Suppression of hTAS2R16 Signaling by Umami Substances
Abstract
:1. Introduction
2. Results and Discussion
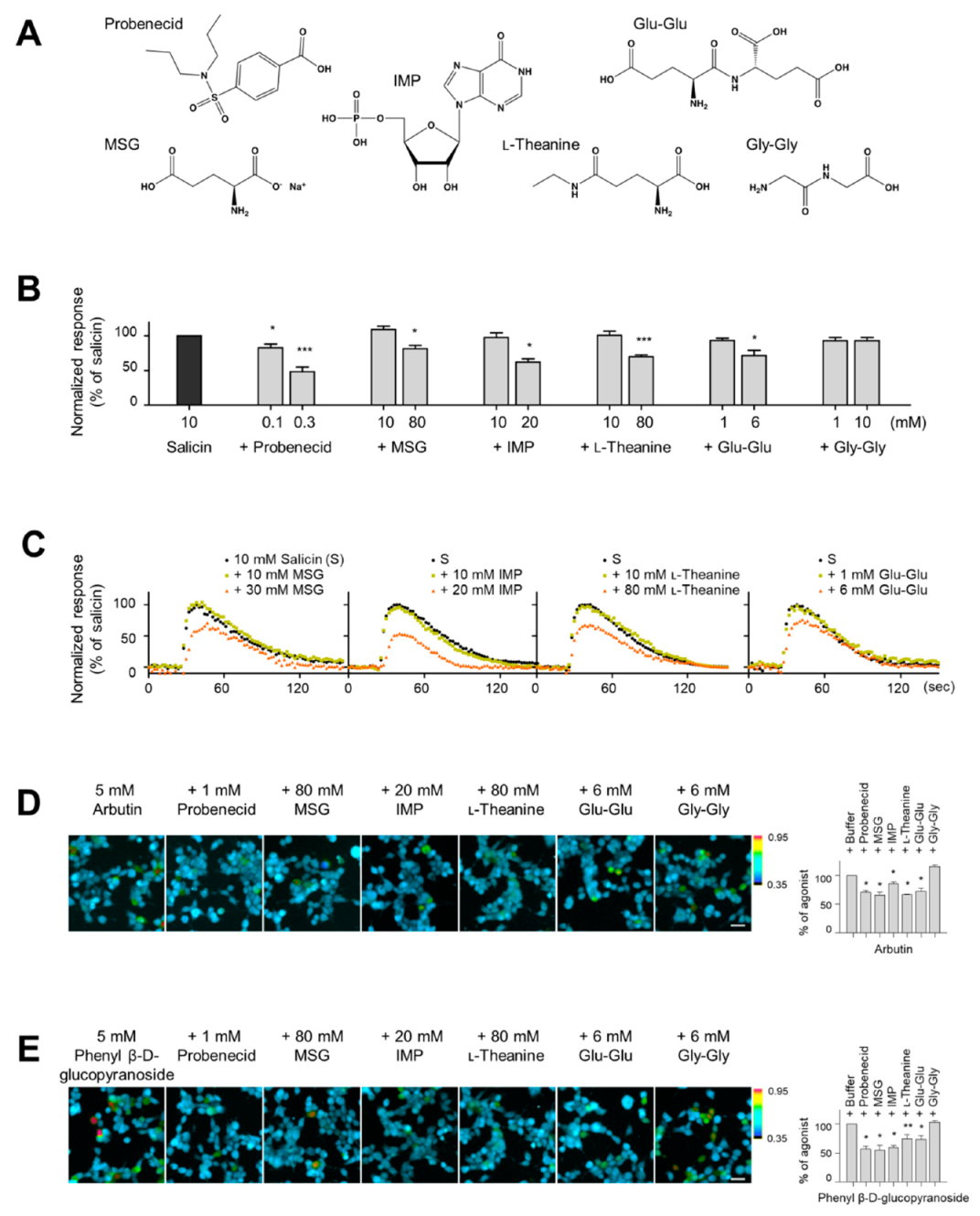
2.1. Effect of MSG, IMP, and l-Theanine on Ligand Binding to the hTAS2R16
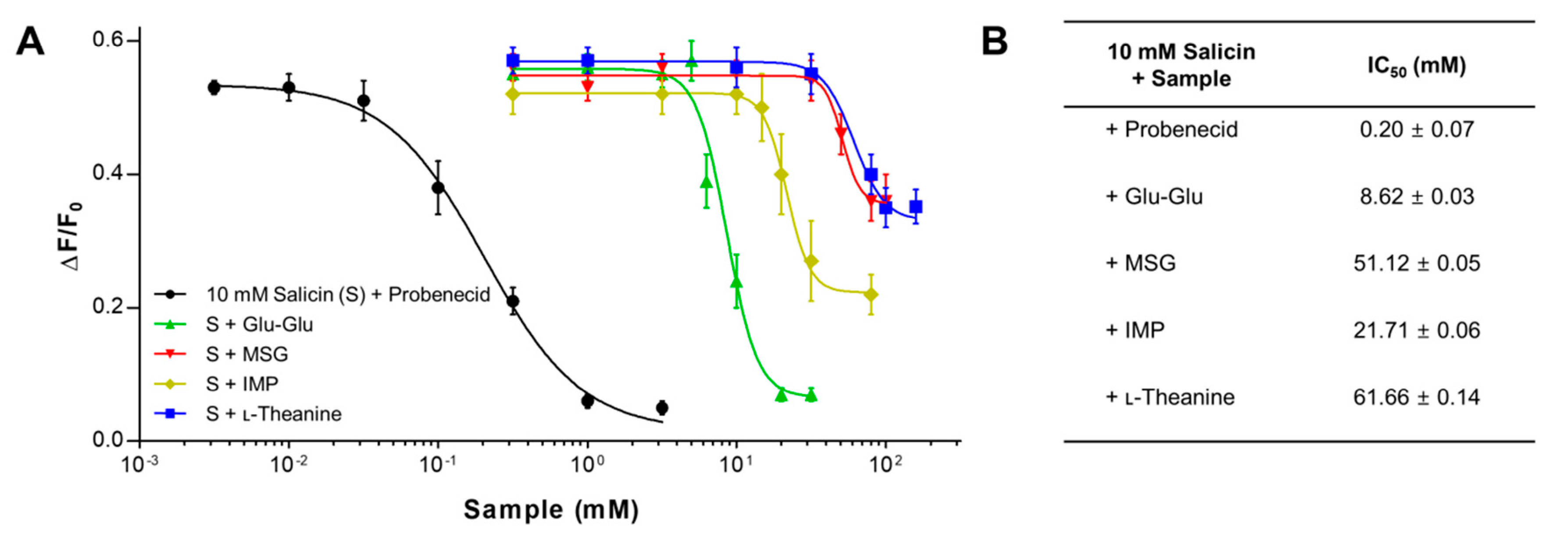
2.2. The Inhibitory Potency of Umami Substances on Salicin Binding to the hTAS2R16
2.3. Mechanism of Antagonism of the hTAS2R16 by Umami Substances
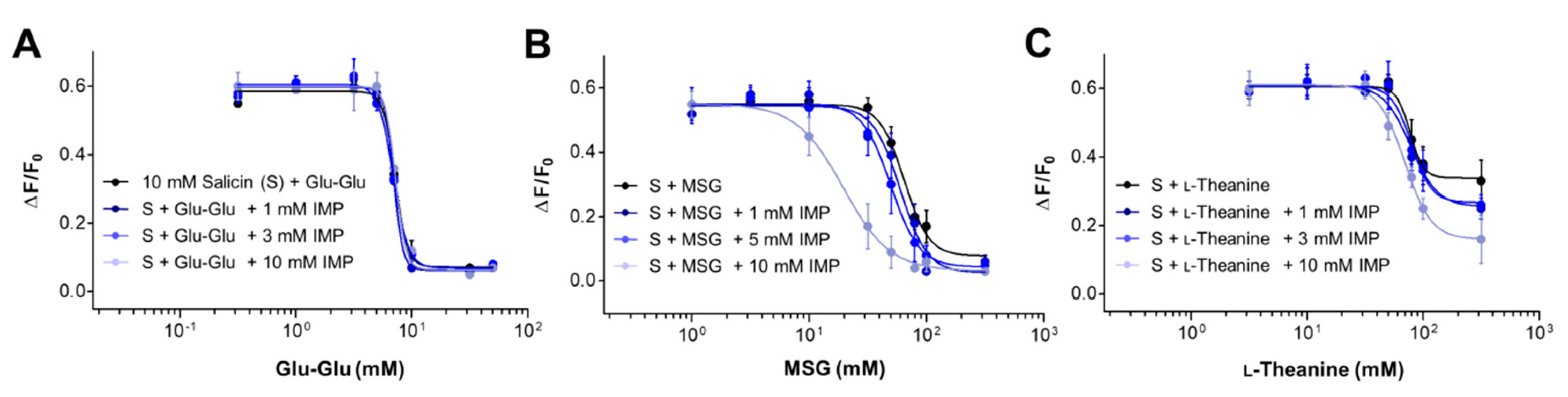
2.4. Synergism between Umami Substances on the Suppression of Bitterness
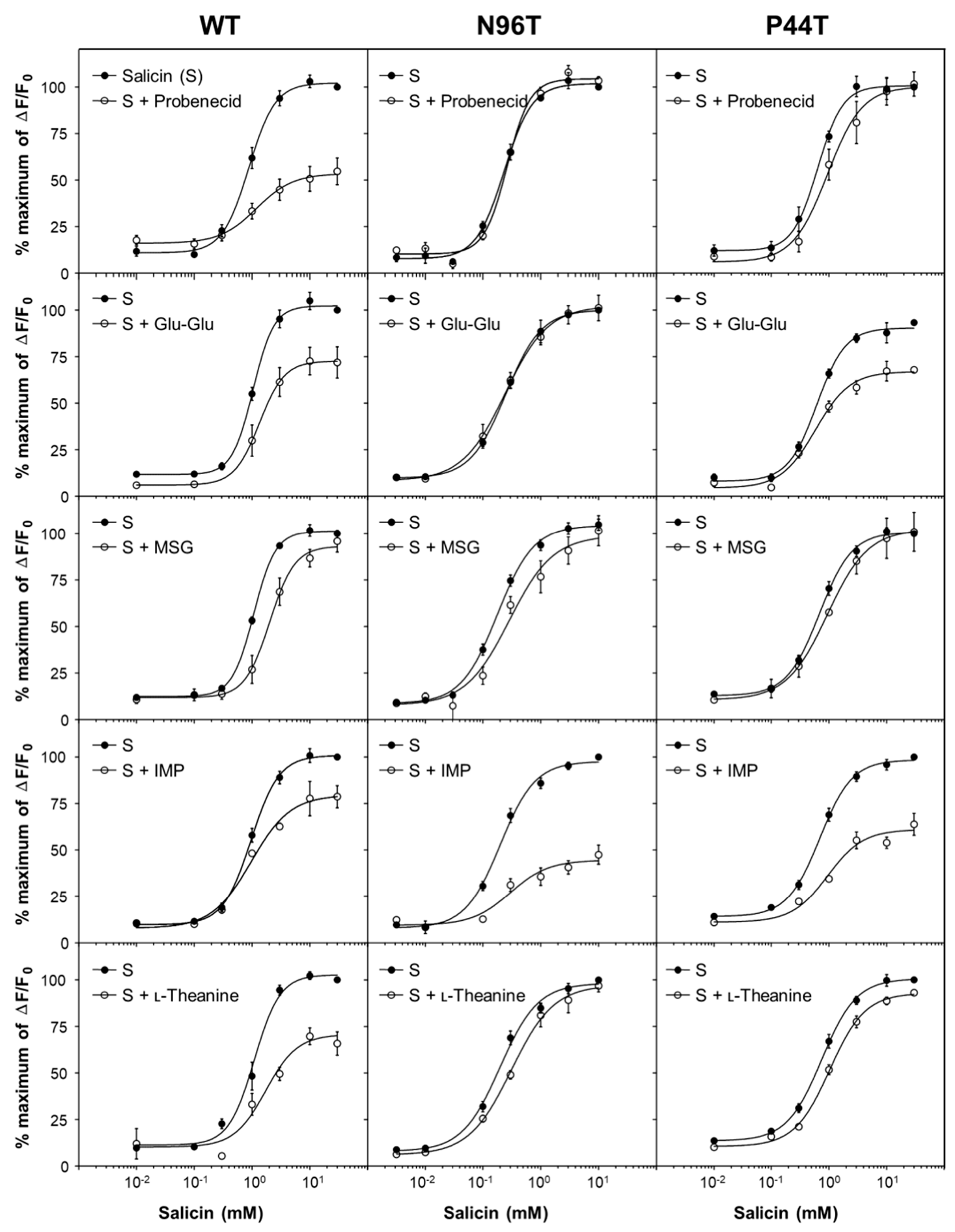
2.5. Point Mutant Analysis Using Two Known Probenecid-Insensitive Mutants, hTAS2R16 N96T and P44T, for the Potential Binding Sites of Umami Substances
3. Materials and Methods
3.1. Materials
3.2. Cell Culture and Transfection
3.3. Measurement of Cellular Ca2+ Responses in HEK293 Cells Expressing Wild-Type and Mutant hTAS2R16 by Cell-Based Assay
3.4. Measurement of Cellular Ca2+ Responses in HEK293T Cells Expressing hTAS2R by Calcium Imaging Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J. Common Sense about Taste: From Mammals to Insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jin, H.; Zhang, W.; Ding, C.; O’Keeffe, S.; Ye, M.; Zuker, C.S. Sour Sensing from the Tongue to the Brain. Cell 2019, 179, 392–402.e15. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Wilson, C.E.; Tu, Y.-H.; Joshi, N.R.; Kinnamon, S.C.; Liman, E.R. Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1. Curr. Boil. 2019, 29, 3647–3656.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Basic properties of umami and effects on humans. Physiol. Behav. 1991, 49, 833–841. [Google Scholar] [CrossRef]
- DuBois, G.E.; Prakash, I. Non-Caloric Sweeteners, Sweetness Modulators, and Sweetener Enhancers. Annu. Rev. Food Sci. Technol. 2012, 3, 353–380. [Google Scholar] [CrossRef]
