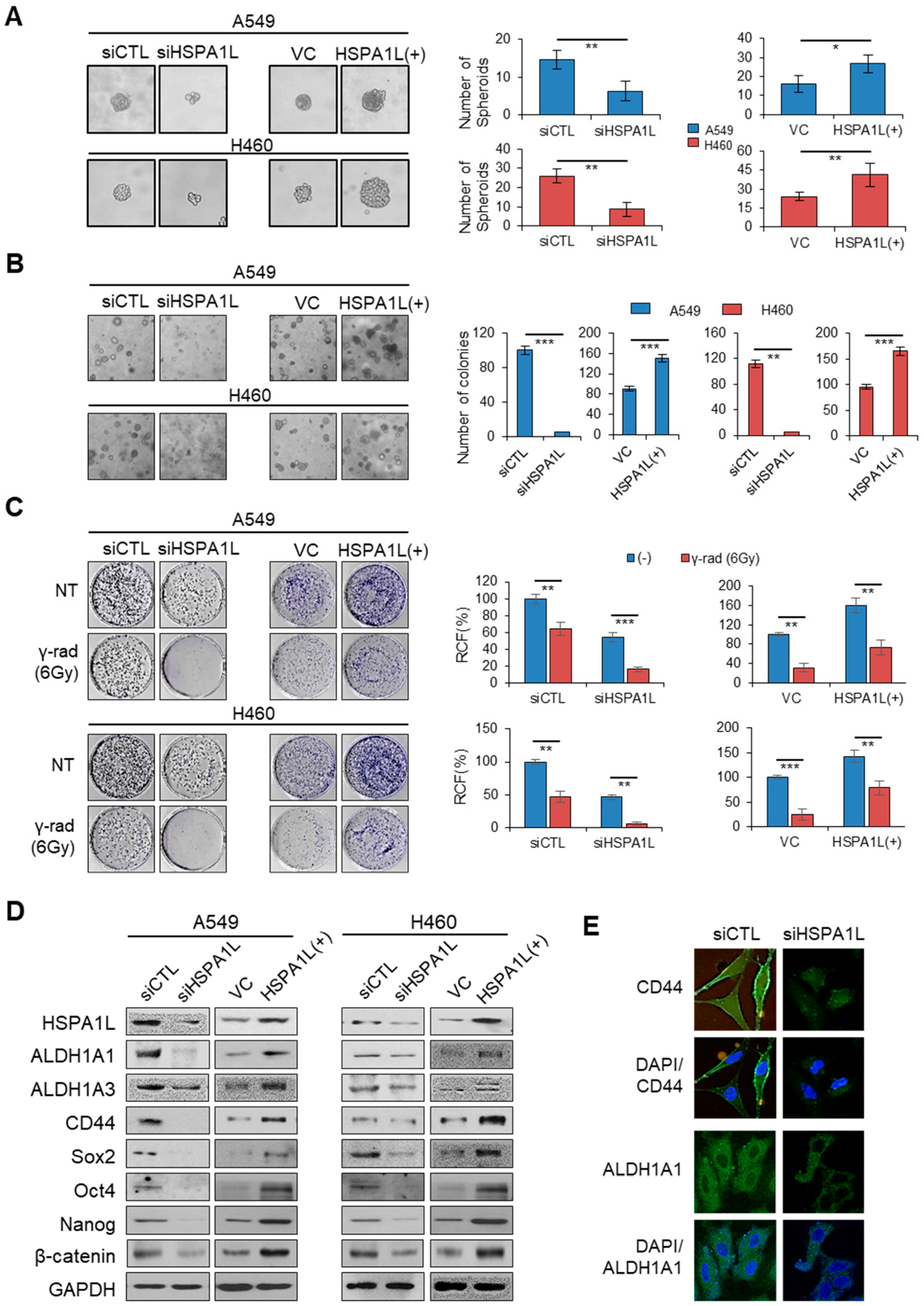
2.2. HSPA1L Promoted Self-Renewal and Tumorigenic Capacity in Lung Cancer Cells
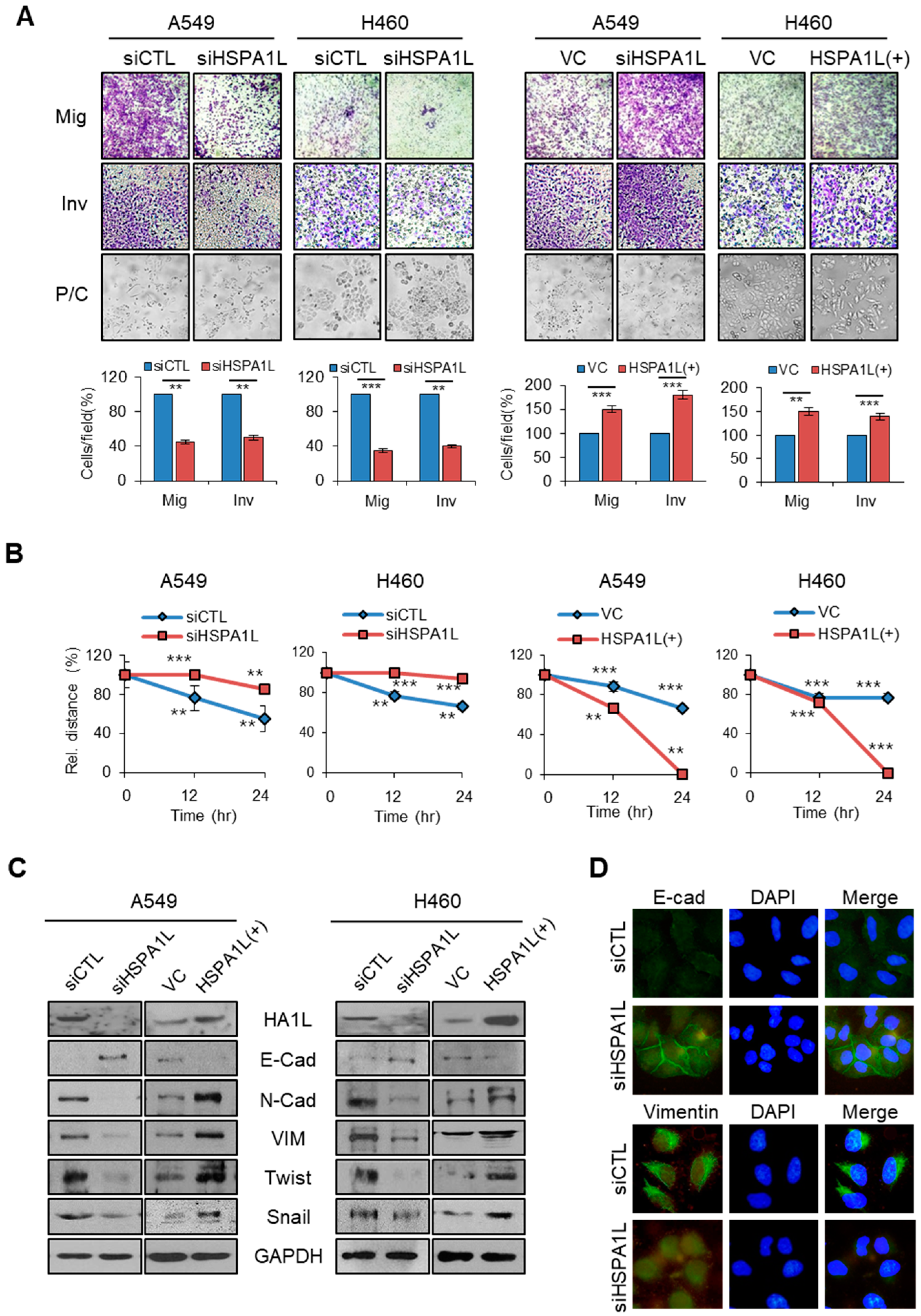
2.3. HSPA1L Promoted Migratory and Invasive Properties in Lung Cancer Cells via Epithelial-Mesenchymal Transition (EMT)
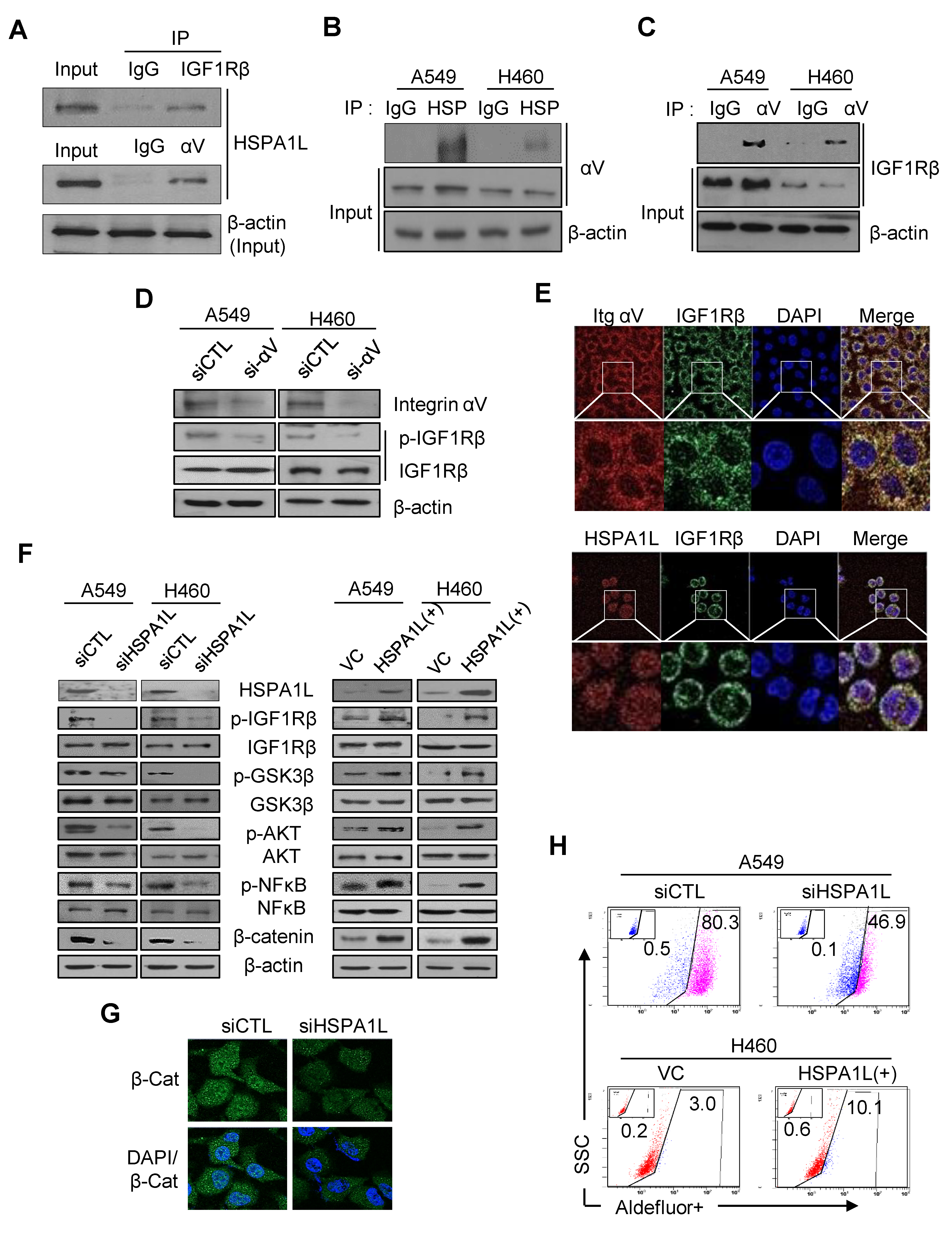
2.4. HSPA1L/Integrin αV Was Involved in the Activation of IGF1Rβ and its Downstream Signals, AKT/NF-κB p65 and AKT/GSK3β/β-Catenin Pathway
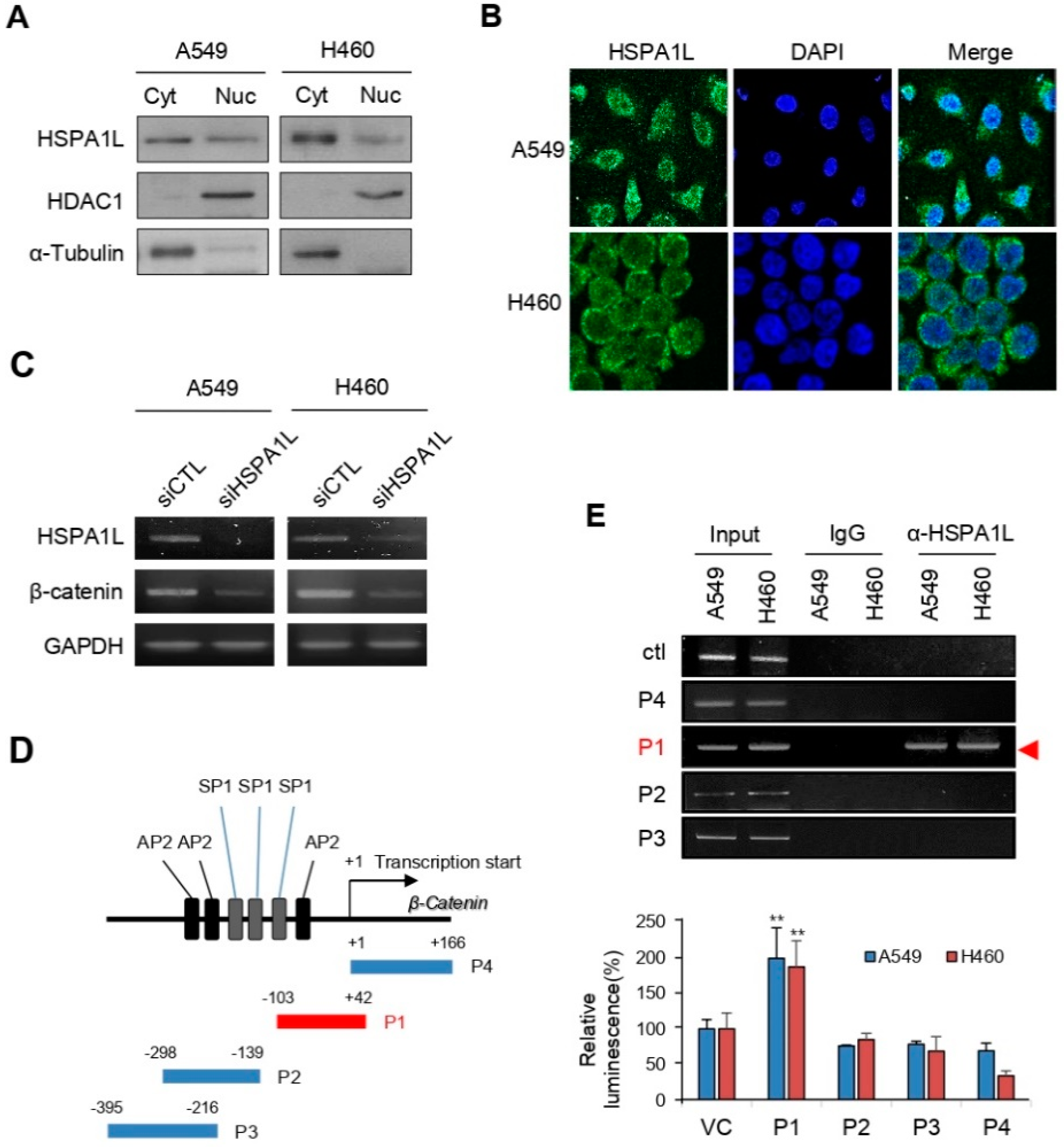
2.5. HSPA1L Bound Directly to the Specific Promoter Region of β-Catenin in the Nucleus and Functioned as a Transcription Activator of β-Catenin
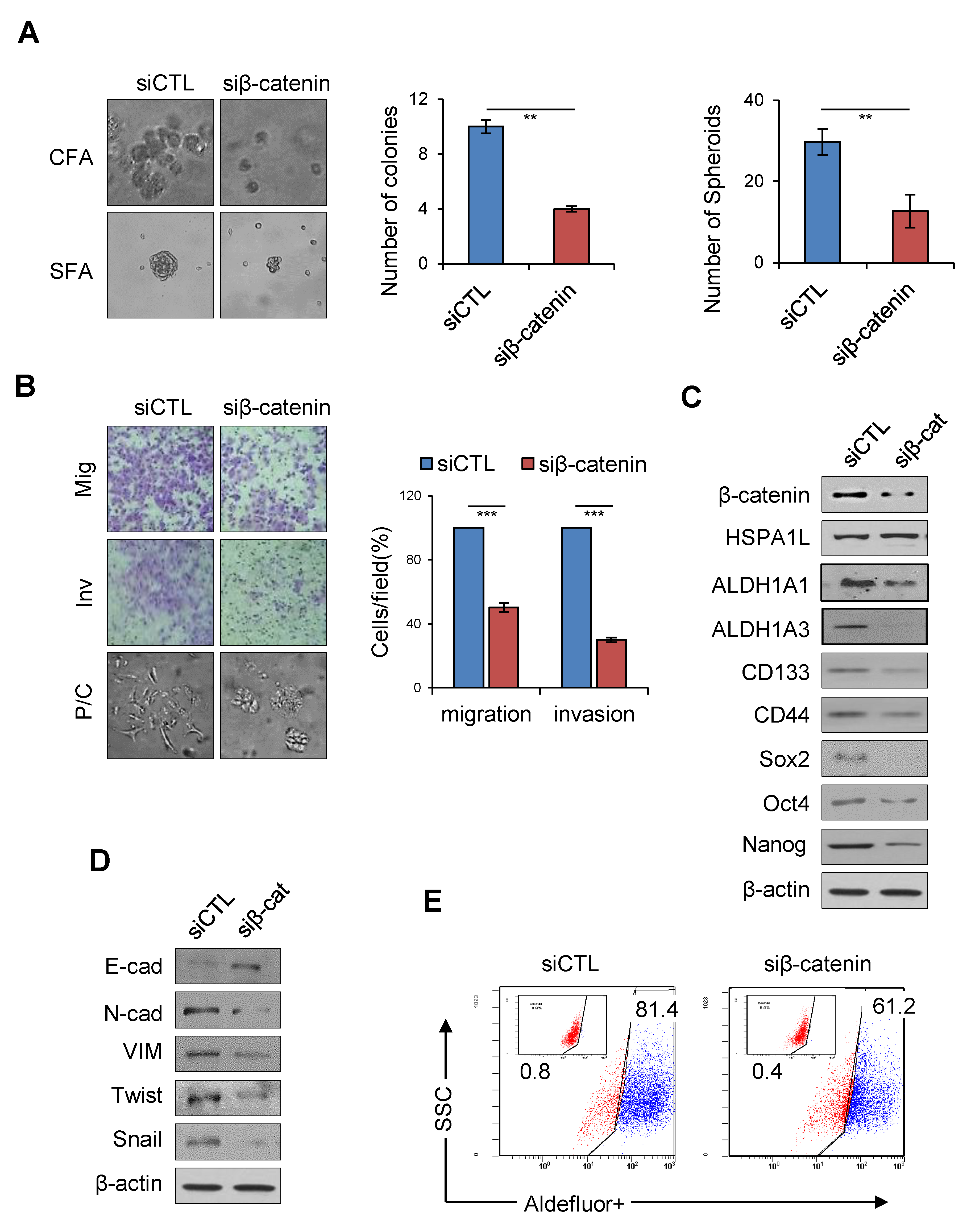
2.6. HSPA1L/β-Catenin Axis Was Involved in EMT-Associated CSC Characteristics by Regulating ALDH1 Expression
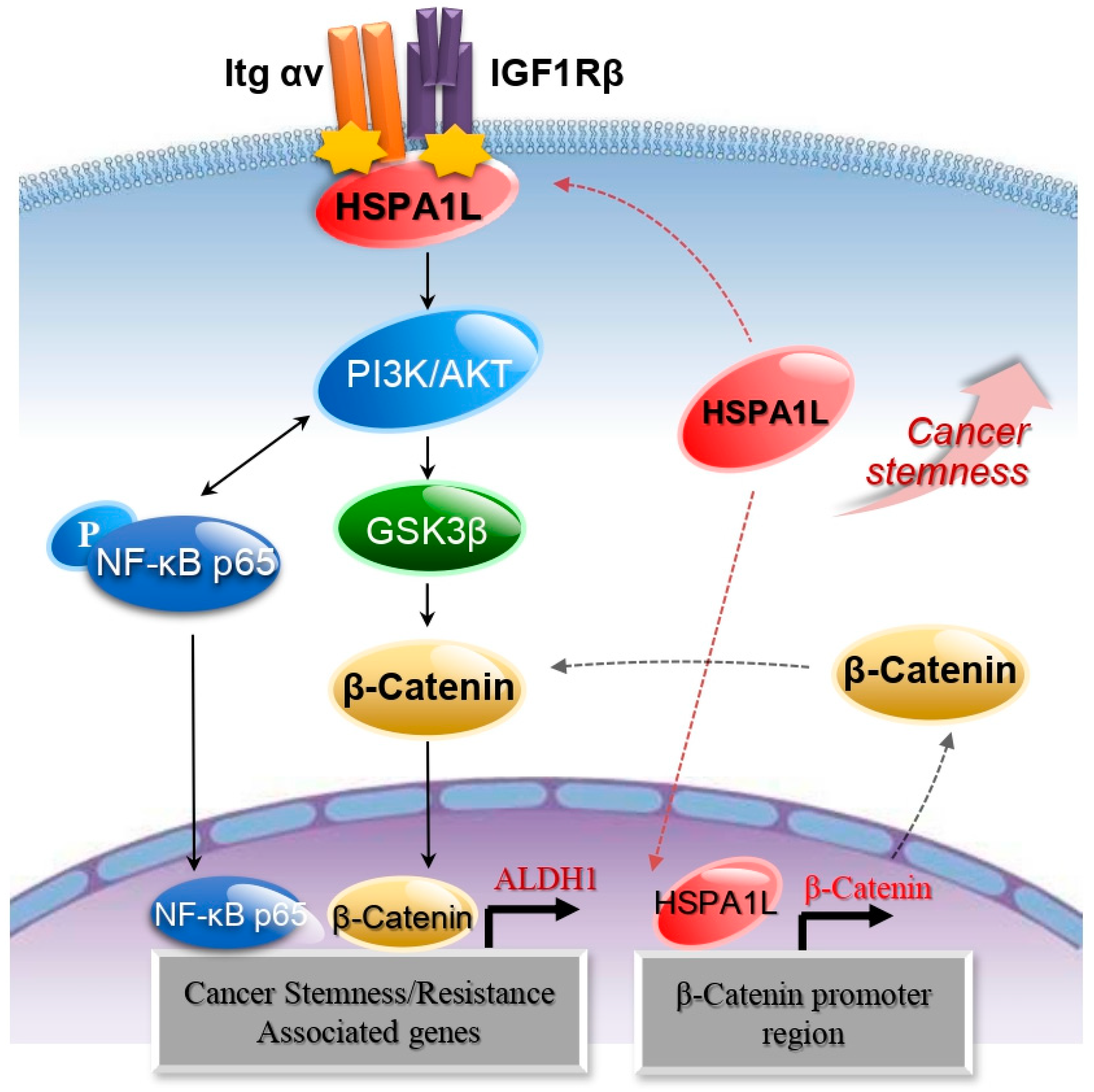
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Small Interfering RNA (siRNA) Transfection
4.3. Construction and Transfection of the HSPA1L Overexpression Vector
4.4. Anchorage-Dependent Colony-Formation (Soft Agar Assay) Assays
4.5. Sphere-Formation Assays
4.6. Wound-Healing Assays
4.7. Migration Assays
4.8. Invasion Assay
4.9. Western Blot Analysis
4.10. ALDEFLUOR Assay and FACS
4.11. Fluorescence Microscopy
4.12. Immunoprecipitation
4.13. Chromatin Immunoprecipitation (ChIP) Assays
4.14. Preparation of Nuclear/Cytoplasmic Fractions
4.15. Soft Agar Colony-Formation Assay
4.16. CSC Sorting from the A549 NSCLC Cell Line
