Ethionamide Preconditioning Enhances the Proliferation and Migration of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
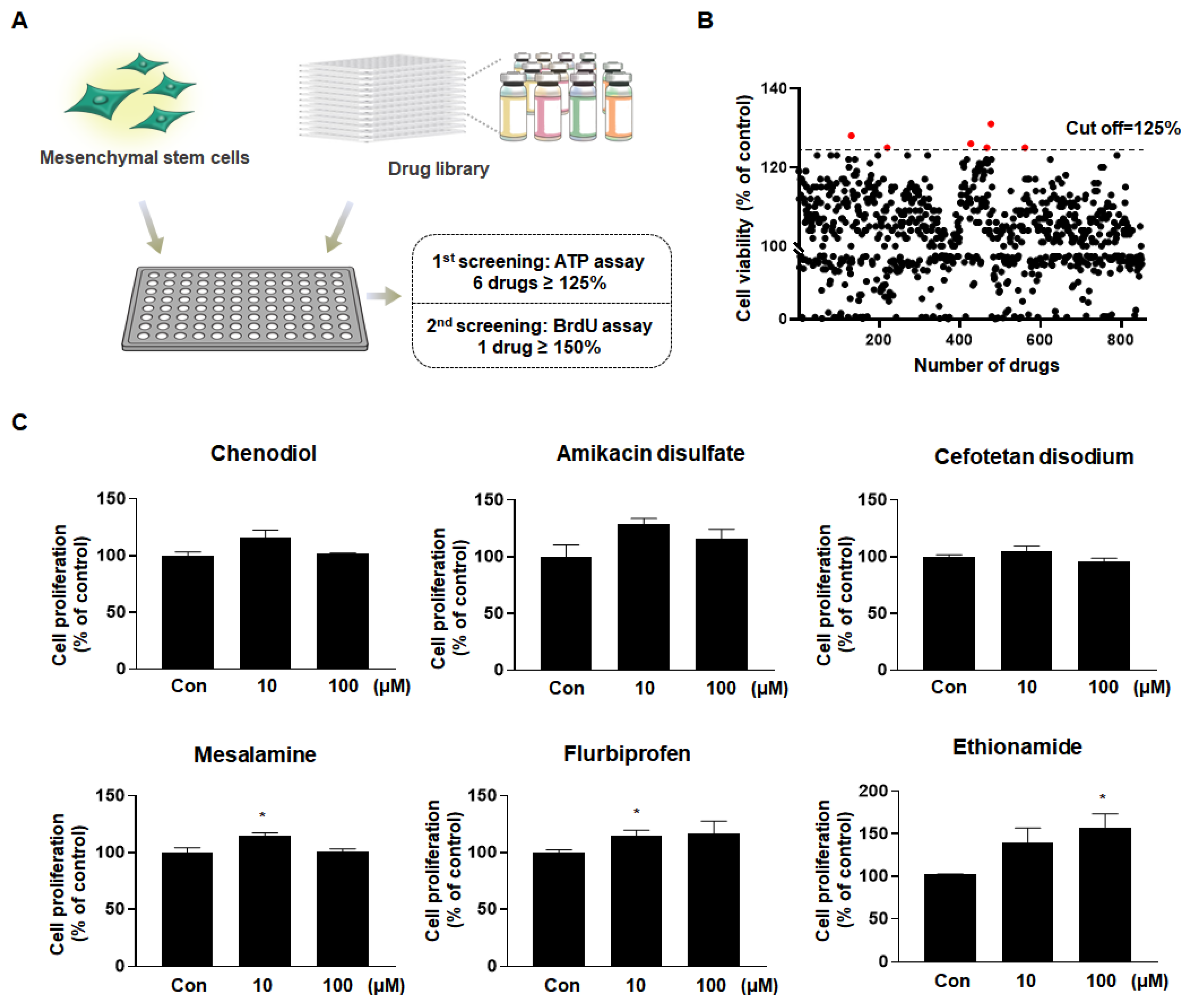
2.1. Ethionamide Was Selected for the Preconditioning of MSCs
2.2. Optimum Preconditioning Condition of Ethionamide Was Determined Based on the Concentration and Incubation Period of Ethionamide
2.3. Ethionamide Enhanced the Proliferation of Mscs via Activation of PI3K/Akt and MEK/ERK1/2 Signaling Pathways
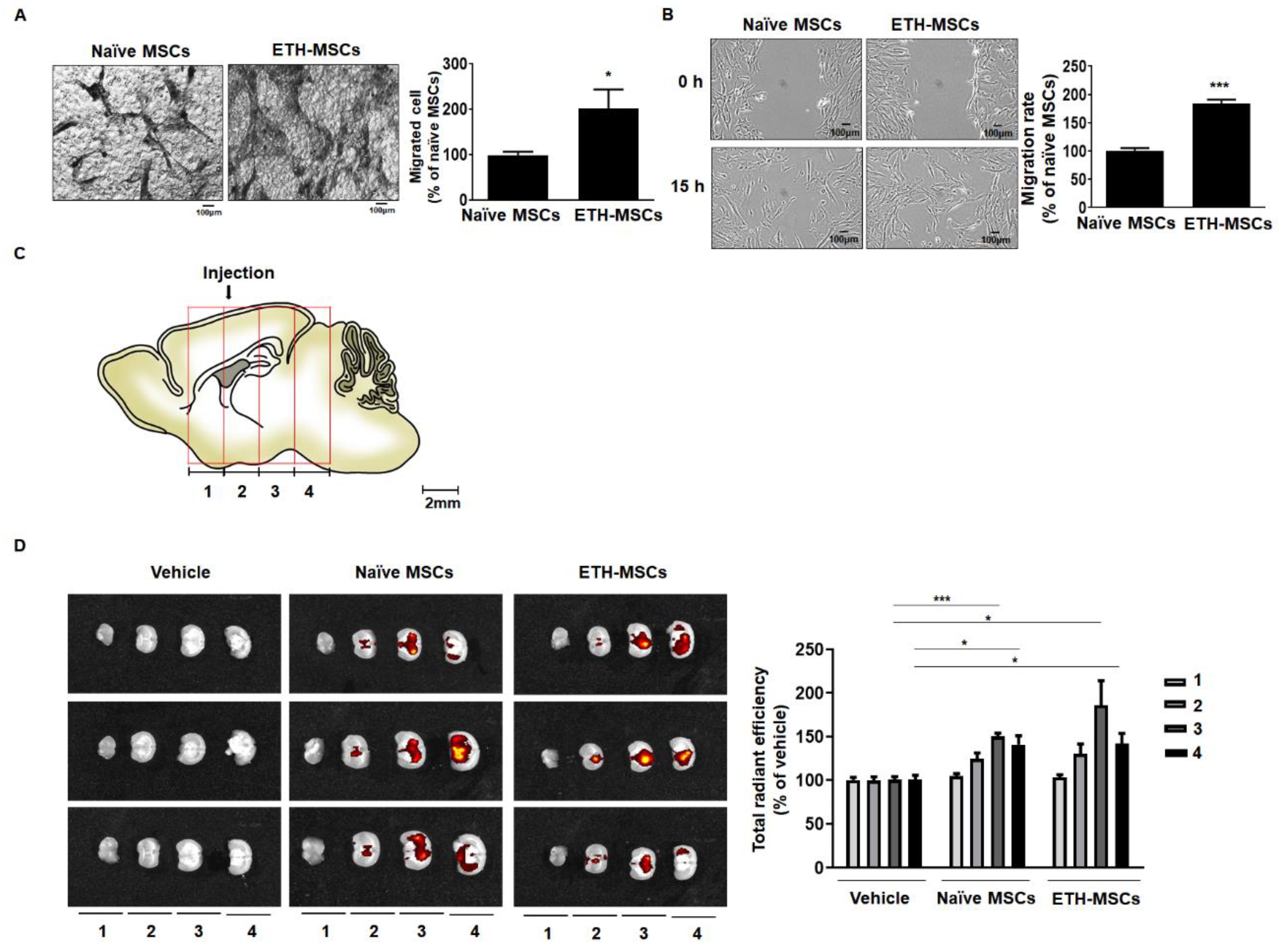
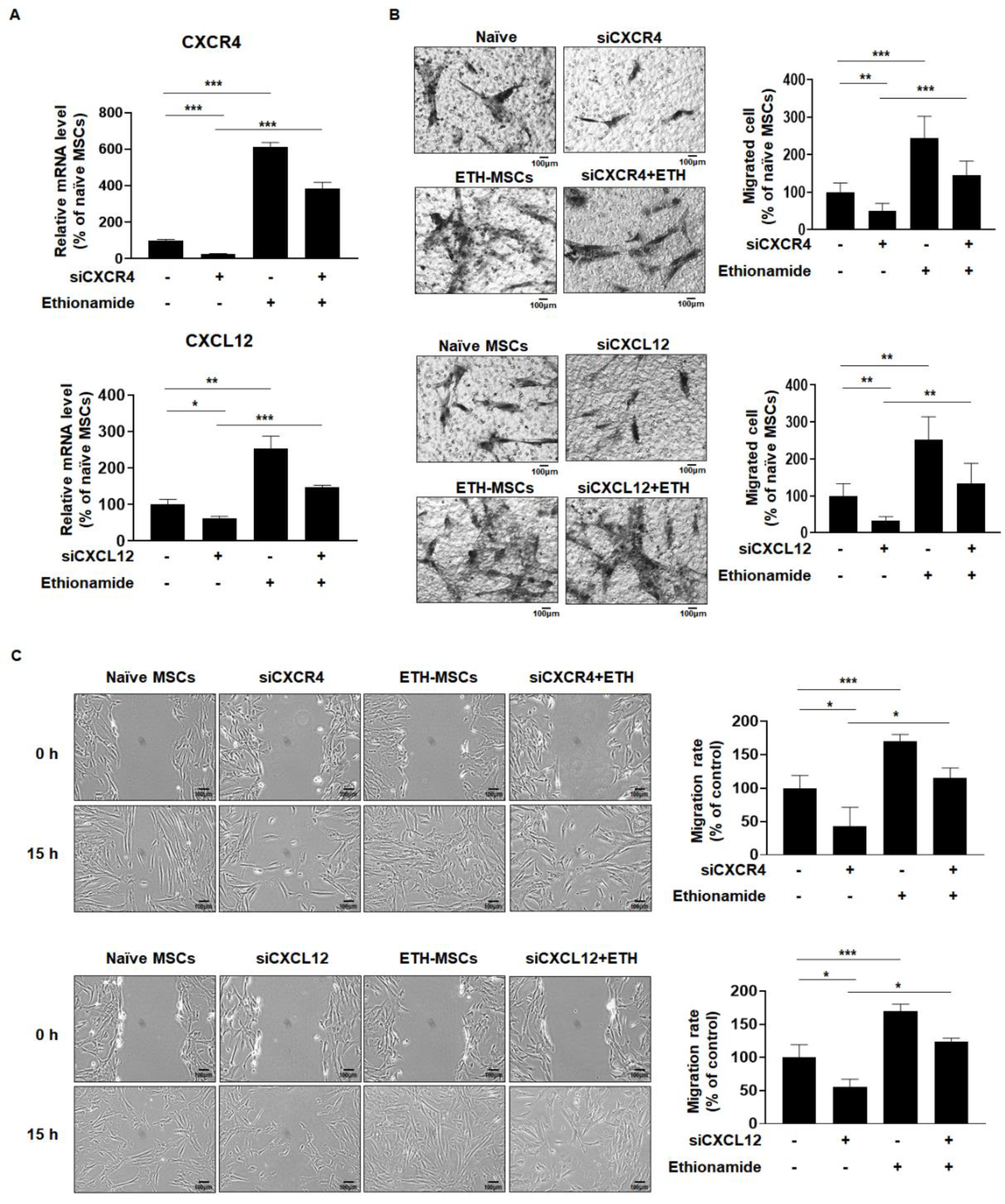
2.4. Ethionamide Increased the Migration Ability of Mscs via Expression of Migration-Related Genes
2.5. Ethionamide Increased the Secretion of Paracrine Factors
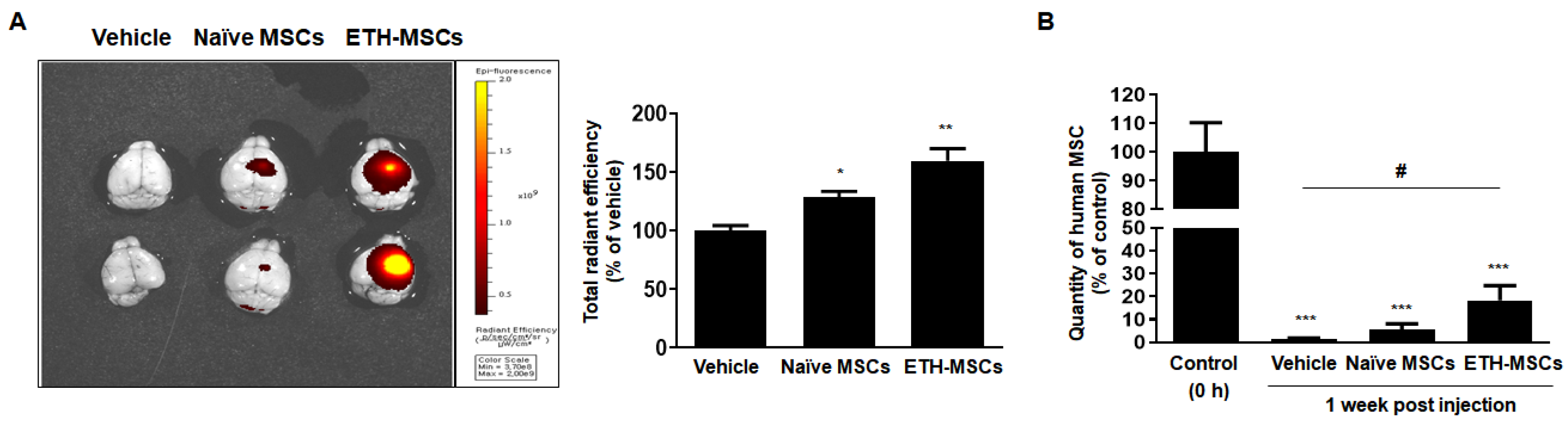
2.6. Ethionamide Enhanced the Survival of Mscs In Vivo
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Cell Culture and Treatment
4.3. Measurement of Cell Viability and Proliferation
4.4. Fluorescence-Activated Cell Sorting (FACS) Analysis
4.5. Differentiation of MSCs
4.6. Western Blotting
4.7. Measurement of Migration In Vitro
4.8. RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Small Interfering RNA
4.10. Quantification of Mscs Migration and Survival In Vivo
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goldring, C.E.P.; Duffy, P.A.; Benvenisty, N.; Andrews, P.W.; Ben-David, U.; Eakins, R.; French, N.; Hanley, N.A.; Kelly, L.; Kitteringham, N.R.; et al. Assessing the safety of stem cell therapeutics. Cell Stem Cell 2011, 8, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar]
- Giachino, C.; Basak, O.; Taylor, V. Stem Cells in Regenerative Medicine Chapter 8. Stem Cells Regen. Med. 2009, 482, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Sibov, T.T.; Severino, P.; Marti, L.C.; Pavon, L.F.; Oliveira, D.M.; Tobo, P.R.; Campos, A.H.; Paes, A.T.; Amaro, E.; Gamarra, L.F.; et al. Mesenchymal stem cells from umbilical cord blood: Parameters for isolation, characterization and adipogenic differentiation. Cytotechnology 2012, 64, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Trubiani, O.; Pizzicannella, J.; Caputi, S.; Marchisio, M.; Mazzon, E.; Paganelli, R.; Paganelli, A.; Diomede, F. Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives. Stem Cells Dev. 2019, 28, 995–1003. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Anscombe G E M—The Collected Philosophical Papers of G E M Anscombe Vol 2 Metaphysics and The Philosophy of Mind (Blackwell,1981). Methods 2005, 45, 1–124. [Google Scholar] [CrossRef]