- Keast, R.; Breslin, A.P.A.S. An overview of binary taste–taste interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Kroeze, J.H.; Bartoshuk, L.M. Bitterness suppression as revealed by split-tongue taste stimulation in humans. Physiol. Behav. 1985, 35, 779–783. [Google Scholar] [CrossRef]
- Rhyu, M.-R.; Song, A.-Y.; Kim, E.-Y.; Son, H.-J.; Kim, Y.; Mummalaneni, S.; Qian, J.; Grider, J.R.; Lyall, V. Kokumi Taste Active Peptides Modulate Salt and Umami Taste. Nutrients 2020, 12, 1198. [Google Scholar] [CrossRef]
- Tokita, K.; Boughter, J.D., Jr. Sweet-bitter and umami-bitter taste interactions in single parabrachial neurons in C57BL/6J mice. J. Neurophysiol. 2012, 108, 2179–2190. [Google Scholar] [CrossRef]
- Katsumata, T.; Nakakuki, H.; Tokunaga, C.; Fujii, N.; Egi, M.; Phan, T.-H.T.; Mummalaneni, S.; DeSimone, J.A.; Lyall, V. Effect of Maillard Reacted Peptides on Human Salt Taste and the Amiloride-Insensitive Salt Taste Receptor (TRPV1t). Chem. Senses 2008, 33, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Je, J.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Amino acid changes in fermented oyster (Crassostrea gigas) sauce with different fermentation periods. Food Chem. 2005, 91, 15–18. [Google Scholar] [CrossRef]
- Kato, H.; Nishimura, T. Taste Components and Conditioning of Beef, Pork, and Chicken. In Umami: A Basic Taste; Marcel Dekker, Inc.: New York, NY, USA, 1987; pp. 289–306. [Google Scholar]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste. Mech. Enzymol. Bridg. Struct. Funct. 1989, 388, 158–174. [Google Scholar] [CrossRef]
