Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents—Pathophysiology and Biomarkers—A Review
Abstract
:1. Introduction
2. Epidemiology of Acute Kidney Injury
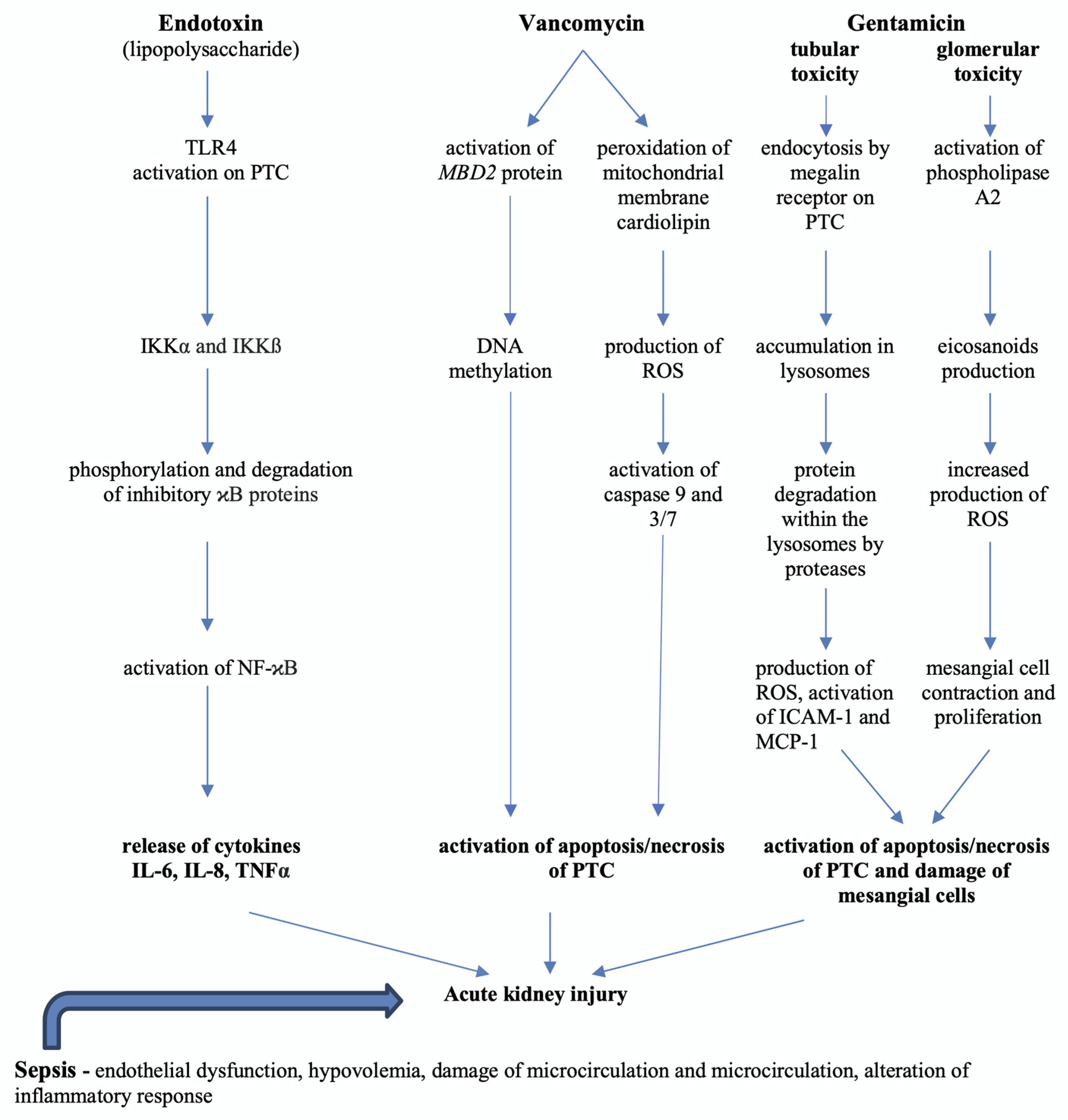
3. Pathophysiology of Sepsis-Induced Acute Kidney Injury
4. Biomarkers of Sepsis-Induced Acute Kidney Injury
5. MiRNAs as Biomarkers of Septic Acute Kidney Injury
6. Medication-Induced AKI in Septic Patients
7. Vancomycin-Induced Nephrotoxicity Pathophysiology and Biomarkers
8. Gentamicin-Induced Nephrotoxicity Pathophysiology and Biomarkers
9. MicroRNAs Associated with AKI Induced by Sepsis or Nephrotoxic Antibiotic Therapy
9.1. miR-15a-5p
9.2. miR-192-5p
9.3. miR-155-5p
9.4. miR-486-5p
9.5. miR-423-5p
10. Therapeutic Approaches in Septic Patients with AKI
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar]
- Jiang, L.; Zhu, Y.; Luo, X.; Wen, Y.; Du, B.; Wang, M.; Zhao, Z.; Yin, Y.; Zhu, B.; Xi, X. Epidemiology of acute kidney injury in intensive care units in Beijing: The multi-center BAKIT study. BMC Nephrol. 2019, 20, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef]
- Gómez, H.; Kellum, J.A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 2016, 22, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar]
- Anders, H.J.; Banas, B.; Schlöndorff, D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J. Am. Soc. Nephrol. 2004, 15, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar]
- Morrell, E.D.; Kellum, J.A.; Pastor-Soler, N.M.; Hallows, K.R. Septic acute kidney injury: Molecular mechanisms and the importance of stratification and targeting therapy. Crit. Care 2014, 18, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q. Novel strategy for septic acute kidney injury rescue: Maintenance of the tubular integrity. Kidney Int. 2020, 97, 847–849. [Google Scholar] [PubMed]
- Nakano, D.; Kitada, K.; Wan, N.; Zhang, Y.; Wiig, H.; Wararat, K.; Yanagita, M.; Lee, S.; Jia, L.; Titze, J.M.; et al. Lipopolysaccharide induces filtrate leakage from renal tubular lumina into the interstitial space via a proximal tubular Toll-like receptor 4-dependent pathway and limits sensitivity to fluid therapy in mice. Kidney Int. 2020, 97, 904–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017, 55, 1074–1089. [Google Scholar] [CrossRef]
- Klein, S.J.; Brandtner, A.K.; Lehner, G.F.; Ulmer, H.; Bagshaw, S.M.; Wiedermann, C.J.; Joannidis, M. Biomarkers for prediction of renal replacement therapy in acute kidney injury: A systematic review and meta-analysis. Intensive Care Med. 2018, 44, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury-pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef]
- Teo, S.H.; Endre, Z.H. Biomarkers in acute kidney injury (AKI). Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 331–344. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, J.L.; Nin, N.; Cardinal-Fernandez, P.; Rojas, Y.; de Paula, M.; Granados, R.; Martínez-Caro, L.; Ruíz-Cabello, J.; Lorente, J.A. Identification of novel metabolomic biomarkers in an experimental model of septic acute kidney injury. Am. J. Physiol. Renal Physiol. 2019, 316, F54–F62. [Google Scholar] [CrossRef] [Green Version]