- Wang, H.-S.; Hung, S.-C.; Peng, S.-T.; Huang, C.-C.; Wei, H.-M.; Guo, Y.-J.; Fu, Y.-S.; Lai, M.-C.; Chen, C.-C. Mesenchymal Stem Cells in the Wharton’s Jelly of the Human Umbilical Cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, G.; Xie, X.; Li, X.; Chen, Y.; De Isla, N.; Huselstein, C.; Stoltz, J.-F.; Li, Y. Immunomodulatory function of mesenchymal stem cells: Regulation and application. J. Cell. Immunother. 2018, 4, 1–3. [Google Scholar] [CrossRef]
- Iyer, S.S.; Rojas, M. Anti-infl ammatory effects of mesenchymal stem cells: Novel. Expert. Opin. Biol. Ther. 2008, 8, 569–582. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.; Bakar, E.; Cerkezkayabekir, A.; Sanal, F.; Ulucam, E.; Subaşı, C.; Karaöz, E. Mesenchymal stem cells increase antioxidant capacity in intestinal ischemia/reperfusion damage. J. Pediatr. Surg. 2017, 52, 1196–1206. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.; Chang, E.H.; Kim, J.H.; Hwang, J.W.; Kim, J.Y.; Kyung, J.W.; Kim, S.H.; Oh, J.S.; Shim, S.M.; et al. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer’s disease model. Stem Cells Dev. 2015, 24, 2378–2390. [Google Scholar] [CrossRef] [Green Version]
- Park, S.E.; Lee, J.; Chang, E.H.; Kim, J.H.; Sung, J.H.; Na, D.L.; Chang, J.W. Activin A secreted by human mesenchymal stem cells induces neuronal development and neurite outgrowth in an in vitro model of Alzheimer’s disease: Neurogenesis induced by MSCs via activin A. Arch. Pharm. Res. 2016, 39, 1171–1179. [Google Scholar] [CrossRef]
- Lim, J.; Kim, Y.; Heo, J.; Kim, K.H.; Lee, S.; Lee, S.W.; Kim, K.; Kim, I.G.; Shin, D.M. Priming with ceramide-1 phosphate promotes the therapeutic effect of mesenchymal stem/stromal cells on pulmonary artery hypertension. Biochem. Biophys. Res. Commun. 2016, 473, 35–41. [Google Scholar] [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Albareda, J.; Prades, M.; Gosálvez, J.; Roy, R.; Zaragoza, P.; Martín-Burriel, I.; et al. Priming Equine Bone Marrow-Derived Mesenchymal Stem Cells with Proinflammatory Cytokines: Implications in Immunomodulation-Immunogenicity Balance, Cell Viability, and Differentiation Potential. Stem Cells Dev. 2017, 26, 15–24. [Google Scholar] [CrossRef]