- Maehashi, K.; Matsuzaki, M.; Yamamoto, Y.; Udaka, S. Isolation of peptides from an enzymatic hydrolysate of food proteins and characterization of their taste properties. Biosci. Biotechnol. Biochem. 1999, 63, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T. Low molecular weight peptide in soybean miso. J. Ferment. Technol. 1974, 52, 256–267. [Google Scholar]
- Temussi, P.A. The good taste of peptides. J. Pept. Sci. 2011, 18, 73–82. [Google Scholar] [CrossRef]
- Goodman, M. Peptide homologs, isosteres, and isomers: A general approach to structure-activity relationships. Biopolymers 1985, 24, 137–155. [Google Scholar] [CrossRef]
- Fujimaki, M.; Yamashita, M.; Arai, S.; Kato, H. Plastein reaction: Its application to debittering of proteolyzates. Agric. Biol. Chem. 1970, 34, 483–484. [Google Scholar] [CrossRef]
- Yamashita, M.; Arai, S.; Matsuyama, J.; Gonda, M.; Kato, H.; Fujimaki, M. Enzymatic Modification of Proteins in Foodstuffs: Part III. Phenomenal Survey on α-Chymotryptic Plastein Synthesis from Peptic Hydrolyzate of Soy Protein Part IV. Bitter Dipeptides as Plastein-building Blocks Debittering of Peptic Proteolyzate with α-Chymotrypsin. Agric. Biol. Chem. 1970, 34, 1484–1500. [Google Scholar]
- Arai, S.; Yamashita, M.; Fujimaki, M. Glutamyl oligopeptides as factors responsible for tastes of a proteinase-modified soybean protein. Agric. Biol. Chem. 1972, 36, 1253–1256. [Google Scholar] [CrossRef]
- Noguchi, M.; Yamashita, M.; Arai, S.; Fujimaki, M. On the bitter-masking activity of a glutamic acid-rich Oligopeptide fraction. J. Food Sci. 1975, 40, 367–369. [Google Scholar] [CrossRef]
- Yamashita, M.; Arai, S.; Kokubo, S.; Aso, K.; Fujimaki, M. Synthesis and characterization of a glutamic acid enriched plastein with greater solubility. J. Agric. Food Chem. 1975, 23, 27–30. [Google Scholar] [CrossRef]