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar] [PubMed]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Han, J.; Marcar, L.; Black, J.; Liu, Q.; Li, X.; Nagulapalli, K.; Sequist, L.V.; Mak, R.H.; Benes, C.H.; et al. Radiation resistance in KRAS-mutated lung cancer is enabled by stem-like properties mediated by an osteopontin–EGFR pathway. Cancer Res. 2017, 77, 2018–2028. [Google Scholar] [CrossRef]
- Maitland, N.J.; Collins, A.T. Prostate cancer stem cells: A new target for therapy. J. Clin. Oncol. 2008, 26, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Shtivelman, E.; Hensing, T.; Simon, G.R.; Dennis, P.A.; Otterson, G.A.; Bueno, R.; Salgia, R. Molecular pathways and therapeutic targets in lung cancer. Oncotarget 2014, 5, 1392. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transnatl. Lung Cancer Res. 2016, 5, 288. [Google Scholar] [CrossRef]
- Mihatsch, J.; Toulany, M.; Bareiss, P.M.; Grimm, S.; Lengerke, C.; Kehlbach, R.; Rodemann, H.P. Selection of radioresistant tumor cells and presence of ALDH1 activity in vitro. Radiother. Oncol. 2011, 99, 300–306. [Google Scholar] [CrossRef]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Reid, L.M. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Weng, J.J.; Cheng, C.T.; Wu, R.C.; Huang, S.C.; Wu, C.E.; Chung, Y.H.; Liu, C.Y.; Chang, M.H.; Chen, M.H.; et al. ALDH1A3, the major aldehyde dehydrogenase isoform in human cholangiocarcinoma cells, affects prognosis and gemcitabine resistance in cholangiocarcinoma patients. Clin. Cancer Res. 2016, 22, 4225–4235. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Schott, A. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem. Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Jiang, F.; Qiu, Q.; Khanna, A.; Todd, N.W.; Deepak, J.; Xing, L.; Katz, R.L. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009, 7, 330–338. [Google Scholar] [CrossRef]
- Bajramović, J.J.; Bsibsi, M.; Geutskens, S.B.; Hassankhan, R.; Verhulst, K.C.; Stege, G.J.; van Noort, J.M. Differential expression of stress proteins in human adult astrocytes in response to cytokines. J. Neuroimmunol. 2000, 106, 14–22. [Google Scholar] [CrossRef]
- Georgopoulos, C.; Welch, W.J. Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 1993, 9, 601–634. [Google Scholar] [CrossRef]
- Anderton, S.M.; van der Zee, R.; Prakken, B.; Noordzij, A.; van Eden, W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J. Exp. Med. 1995, 181, 943–952. [Google Scholar] [CrossRef]
- Stocki, P.; Wang, X.N.; Dickinson, A.M. Inducible heat shock protein 70 reduces T cell responses and stimulatory capacity of monocyte-derived dendritic cells. J. Biol. Chem. 2012, 287, 12387–12394. [Google Scholar] [CrossRef]
- Malago, J.J.; Koninkx, J.F.; van Dijk, J.E. The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones. 2002, 7, 191. [Google Scholar] [CrossRef]