- Peterson, K.M.; Aly, A.; Lerman, A.; Lerman, L.O.; Rodriguez-Porcel, M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: Role of oxidative stress. Life Sci. 2011, 88, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xue, W.; Ge, G.; Luo, X.; Li, Y.; Xiang, H.; Ding, X.; Tian, P.; Tian, X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem. Biophys. Res. Commun. 2010, 401, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Chio, C.-C.; Cheong, C.-U.; Chao, C.-M.; Cheng, B.-C.; Lin, M.-T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2012, 124, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Walczak, P.; Lukomska, B.; Janowski, M. Genetic Engineering of Mesenchymal Stem Cells to Induce Their Migration and Survival. Stem Cells Int. 2016, 2016, 4956063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyse, R.D.; Dunbar, G.L.; Rossignol, J. Use of genetically modified mesenchymal stem cells to treat neurodegenerative diseases. Int. J. Mol. Sci. 2014, 15, 1719–1745. [Google Scholar] [CrossRef] [Green Version]
- Stucky, E.C.; Schloss, R.S.; Yarmush, M.L.; Shreiber, D.I. Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response. Cytotherapy 2015, 17, 1353–1364. [Google Scholar] [CrossRef] [Green Version]
- Blocki, A.; Beyer, S.; Dewavrin, J.Y.; Goralczyk, A.; Wang, Y.; Peh, P.; Ng, M.; Moonshi, S.S.; Vuddagiri, S.; Raghunath, M.; et al. Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long-term retention of MSCs in infarcted myocardium. Biomaterials 2015, 53, 12–24. [Google Scholar] [CrossRef]
- Zhou, H.; Li, D.; Shi, C.; Xin, T.; Yang, J.; Zhou, Y.; Hu, S.; Tian, F.; Wang, J.; Chen, Y. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci. Rep. 2015, 5, 12898. [Google Scholar] [CrossRef]
- Tsai, L.K.; Wang, Z.; Munasinghe, J.; Leng, Y.; Leeds, P.; Chuang, D.M. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke 2011, 42, 2932–2939. [Google Scholar] [CrossRef]