- Chebotareva, N.; Bobkova, I.; Shilov, E. Heat shock proteins and kidney disease: Perspectives of HSP therapy. Cell Stress Chaperones 2017, 22, 319–343. [Google Scholar] [CrossRef]
- Morales-Buenrostro, L.E.; Salas-Nolasco, O.I.; Barrera-Chimal, J.; Casas-Aparicio, G.; Irizar-Santana, S.; Pérez-Villalva, R.; Bobadilla, N.A. Hsp72 is a novel biomarker to predict acute kidney injury in critically ill patients. PLoS ONE 2014, 9, e109407. [Google Scholar] [CrossRef]
- Dozmorov, M.G.; Giles, C.B.; Koelsch, K.A.; Wren, J.D. Systematic classification of non-coding RNAs by epigenomic similarity. BMC Bioinform. 2013, 14, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, P.C.; Chen, C.C.; Chen, Y.C.; Chang, Y.S.; Chu, P.H. MicroRNAs in acute kidney injury. Hum. Genom. 2016, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Giza, D.E.; Fuentes-Mattei, E.; Bullock, M.D.; Tudor, S.; Goblirsch, M.J.; Fabbri, M.; Lupu, F.; Yeung, S.J.; Vasilescu, C.; Calin, G.A. Cellular and viral microRNAs in sepsis: Mechanisms of action and clinical applications. Cell Death Differ. 2016, 23, 1906–1918. [Google Scholar] [CrossRef]
- Benz, F.; Roy, S.; Trautwein, C.; Roderburg, C.; Luedde, T. Circulating MicroRNAs as Biomarkers for Sepsis. Int. J. Mol. Sci. 2016, 17, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Liu, Z.; Wang, X.; Qiu, C.; Zheng, S. MiR-21-3p Plays a Crucial Role in Metabolism Alteration of Renal Tubular Epithelial Cells during Sepsis Associated Acute Kidney Injury via AKT/CDK2-FOXO1 Pathway. Biomed. Res. Int. 2019, 2019, 2821731. [Google Scholar] [CrossRef] [Green Version]
- Ge, Q.M.; Huang, C.M.; Zhu, X.Y.; Bian, F.; Pan, S.M. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS ONE 2017, 12, e0173292. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Inagi, R. Mitochondria: A therapeutic target in acute kidney injury. Nephrol. Dial. Transplant. 2016, 31, 1062–1069. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, J.; Jing, Y.; Zhang, J. MiR-106a aggravates sepsis-induced acute kidney injury by targeting THBS2 in mice model. Acta Cir. Bras. 2019, 34, e201900602. [Google Scholar] [CrossRef] [Green Version]
- Taber, S.S.; Pasko, D.A. The epidemiology of drug-induced disorders: The kidney. Expert Opin. Drug Saf. 2008, 7, 679–690. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Wilhelm-Leen, E.; Montez-Rath, M.E.; Chertow, G. Estimating the Risk of Radiocontrast-Associated Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perazella, M.A.; Markowitz, G.S. Drug-induced acute interstitial nephritis. Nat. Rev. Nephrol. 2010, 6, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martinek, A.; Zadrazil, J.; Teplan, V. Acute toxic kidney injury. Ren. Fail. 2019, 41, 576–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arimura, Y.; Yano, T.; Hirano, M.; Sakamoto, Y.; Egashira, N.; Oishi, R. Mitochondrial superoxide production contributes to vancomycin-induced renal tubular cell apoptosis. Free Radic. Biol. Med. 2012, 52, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Perazella, M.A. PPIs and kidney disease: From AIN to CKD. J. Nephrol. 2016, 29, 611–616. [Google Scholar] [CrossRef]