- Arai, S. The Bitter Flavor Due to Peptides or Protein Hydrolysates and Its Control by Bitterness-Masking with Acidic Oligopeptides; Elsevier BV: Amsterdam, The Netherlands, 1980; pp. 133–147. [Google Scholar]
- Adler, E.; Hoon, M.A.; Mueller, K.L.; Chandrashekar, J.; Ryba, N.J.; Zuker, C.S. A novel family of mammalian taste receptors. Cell 2000, 100, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bufe, B.; Hofmann, T.; Krautwurst, D.; Raguse, J.-D.; Meyerhof, W. The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat. Genet. 2002, 32, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs Function as Bitter Taste Receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Matsunami, H.; Montmayeur, J.-P.; Buck, L.B. A family of candidate taste receptors in human and mouse. Nature 2000, 404, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Booth, B.J.; Losee, M.L.; Pecore, S.D.; Warwick, Z.S. Bitterness of sweeteners as a function of concentration. Brain Res. Bull. 1995, 36, 505–513. [Google Scholar] [CrossRef]
- Kuhn, C.; Bufe, B.; Winnig, M.; Hofmann, T.; Frank, O.; Behrens, M.; Lewtschenko, T.; Slack, J.P.; Ward, C.D.; Meyerhof, W. Bitter Taste Receptors for Saccharin and Acesulfame K. J. Neurosci. 2004, 24, 10260–10265. [Google Scholar] [CrossRef] [Green Version]
- Greene, T.A.; Alarcon, S.; Thomas, A.; Berdougo, E.; Doranz, B.J.; Breslin, P.A.S.; Rucker, J.B. Probenecid Inhibits the Human Bitter Taste Receptor TAS2R16 and Suppresses Bitter Perception of Salicin. PLoS ONE 2011, 6, e20123. [Google Scholar] [CrossRef]