- Müller, M.; Raabe, O.; Addicks, K.; Wenisch, S.; Arnhold, S. Effects of non-steroidal anti-inflammatory drugs on proliferation, differentiation and migration in equine mesenchymal stem cells. Cell Biol. Int. 2011, 35, 235–248. [Google Scholar] [CrossRef]
- Johnsson, K.; King, D.S.; Schultz, P.G. Studies on the Mechanism of Action of Isoniazid and Ethionamide in the Chemotherapy of Tuberculosis. J. Am. Chem. Soc. 1995, 117, 5009–5010. [Google Scholar] [CrossRef]
- Vale, N.; Gomes, P.; Santos, H.A. Metabolism of the antituberculosis drug ethionamide. Curr. Drug Metab. 2013, 14, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 Activation Controls Cell Proliferation and Cell Death Is Subcellular Localization the Answer. Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Selectivity and therapeutic inhibition of kinases: To be or not to be? Nat. Immunol. 2009, 10, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Belinda Ding, B.; Yu, J.; Yu, R.Y.-L.; Mendez, L.M.; Shaknovich, R.; Zhang, Y.; Cattoretti, G.; Hilda Ye, B. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. J. Am. Soc. Hematol. 2008, 111, 1515–1523. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Yong, X.; Li, C.; Lü, M.; Liu, D.; Chen, L.; Hu, J.; Teng, M.; Zhang, D.; Fan, Y.; et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J. Surg. Res. 2013, 183, 427–434. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Wang, H.W.; Chang, S.J.; Liao, K.H.; Lee, I.H.; Lin, W.S.; Wu, C.H.; Lin, W.Y.; Cheng, S.M. Mesenchymal Stem Cells from Human Umbilical Cord Express Preferentially Secreted Factors Related to Neuroprotection, Neurogenesis, and Angiogenesis. PLoS ONE 2013, 8, e72604. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, A.; Kemp, K.; Ginty, M.; Hares, K.; Mallam, E.; Scolding, N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009. [Google Scholar] [CrossRef] [Green Version]
- Pollock, K.; Dahlenburg, H.; Nelson, H.; Fink, K.D.; Cary, W.; Hendrix, K.; Annett, G.; Torrest, A.; Deng, P.; Gutierrez, J.; et al. Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in huntington’s disease mouse models. Mol. Ther. 2016. [Google Scholar] [CrossRef]