- Ong, L.Z.; Tambyah, P.A.; Lum, L.H.; Low, Z.J.; Cheng, I.; Murali, T.M.; Wan, M.Q.; Chua, H.R. Aminoglycoside-associated acute kidney injury in elderly patients with and without shock. J. Antimicrob. Chemother. 2016, 71, 3250–3257. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2009, 29, 1275–1279. [Google Scholar] [CrossRef]
- Zamoner, W.; Prado, I.R.S.; Balbi, A.L.; Ponce, D. Vancomycin dosing, monitoring and toxicity: Critical review of the clinical practice. Clin. Exp. Pharmacol. Physiol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Executive Summary: Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2020, 40, 363–367. [Google Scholar]
- Chavada, R.; Ghosh, N.; Sandaradura, I.; Maley, M.; Van Hal, S.J. Establishment of an AUC0-24 Threshold for Nephrotoxicity Is a Step towards Individualized Vancomycin Dosing for Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Hanrahan, T.P.; Kotapati, C.; Roberts, M.J.; Rowland, J.; Lipman, J.; Roberts, J.A.; Udy, A. Factors associated with vancomycin nephrotoxicity in the critically ill. Anaesth. Intensive Care 2015, 43, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, Y.; Yano, T.; Hanada, Y.; Takeshita, A.; Inagaki, F.; Masuda, S.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur. J. Pharmacol. 2017, 800, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kane-Gill, S.L.; Ostermann, M.; Shi, J.; Joyce, E.L.; Kellum, J.A. Evaluating Renal Stress Using Pharmacokinetic Urinary Biomarker Data in Critically Ill Patients Receiving Vancomycin and/or Piperacillin-Tazobactam: A Secondary Analysis of the Multicenter Sapphire Study. Drug Saf. 2019, 42, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.J.; Prozialeck, W.C.; Lodise, T.P.; Venkatesan, N.; O’Donnell, J.N.; Pais, G.; Cluff, C.; Lamar, P.C.; Neely, M.N.; Gulati, A.; et al. Evaluation of Vancomycin Exposures Associated with Elevations in Novel Urinary Biomarkers of Acute Kidney Injury in Vancomycin-Treated Rats. Antimicrob. Agents Chemother. 2016, 60, 5742–5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, H.M.; Qin, X.L.; Liu, T.T.; Wei, W.X.; Cheng, D.H.; Lu, H.; Guo, Q.; Jing, L. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as early biomarkers for predicting vancomycin-associated acute kidney injury: A prospective study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4203–4213. [Google Scholar] [PubMed]