- Slack, J.P.; Brockhoff, A.; Batram, C.; Menzel, S.; Sonnabend, C.; Born, S.; Galindo, M.M.; Kohl, S.; Thalmann, S.; Ostopovici-Halip, L.; et al. Modulation of Bitter Taste Perception by a Small Molecule hTAS2R Antagonist. Curr. Boil. 2010, 20, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Merritt, J.E.; McCarthy, S.A.; Davies, M.P.A.; Moores, K.E. Use of fluo-3 to measure cytosolic Ca2+ in platelets and neutrophils. Loading cells with the dye, calibration of traces, measurements in the presence of plasma, and buffering of cytosolic Ca2+. Biochem. J. 1990, 269, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Son, H.J.; Kim, Y.; Misaka, T.; Rhyu, M.-R. Umami–bitter interactions: The suppression of bitterness by umami peptides via human bitter taste receptor. Biochem. Biophys. Res. Commun. 2015, 456, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, E.-Y.; Son, H.J.; Lee, J.-J.; Choi, Y.-H.; Rhyu, M.-R. Identification of a key umami-active fraction in modernized Korean soy sauce and the impact thereof on bitter-masking. Food Chem. 2017, 233, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.E.; Beauchamp, G.K. Flavor Modification by Sodium Chloride and Monosodium Glutamate. J. Food Sci. 1994, 59, 682–686. [Google Scholar] [CrossRef]
- Woskow, M.H. Selectivity in flavor modification by 5’-ribonucleotides. Food Technol. 1969, 23, 1364–1367. [Google Scholar]
- Kuninaka, A. Studies on Taste of Ribonucleic Acid Derivatives. J. Agric. Chem. Soc. Jpn. 1960, 34, 489–492. [Google Scholar] [CrossRef]
- Schiffman, S.; Frey, A.; Luboski, J.; Foster, M.; Erickson, R. Taste of glutamate salts in young and elderly subjects: Role of inosine 5′-monophosphate and ions. Physiol. Behav. 1991, 49, 843–854. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ninomiya, K. What is umami? Food Rev. Int. 1998, 14, 123–138. [Google Scholar] [CrossRef]
- Narukawa, M.; Morita, K.; Hayashi, Y. L-theanine elicits an umami taste with inosine 5’-monophosphate. Biosci. Biotechnol. Biochem. 2008, 72, 3015–3017. [Google Scholar] [CrossRef]