- Funakoshi, H.; Nakamura, T. Hepatocyte Growth Factor (HGF): Neurotrophic Functions and Therapeutic Implications for Neuronal Injury/Diseases. Curr. Signal. Transduct. Ther. 2012, 6, 156–167. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chen, J.; Yang, M.; Katakowski, M.; Lu, M.; Chopp, M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, K.S.; Park, I.H.; Kim, S.U. Human Neural Stem Cells Over-Expressing VEGF Provide Neuroprotection, Angiogenesis and Functional Recovery in Mouse Stroke Model. PLoS ONE 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011, 70, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadan, O.; Shemesh, N.; Cohen, Y.; Melamed, E.; Offen, D. Adult neurotrophic factor-secreting stem cells: A potential novel therapy for neurodegenerative diseases. Isr. Med. Assoc. J. 2009, 11, 201–204. [Google Scholar]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 2010, 5, 933–946. [Google Scholar] [CrossRef] [Green Version]
- Noronha, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Surgucheva, I.; Shestopalov, V.I.; Surguchov, A. Effect of γ-synuclein silencing on apoptotic pathways in retinal ganglion cells. J. Biol. Chem. 2008, 283, 36377–36385. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Crawford, R.; Chen, C.; Xiao, Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng.—Part. B Rev. 2013, 19, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Liu, G. The PI3K/Akt Signalling Pathway Plays Essential Roles in Mesenchymal Stem Cells. Br. Biomed. Bull. 2017, 5, 301. [Google Scholar]
- Ryu, C.H.; Park, S.A.; Kim, S.M.; Lim, J.Y.; Jeong, C.H.; Jun, J.A.; Oh, J.H.; Park, S.H.; Oh, W.; Jeun, S.-S. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem. Biophys. Res. Commun. 2010, 398, 105–110. [Google Scholar] [CrossRef]
- Yellowley, C.E.; Toupadakis, C.A.; Vapniarsky, N.; Wong, A. Circulating progenitor cells and the expression of Cxcl12, Cxcr4 and angiopoietin-like 4 during wound healing in the murine ear. PLoS ONE 2019, 14, e0222462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellowley, C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair Overview of Fracture Repair. Bonekey Rep. 2013, 2, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Lee, N.K.; Yoo, D.; Lee, J.