- Pais, G.M.; Avedissian, S.N.; O′Donnell, J.N.; Rhodes, N.J.; Lodise, T.P.; Prozialeck, W.C.; Lamar, P.C.; Cluff, C.; Gulati, A.; Fitzgerald, J.C.; et al. Comparative Performance of Urinary Biomarkers for Vancomycin-Induced Kidney Injury According to Timeline of Injury. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, H.; Qiu, S.; Dong, Z.; Xiang, X.; Zhang, D. MBD2 upregulates miR-301a-5p to induce kidney cell apoptosis during vancomycin-induced AKI. Cell Death Dis. 2017, 8, e3120. [Google Scholar] [CrossRef]
- Olbricht, C.J.; Fink, M.; Gutjahr, E. Alterations in lysosomal enzymes of the proximal tubule in gentamicin nephrotoxicity. Kidney Int. 1991, 39, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Gentamicin 40 mg/mL Injection. Available online: https://www.medicines.org.uk/emc/product/6531/smpc (accessed on 7 July 2020).
- Romero, F.; Pérez, M.; Chávez, M.; Parra, G.; Durante, P. Effect of uric acid on gentamicin-induced nephrotoxicity in rats-role of matrix metalloproteinases 2 and 9. Basic Clin. Pharmacol. Toxicol. 2009, 105, 416–424. [Google Scholar] [CrossRef]
- Martínez-Salgado, C.; López-Hernández, F.J.; López-Novoa, J.M. Glomerular nephrotoxicity of aminoglycosides. Toxicol. Appl. Pharmacol. 2007, 223, 86–98. [Google Scholar] [CrossRef]
- Udupa, V.; Prakash, V. Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol. Rep. 2018, 6, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.A.; de Almeida, L.A.; Grossi, M.F.; Tagliati, C.A. In vitro evaluation of biomarkers of nephrotoxicity through gene expression using gentamicin. J. Biochem. Mol. Toxicol. 2018, 32, e22189. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Zarybnicky, T.; Omi, T.; Shirai, Y.; Toyokuni, S.; Oda, S.; Yokoi, T. A scrutiny of circulating microRNA biomarkers for drug-induced tubular and glomerular injury in rats. Toxicology 2019, 415, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Aoki, N.; Kuwahara, S.; Hosojima, M.; Kaseda, R.; Goto, S.; Iida, T.; De, S.; Kabasawa, H.; Kaneko, R.; et al. Megalin Blockade with Cilastatin Suppresses Drug-Induced Nephrotoxicity. J. Am. Soc. Nephrol. 2017, 28, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Balakumar, P.; Rohilla, A.; Thangathirupathi, A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol. Res. 2010, 62, 179–186. [Google Scholar] [CrossRef]
- Xu, G.; Mo, L.; Wu, C.; Shen, X.; Dong, H.; Yu, L.; Pan, P.; Pan, K. The miR-15a-5p-XIST-CUL3 regulatory axis is important for sepsis-induced acute kidney injury. Ren. Fail. 2019, 41, 955–966. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; Huang, Z. microRNA-15a-5p participates in sepsis by regulating the inflammatory response of macrophages and targeting TNIP2. Exp. Ther. Med. 2020, 19, 3060–3068. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wan, X.H.; Sang, G.Y.; Zhao, J.D.; Zhu, Q.Y.; Wang, D.M. miR-15a-5p suppresses endometrial cancer cell growth via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4810–4818. [Google Scholar]
- Chen, D.; Wu, D.; Shao, K.; Ye, B.; Huang, J.; Gao, Y. MiR-15a-5p negatively regulates cell survival and metastasis by targeting CXCL10 in chronic myeloid leukemia. Am. J. Transl. Res. 2017, 9, 4308–4316. [Google Scholar]