- Qu, B.; Jia, Y.; Liu, Y.; Wang, H.; Ren, G.; Wang, H. The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: A literature review. Cell Stress Chaperones. 2015, 20, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Brocchieri, L.; de Macario, E.C.; Macario, A.J. HSP70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 2008, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Vydra, N.; Malusecka, E.; Jarzab, M.; Lisowska, K.; Glowala-Kosinska, M.; Benedyk, K.; Widlak, W. Spermatocyte-specific expression of constitutively active heat shock factor 1 induces HSP70i-resistant apoptosis in male germ cells. Cell Death Differ. 2006, 13, 212–222. [Google Scholar] [CrossRef]
- Williams, P.A.; Kobilnyk, H.E.; McMillan, E.A.; Strochlic, T.I. MAPKAP kinase 2–mediated phosphorylation of HspA1L protects male germ cells from heat stress–induced apoptosis. Cell Stress Chaperones. 2019, 24, 1127–1136. [Google Scholar] [CrossRef]
- Kohan, L.; Tabiee, O.; Sepahi, N. HSPA1L and HSPA1B gene polymorphisms and haplotypes are associated with idiopathic male infertility in Iranian population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 57–61. [Google Scholar] [CrossRef]
- Ciftci, H.; Celepkolo, B.; Dilmec, F.; Koksal, M.; Yeni, E.; Yagmur, I.; Gumus, K. Genetic polymorphisms of hspa1b and hspa1l in infertile men. J. Pak. Med. Assoc. 2015, 65, 701–704. [Google Scholar]
- Guo, H.; Deng, Q.; Wu, C.; Hu, L.; Wei, S.; Xu, P.; Shen, H. Variations in HSPA1B at 6p21. 3 are associated with lung cancer risk and prognosis in Chinese populations. Cancer Res. 2011, 71, 7576–7586. [Google Scholar] [CrossRef]