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L. Lowering the concentration affects the migration and viability of intracerebroventricular-delivered human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 493, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Jung, N.Y.; Lee, N.K.; Lee, J.; Hyung, B.; Myeong, S.H.; Kim, H.S.; Suh, Y.L.; Lee, J., Il; Cho, K.R.; et al. Distribution of human umbilical cord blood–derived mesenchymal stem cells (hUCB-MSCs) in canines after intracerebroventricular injection. Neurobiol. Aging 2016, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, A.J.; Greenberg, M.E. CREB: A Stimulus-Induced Transcription Factor Activated by A Diverse Array of Extracellular Signals. Annu. Rev. Biochem. 1999, 68, 821–861. [Google Scholar] [CrossRef]
- Begni, V.; Riva, M.A.; Cattaneo, A. Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin. Sci. 2017, 131, 123–138. [Google Scholar] [CrossRef]
- Pradhan, J.; Noakes, P.G.; Bellingham, M.C. The Role of Altered BDNF/TrkB Signaling in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2019, 13, 1–16. [Google Scholar] [CrossRef]
- Bai, J.; Jiang, X.; He, M.; Chan, B.C.B.; Wong, A.O.L. Novel Mechanisms for IGF-I Regulation by Glucagon in Carp Hepatocytes: Up-Regulation of HNF1α and CREB Expression via Signaling Crosstalk for IGF-I Gene Transcription. Front. Endocrinol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Gu, Z.; Wu, M.; Yang, Y.; Zhang, J.; Ou, J.; Zuo, Z.; Wang, J.; Chen, Y. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1α via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res. Ther. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Motoki, T.; Sugiura, Y.; Matsumoto, Y.; Tsuji, T.; Kubota, S.; Takigawa, M.; Gohda, E. Induction of hepatocyte growth factor expression by maleic acid in human fibroblasts through MAPK activation. J. Cell. Biochem. 2008, 104, 1465–1476. [Google Scholar] [CrossRef]
- Park, H.-J.; Choi, Y.-H.; Cho, Y.J.; Henson, P.M.; Kang, J.L. RhoA-mediated signaling up-regulates hepatocyte growth factor gene and protein expression in response to apoptotic cells. J. Leukoc. Biol. 2011, 89, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, H.K.; Ashraf, M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J. Mol. Cell. Cardiol. 