- Shang, J.; He, Q.; Chen, Y.; Yu, D.; Sun, L.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. miR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J. Cell. Physiol. 2019, 234, 9746–9755. [Google Scholar] [CrossRef]
- Caserta, S.; Kern, F.; Cohen, J.; Drage, S.; Newbury, S.F.; Llewelyn, M.J. Circulating Plasma microRNAs can differentiate Human Sepsis and Systemic Inflammatory Response Syndrome (SIRS). Sci. Rep. 2016, 6, 28006. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Mengozzi, M.; Kern, F.; Newbury, S.F.; Ghezzi, P.; Llewelyn, M.J. Severity of Systemic Inflammatory Response Syndrome Affects the Blood Levels of Circulating Inflammatory-Relevant MicroRNAs. Front. Immunol. 2018, 8, 1977. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Wen, D.; Zhao, Q.; Shen, P.Y.; Shi, H.; Zhao, Q.; Chen, Y.X.; Zhang, W. Urinary MicroRNA-30c-5p and MicroRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury. Exp. Biol. Med. 2017, 242, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Jiang, Z.; Yang, X.; Lin, J.; Cai, Q.; Li, X. Circular RNA HIPK3 contributes to hyperglycemia and insulin homeostasis by sponging miR-192-5p and upregulating transcription factor forkhead box O1. Endocr. J. 2020, 67, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.A.; Wang, F.; Liu, Y.; Kriegel, A.J.; Geurts, A.M.; Usa, K.; Xue, H.; Wang, D.; Kong, Y.; Liang, M. MiR-192-5p in the Kidney Protects Against the Development of Hypertension. Hypertension 2019, 73, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Li, H.; Wang, S.; Xiang, X.; Zhang, D. p53 activates miR-192-5p to mediate vancomycin induced AKI. Sci. Rep. 2016, 6, 38868. [Google Scholar] [CrossRef] [Green Version]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Roßmanith, E.; Lang, I.; Falkenhagen, D. miR-146a, miR-146b, and miR-155 increase expression of IL-6 and IL-8 and support HSP10 in an In vitro sepsis model. PLoS ONE 2017, 12, e0179850. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.; Hu, R.; Runtsch, M.C.; Kagele, D.A.; Mosbruger, T.L.; Tolmachova, T.; Seabra, M.C.; Round, J.L.; Ward, D.M.; O’Connell, R.M. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 2015, 6, 7321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.J.; Le, T.T.; Dobbin, C.A.; Banovic, T.; Howard, C.B.; Flores, F.D.M.L.; Vanags, D.; Naylor, D.J.; Hill, G.R.; Suhrbier, A. Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J. Biol. Chem. 2005, 280, 4037–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikumar, J.; Hoffmann, D.; Kim, T.M.; Gonzalez, V.R.; Zhang, Q.; Goering, P.L.; Brown, R.P.; Bijol, V.; Park, P.J.; Waikar, S.S.; et al. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol. Sci. 2012, 129, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Chen, B.; Chen, C.; Li, H.; Wang, D.; Tan, Y.; Weng, H. CircNr1h4 regulates the pathological process of renal injury in salt-sensitive hypertensive mice by targeting miR-155-5p. J. Cell. Mol. Med. 2020, 24, 1700–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zheng, Z.J.; Jia, Y.J.; Yang, Y.L.; Xue, Y.M. Role of p53/miR-155-5p/sirt1 loop in renal tubular injury of diabetic kidney disease. J. Transl. Med. 2018, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Viñas, J.L.; Burger, D.; Zimpelmann, J.; Haneef, R.; Knoll, W.; Campbell, P.; Gutsol, A.; Carter, A.; Allan, D.S.; Burns, K.D. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 2016, 90, 1238–1250. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, R.; Workeneh, B.; Dong, Y.; Wang, X.; Hu, Z. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int. 2012, 82, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Regmi, A.; Liu, G.; Zhong, X.; Hu, S.; Ma, R.; Gou, L.; Zafar, M.I.; Chen, L. Evaluation of Serum microRNAs in Patients with Diabetic Kidney Disease: A Nested Case-Controlled Study and Bioinformatics Analysis. Med. Sci. Monit. 2019, 25, 1699–1708. [Google Scholar] [CrossRef]
- Chai, X.; Si, H.; Song, J.; Chong, Y.; Wang, J.; Zhao, G. miR-486-5p Inhibits Inflammatory Response, Matrix Degradation and Apoptosis of Nucleus Pulposus Cells through Directly Targeting FOXO1 in Intervertebral Disc Degeneration. Cell. Physiol. Biochem. 2019, 52, 109–118. [Google Scholar]
- Yuan, X.P.; Liu, L.S.; Chen, C.B.; Zhou, J.; Zheng, Y.T.; Wang, X.P.; Han, M.; Wang, C.X. MicroRNA-423-5p facilitates hypoxia/reoxygenation-induced apoptosis in renal proximal tubular epithelial cells by targeting GSTM1 via endoplasmic reticulum stress. Oncotarget 2017, 8, 82064–82077. [Google Scholar] [CrossRef]
- Wang, W.; Gao, J.; Wang, F. MiR-663a/MiR-423-5p are involved in the pathogenesis of lupus nephritis via modulating the activation of NF-κB by targeting TNIP2. Am. J. Transl. Res. 2017, 9, 3796–3803. [Google Scholar]
- Xu, Y.; Zhang, J.; Fan, L.; He, X. miR-423-5p suppresses high-glucose-induced podocyte injury by targeting Nox4. Biochem. Biophys. Res. Commun. 2018, 505, 339–345. [Google Scholar] [CrossRef]
- Montomoli, J.; Donati, A.; Ince, C. Acute Kidney Injury and Fluid Resuscitation in Septic Patients: Are We Protecting the Kidney? Nephron 2019, 143, 170–173. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.E.; Prowle, J.R. Fluid Overload. Crit. Care Clin. 2015, 31, 803–821. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudry, S.; Hajage, D.; Benichou, N.; Chaïbi, K.; Barbar, S.; Zarbock, A.; Lumlertgul, N.; Wald, R.; Bagshaw, S.M.; Srisawat, N.; et al. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: A systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2020, 395, 1506–1515. [Google Scholar] [CrossRef]
- Gaudry, S.; Hajage, D.; Schortgen, F.; Martin-Lefevre, L.; Pons, B.; Boulet, E.; Boyer, A.; Chevrel, G.; Lerolle, N.; Carpentier, D.; et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N. Engl. J. Med. 2016, 375, 122–133. [Google Scholar] [CrossRef]
- Karkar, A.; Ronco, C. Prescription of CRRT: A pathway to optimize therapy. Ann. Intensive Care 2020, 10, 32. [Google Scholar] [CrossRef]