- Jeruzal-Świątecka, J.; Fendler, W.; Pietruszewska, W. Clinical Role of Extraoral Bitter Taste Receptors. Int. J. Mol. Sci. 2020, 21, 5156. [Google Scholar] [CrossRef]
- Arai, S.; Yamashita, M.; Noguchi, M.; Fujimaki, M. Tastes of L-Glutamyl Oligopeptides in Relation to Their Chromatographic Properties. Agric. Boil. Chem. 1973, 37, 151–156. [Google Scholar] [CrossRef]
- Vauquelin, G.; Van Liefde, I.; Vanderheyden, P. Models and methods for studying insurmountable antagonism. Trends Pharmacol. Sci. 2002, 23, 514–518. [Google Scholar] [CrossRef]
- Vauquelin, G.; Van Liefde, I.; Birzbier, B.B.; Vanderheyden, P.M.L. New insights in insurmountable antagonism. Fundam. Clin. Pharmacol. 2002, 16, 263–272. [Google Scholar] [CrossRef]
- Zhang, F.; Klebansky, B.; Fine, R.M.; Xu, H.; Pronin, A.; Liu, H.; Tachdjian, C.; Li, X. Molecular mechanism for the umami taste synergism. Proc. Natl. Acad. Sci. USA 2008, 105, 20930–20934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, H.J.; Kim, Y.; Misaka, T.; Noh, B.S.; Rhyu, M.-R. Activation of the Chemosensory Ion Channels TRPA1 and TRPV1 by Hydroalcohol Extract of Kalopanax pictus Leaves. Biomol. Ther. 2012, 20, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Ammon, C.; Schafer, J.; Kreuzer, O.; Meyerhof, W. Presence of a Plasma Membrane Targeting Sequence in the Amino-Terminal Region of the Rat Somatostatin Receptor 3. Arch. Physiol. Biochem. 2002, 110, 137–145. [Google Scholar] [CrossRef]
- Ueda, T.; Ugawa, S.; Yamamura, H.; Imaizumi, Y.; Shimada, S. Functional Interaction between T2R Taste Receptors and G-Protein α Subunits Expressed in Taste Receptor Cells. J. Neurosci. 2003, 23, 7376–7380. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Misaka, T.; Ishiguro, M.; Masuda, K.; Sugawara, T.; Ito, K.; Kobayashi, T.; Matsuo, S.; Ishimaru, Y.; Asakura, T.; et al. Characterization of the beta-d-glucopyranoside binding site of the human bitter taste receptor hTAS2R16. J. Biol. Chem. 2010, 285, 28373–28378. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Misaka, T.; Nagai, T.; Ishimaru, Y.; Matsuo, S.; Asakura, T.; Abe, K. pH-Dependent Inhibition of the Human Bitter Taste Receptor hTAS2R16 by a Variety of Acidic Substances. J. Agric. Food Chem. 2009, 57, 2508–2514. [Google Scholar] [CrossRef]
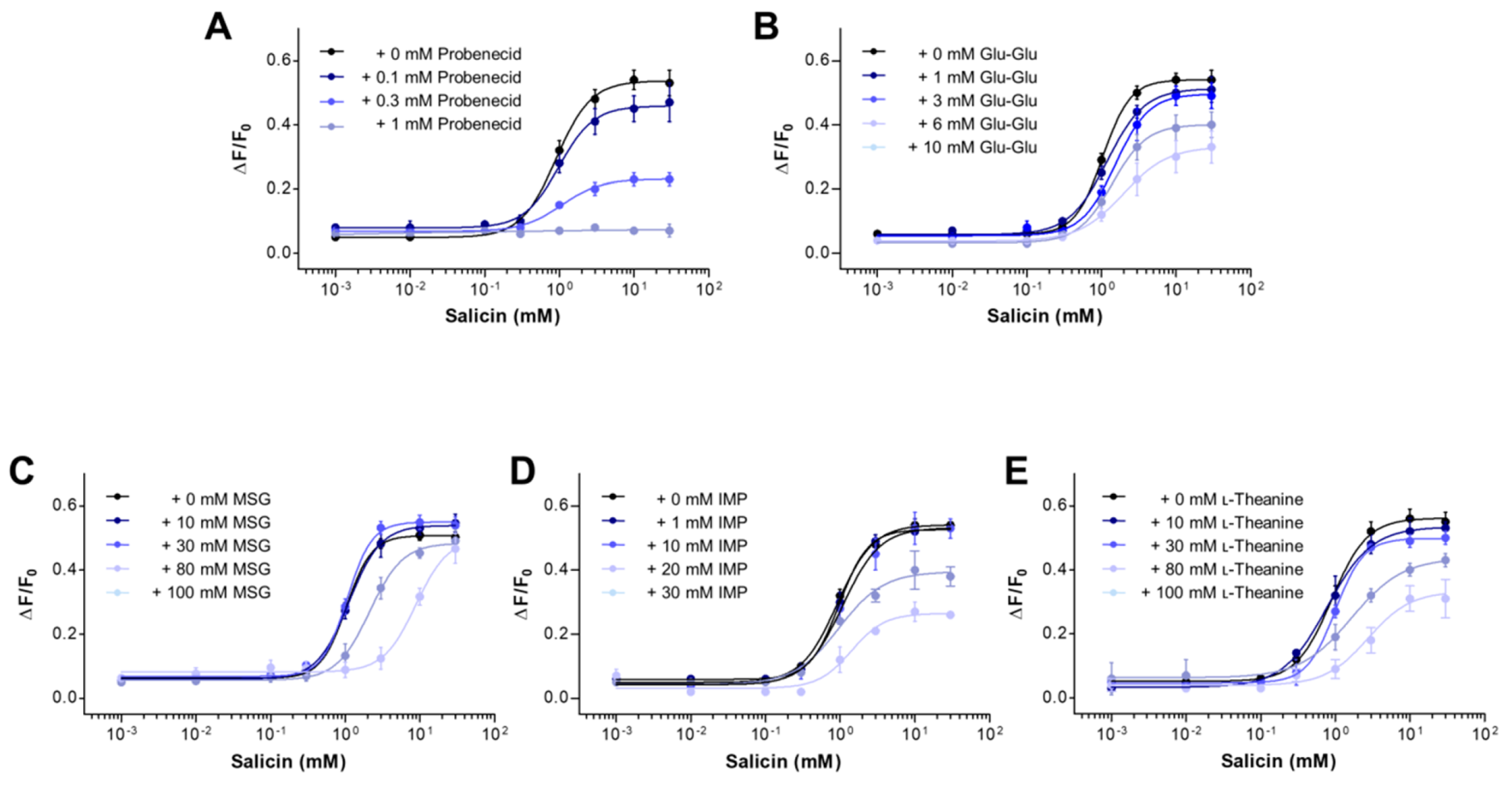
| Salicin (0–30 mM) + Sample (mM) | EC50 (mM) | Maximal Response (ΔF/F0) | |
|---|---|---|---|
| + Probenecid | 0 | 0.90 ± 0.06 † | 0.54 |
| 0.1 | 0.98 ± 0.10 | 0.47 | |
| 0.3 | 1.07 ± 0.11 | 0.23 | |
| 1 | NE ‡ | 0.07 | |
| + Glu-Glu | 0 | 1.04 ± 0.03 | 0.54 |
| 1 | 1.18 ± 0.05 | 0.51 | |
| 3 | 1.53 ± 0.06 | 0.49 | |
| 6 | 1.40 ± 0.11 | 0.40 | |
| 10 | 1.96 ± 0.15 | 0.33 | |
| + MSG | 0 | 1.04 ± 0.02 | 0.51 |
| 10 | 1.12 ± 0.05 | 0.55 | |
| 30 | 1.06 ± 0.03 | 0.54 | |
| 80 | 2.13 ± 0.05 | 0.49 | |
| 100 | 8.83 ± 0.09 | 0.47 | |
| + IMP | 0 | 0.92 ± 0.03 | 0.54 |
| 1 | 0.97 ± 0.04 | 0.53 | |
| 10 | 1.73 ± 0.06 | 0.53 | |
| 20 | 1.45 ± 0.13 | 0.40 | |
| 30 | 1.95 ± 0.09 | 0.27 | |
| + l-Theanine | 0 | 0.89 ± 0.05 | 0.56 |
| 10 | 0.78 ± 0.06 | 0.53 | |
| 30 | 1.00 ± 0.04 | 0.50 | |
| 80 | 1.65 ± 0.14 | 0.43 | |
| 100 | 2.74 ± 0.17 | 0.31 | |
| 10 mM Salicin + Sample | + IMP (mM) | IC50 (mM) |
|---|---|---|
| + Glu-Glu | 0 | 7.25 ± 0.02 |
| 1 | 7.03 ± 0.01 | |
| 3 | 6.97 ± 0.02 | |
| 10 | 7.28 ± 0.02 | |
| + MSG | 0 | 64.67 ± 0.04 |
| 1 | 59.85 ± 0.05 | |
| 5 | 49.65 ± 0.06 | |
| 10 | 19.51 ± 0.09 | |
| + l-Theanine | 0 | 77.33 ± 0.05 |
| 1 | 78.13 ± 0.06 | |