2008, 45, 554–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-B.; Chen, H.; Chen, H.-Q.; Zhu, M.-F.; Hu, X.-Y.; Wang, Y.-P.; Jiang, Z.; Xu, Y.-C.; Xiang, M.-X.; Wang, J.-A. Angiopoietin-1 preconditioning enhances survival and functional recovery of mesenchymal stem cell transplantation. J. Zhejiang Univ. Sci. B. 2012, 13, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Jin, K.S.; Bang, O.Y.; Kim, B.J.; Park, S.J.; Lee, N.H.; Yoo, K.H.; Koo, H.H.; Sung, K.W. Differential Migration of Mesenchymal Stem Cells to Ischemic Regions after Middle Cerebral Artery Occlusion in Rats. PLoS ONE 2015, 10, e0134920. [Google Scholar] [CrossRef] [Green Version]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.W.; Lee, N.K.; Yang, J.H.; Son, H.J.; Bang, S.I.; Chang, J.W.; Na, D.L. A comparison of immune responses exerted following syngeneic, allogeneic, and xenogeneic transplantation of mesenchymal stem cells into the mouse brain. Int. J. Mol. Sci. 2020, 21, 3052. [Google Scholar] [CrossRef]
- Ericsson, C.J. Case reports. Angle Orthod. 1969, 39, 246–255. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.-H.; Myeong, S.H.; Son, H.J.; Hwang, J.W.; Lee, N.K.; Chang, J.W.; Na, D.L. Ethionamide Preconditioning Enhances the Proliferation and Migration of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 7013. https://doi.org/10.3390/ijms21197013
Lee N-H, Myeong SH, Son HJ, Hwang JW, Lee NK, Chang JW, Na DL. Ethionamide Preconditioning Enhances the Proliferation and Migration of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2020; 21(19):7013. https://doi.org/10.3390/ijms21197013
Chicago/Turabian StyleLee, Na-Hee, Su Hyeon Myeong, Hyo Jin Son, Jung Won Hwang, Na Kyung Lee, Jong Wook Chang, and Duk L. Na. 2020. "Ethionamide Preconditioning Enhances the Proliferation and Migration of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 21, no. 19: 7013. https://doi.org/10.3390/ijms21197013
APA StyleLee, N. -H., Myeong, S. H., Son, H. J., Hwang, J. W., Lee, N. K., Chang, J. W., & Na, D. L. (2020). Ethionamide Preconditioning Enhances the Proliferation and Migration of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences, 21(19), 7013. https://doi.org/10.3390/ijms21197013