- Romagnoli, S.; Ricci, Z.; Ronco, C. CRRT for sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 2018, 24, 483–492. [Google Scholar] [CrossRef]
- Petejova, N.; Martinek, A.; Zahalkova, J.; Duricova, J.; Brozmannova, H.; Urbanek, K.; Grundmann, M.; Plasek, J.; Kacirova, I. Vancomycin pharmacokinetics during high-volume continuous venovenous hemofiltration in critically ill septic patients. Biomed. Pap. Med. Faculty Univ. Palacky Olomouc Czech Repub. 2014, 158, 65–72. [Google Scholar] [CrossRef]
- Petejova, N.; Zahalkova, J.; Duricova, J.; Kacirova, I.; Brozmanova, H.; Urbanek, K.; Grundmann, M.; Martinek, A. Gentamicin pharmacokinetics during continuous venovenous hemofiltration in critically ill septic patients. J. Chemother. 2012, 24, 107–112. [Google Scholar] [CrossRef]

| Medications (Agents) | Clinical and Histological Presentation of Renal Toxicity |
|---|---|
| Antimicrobials Aminoglycosides Glycopeptides (vancomycin) Polymyxins | acute tubular necrosis, apoptosis, necroptosis, acute oxidative stress, cycle cell arrest |
| Antimicrobials β-lactams Sulphonamides Macrolides Antiretrovirals (Acyclovir) Rifampin Fluoroquinolones Chloramphenicol Other medications Diuretics NSAIDs Proton pump inhibitors | acute tubulointerstitial nephritis |
| Sulphonamides Antiretrovirals | acute crystalline nephropathy |
| High-osmolar iodine radiocontrast agents Hydroxyethyl starches and gelatine solutions | increase in ROS production, vasoconstriction, osmotic nephrosis |
| NSAIDs | altered renal hemodynamics |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petejova, N.; Martinek, A.; Zadrazil, J.; Kanova, M.; Klementa, V.; Sigutova, R.; Kacirova, I.; Hrabovsky, V.; Svagera, Z.; Stejskal, D. Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents—Pathophysiology and Biomarkers—A Review. Int. J. Mol. Sci. 2020, 21, 7115. https://doi.org/10.3390/ijms21197115
Petejova N, Martinek A, Zadrazil J, Kanova M, Klementa V, Sigutova R, Kacirova I, Hrabovsky V, Svagera Z, Stejskal D. Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents—Pathophysiology and Biomarkers—A Review. International Journal of Molecular Sciences. 2020; 21(19):7115. https://doi.org/10.3390/ijms21197115
Chicago/Turabian StylePetejova, Nadezda, Arnost Martinek, Josef Zadrazil, Marcela Kanova, Viktor Klementa, Radka Sigutova, Ivana Kacirova, Vladimir Hrabovsky, Zdenek Svagera, and David Stejskal. 2020. "Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents—Pathophysiology and Biomarkers—A Review" International Journal of Molecular Sciences 21, no. 19: 7115. https://doi.org/10.3390/ijms21197115
APA StylePetejova, N., Martinek, A., Zadrazil, J., Kanova, M., Klementa, V., Sigutova, R., Kacirova, I., Hrabovsky, V., Svagera, Z., & Stejskal, D. (2020). Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents—Pathophysiology and Biomarkers—A Review. International Journal of Molecular Sciences, 21(19), 7115. https://doi.org/10.3390/ijms21197115