| 3 | 79.86 ± 0.06 | |
| 10 | 69.62 ± 0.06 |
| Sample (mM) | EC50 (mM) | Maximal Response (ΔF/F0) | |||||
|---|---|---|---|---|---|---|---|
| WT | N96T | P44T | WT | N96T | P44T | ||
| + Probenecid | 0 | 0.90 ± 0.04 † | 0.23 ± 0.03 | 0.64 ± 0.06 | 0.54 | 0.68 | 0.56 |
| 0.3 | 1.04 ± 0.19 | 0.26 ± 0.03 | 0.94 ± 0.10 | 0.23 | 0.68 | 0.58 | |
| + Glu-Glu | 0 | 1.04 ± 0.03 | 0.25 ± 0.05 | 0.62 ± 0.04 | 0.54 | 0.61 | 0.62 |
| 6 | 1.35 ± 0.12 | 0.24 ± 0.07 | 0.59 ± 0.07 | 0.40 | 0.65 | 0.42 | |
| + MSG | 0 | 1.07 ± 0.02 | 0.18 ± 0.04 | 0.68 ± 0.03 | 0.51 | 0.66 | 0.56 |
| 80 | 2.03 ± 0.06 | 0.29 ± 0.10 | 0.94 ± 0.14 | 0.49 | 0.65 | 0.57 | |
| + IMP | 0 | 0.96 ± 0.03 | 0.20 ± 0.03 | 0.69 ± 0.04 | 0.54 | 0.66 | 0.61 |
| 20 | 0.97 ± 0.12 | 0.30 ± 0.15 | 0.94 ± 0.13 | 0.40 | 0.28 | 0.37 | |
| + l-Theanine | 0 | 1.10 ± 0.03 | 0.20 ± 0.04 | 0.75 ± 0.04 | 0.56 | 0.63 | 0.59 |
| 80 | 1.73 ± 0.16 | 0.31 ± 0.07 | 1.03 ± 0.03 | 0.43 | 0.66 | 0.56 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhyu, M.-R.; Kim, Y.; Misaka, T. Suppression of hTAS2R16 Signaling by Umami Substances. Int. J. Mol. Sci. 2020, 21, 7045. https://doi.org/10.3390/ijms21197045
Rhyu M-R, Kim Y, Misaka T. Suppression of hTAS2R16 Signaling by Umami Substances. International Journal of Molecular Sciences. 2020; 21(19):7045. https://doi.org/10.3390/ijms21197045
Chicago/Turabian StyleRhyu, Mee-Ra, Yiseul Kim, and Takumi Misaka. 2020. "Suppression of hTAS2R16 Signaling by Umami Substances" International Journal of Molecular Sciences 21, no. 19: 7045. https://doi.org/10.3390/ijms21197045
APA StyleRhyu, M.-R., Kim, Y., & Misaka, T. (2020). Suppression of hTAS2R16 Signaling by Umami Substances. International Journal of Molecular Sciences, 21(19), 7045. https://doi.org/10.3390/ijms21197045