- Medhi, S.; Sarma, M.P.; Asim, M.; Kar, P. Genetic variants of heat shock protein A1L2437 and A1B1267 as possible risk factors for hepatocellular carcinoma in India: 420. J. Viral. Hepatitis 2013, 201, e141–e147. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Campbell, L.L. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, Z.; He, J.; Li, L.; Li, W. Insulin-like growth factor receptor 1 (IGF1R) expression and survival in non-small cell lung cancer patients: A meta-analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 6694. [Google Scholar]
- Xue, M.; Cao, X.; Zhong, Y.; Kuang, D.; Liu, X.; Zhao, Z.; Li, H. Insulin-like growth factor-1 receptor (IGF-1R) kinase inhibitors in cancer therapy: Advances and perspectives. Curr. Pharm. Des. 2012, 18, 2901–2913. [Google Scholar] [CrossRef] [PubMed]
- Hollier, B.G.; Kricker, J.A.; van Lonkhuyzen, D.R.; Leavesley, D.I.; Upton, Z. Substrate-bound insulin-like growth factor (IGF)-I-IGF binding protein-vitronectin-stimulated breast cell migration is enhanced by coactivation of the phosphatidylinositide 3-kinase/AKT pathway by αv-integrins and the IGF-I receptor. Endocrinology 2008, 149, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.R.; Cho, E.W.; Paik, S.G.; Kim, I.G. Hypoxia-induced SM22α in A549 cells activates the IGF1R/PI3K/Akt pathway, conferring cellular resistance against chemo-and radiation therapy. FEBS Lett. 2012, 586, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.Y.; Kim, S.Y.; Choi, S.I.; Kim, K.C.; Cho, E.W.; Kim, I.G. APBB1 reinforces cancer stem cell and epithelial-to-mesenchymal transition by regulating the IGF1R signaling pathway in non-small-cell lung cancer cells. Biochem. Biophys. Res. Commun. 2017, 482, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, B.; Huang, C. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr. Cancer Drug Targets 2007, 7, 305–316. [Google Scholar] [CrossRef]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.C. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef]
- Kotoglou, P.; Kalaitzakis, A.; Vezyraki, P.; Tzavaras, T.; Michalis, L.K.; Dantzer, F.; Angelidis, C. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones 2009, 14, 391–406. [Google Scholar] [CrossRef]
- Cojoc, M.; Peitzsch, C.; Kurth, I.; Trautmann, F.; Kunz-Schughart, L.A.; Telegeev, G.D.; Fuessel, S. Aldehyde dehydrogenase is regulated by β-catenin/TCF and promotes radioresistance in prostate cancer progenitor cells. Cancer Res. 2015, 75, 1482–1494. [Google Scholar] [CrossRef]
- Nomura, M.; Rainusso, N.; Lee, Y.C.; Dawson, B.; Coarfa, C.; Han, R.; Yustein, J.T. Tegavivint and the β-catenin/ALDH axis in chemotherapy-resistant and metastatic osteosarcoma. JNCI J. Natl. Cancer Inst. 2019, 111, 1216–1227. [Google Scholar] [CrossRef]
- Nollet, F.; Berx, G.; Molemans, F.; van Roy, F. Genomic organization of the human β-catenin gene (CTNNB1). Genomics 1996, 32, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Kucia, K.; Owczarek, A.; Suchanek-Raif, R.; Merk, W.; Paul-Samojedny, M.; Kowalski, J. Association Studies of HSPA1A and HSPA1L gene polymorphisms with schizophrenia. Arch. Med. Res. 2018, 49, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Clarimón, J.; Bertranpetit, J.; Boada, M.; Tàrraga, L.; Comas, D. HSP70-2 (HSPA1B) is associated with noncognitive symptoms in late-onset Alzheimer’s disease. J. Geriatr. Psych. Neur. 2003, 16, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat shock proteins: Agents of cancer development and therapeutic targets in anti-cancer therapy. Cells 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Pockley, A.G.; Schmid, T.E.; Schilling, D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015, 368, 179–184. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, Y.S.; Yoon, Y.M.; Yun, C.W.; Yun, S.P.; Kim, S.M.; Lee, S.J. Role of HSPA1L as a cellular prion protein stabilizer in tumor progression via HIF-1α/GP78 axis. Oncogene 2017, 36, 6555–6567. [Google Scholar] [CrossRef]
- Chen, W.J.; Ho, C.C.; Chang, Y.L.; Chen, H.Y.; Lin, C.A.; Ling, T.Y.; Pan, S.H. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 2014, 5, 1–17. [Google Scholar] [CrossRef]
- Shan, J.; Shen, J.; Liu, L.; Xia, F.; Xu, C.; Duan, G.; Xu, Y.; Ma, Q.; Yang, Z.; Zhang, Q.; et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology 2012, 56, 1004–1014. [Google Scholar] [CrossRef]
- Choi, S.I.; Kim, S.Y.; Lee, J.H.; Cho, E.W.; Kim, I.G. TM4SF4 overexpression in radiation-resistant lung carcinoma cells activates IGF1R via elevation of IGF1. Oncotarget 2014, 5, 9823. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Takada, Y.K.; Fujita, M. Crosstalk between insulin-like growth factor (IGF) receptor and integrins through direct integrin binding to IGF1. Cytokine Growth Factor Rev. 2017, 34, 67–72. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence |
|---|---|
| P1-fw | GCCCCTTGTCCTCGCGCGGCGGAA |
| P1-rv | CCCGGGGCCGGGCCAACGCTGCTG |
| P2-fw | GGGGGCCCGGCCTCCCCGATGCAG |
| P2-rv | CCGCCCAGCAGTCTGCTGTGACGG |
| P3-fw | CTGCAGCTGCTCTCCCGG |
| P3-rv | GTGACGGCTGGCGGCTGC |
| P4-fw | AAGCCTCTCGGTCTGTGG |
| P4-rv | CTCAGGGGAACAGGCTCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-I.; Lee, J.-H.; Kim, R.-K.; Jung, U.; Kahm, Y.-J.; Cho, E.-W.; Kim, I.-G. HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription. Int. J. Mol. Sci. 2020, 21, 6957. https://doi.org/10.3390/ijms21186957
Choi S-I, Lee J-H, Kim R-K, Jung U, Kahm Y-J, Cho E-W, Kim I-G. HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription. International Journal of Molecular Sciences. 2020; 21(18):6957. https://doi.org/10.3390/ijms21186957
Chicago/Turabian StyleChoi, Soo-Im, Jei-Ha Lee, Rae-Kwon Kim, Uhee Jung, Yeon-Jee Kahm, Eun-Wie Cho, and In-Gyu Kim. 2020. "HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription" International Journal of Molecular Sciences 21, no. 18: 6957. https://doi.org/10.3390/ijms21186957
APA StyleChoi, S.-I., Lee, J.-H., Kim, R.-K., Jung, U., Kahm, Y.-J., Cho, E.-W., & Kim, I.-G. (2020). HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription. International Journal of Molecular Sciences, 21(18), 6957. https://doi.org/10.3390/ijms21186957





