Abstract
UDP-glycosyltransferases (UGTs) play key roles in modulating plant development and responses to environmental challenges. Previous research reported that the Arabidopsis UDP-glucosyltransferase 74E2 (AtUGT74E2), which transfers glucose to indole-3-butyric acid (IBA), is involved in regulating plant architecture and stress responses. Here, we show novel and distinct roles of UGT74E2 in rice. We found that overexpression of AtUGT74E2 in rice could enhance seed germination. This effect was also observed in the presence of IBA and abscisic acid (ABA), as well as salt and drought stresses. Further investigation indicated that the overexpression lines had lower levels of free IBA and ABA compared to wild-type plants. Auxin signaling pathway gene expression such as for OsARF and OsGH3 genes, as well as ABA signaling pathway genes OsABI3 and OsABI5, was substantially downregulated in germinating seeds of UGT74E2 overexpression lines. Consistently, due to reduced IBA and ABA levels, the established seedlings were less tolerant to drought and salt stresses. The regulation of rice seed germination and stress tolerance could be attributed to IBA and ABA level alterations, as well as modulation of the auxin/ABA signaling pathways by UGT74E2. The distinct roles of UGT74E2 in rice implied that complex and different molecular regulation networks exist between Arabidopsis and rice.
1. Introduction
Seed germination is a critical process during the plant lifecycle, which influences the yield of direct seeding in plants. For crop plants, fast germination and high germination rates are crucial agricultural traits that lead to better growth and high yield. Seed germination can be influenced by many external factors, such as temperature, water availability, light, oxygen, stress conditions, etc. Inner cues, particularly phytohormones, also behave as key regulators to determine seed dormancy and germination. Of these, abscisic acid (ABA) and gibberellins (GAs) are well-investigated plant hormones regarding the control of seed dormancy and germination. Gibberellins (GAs) contribute to break seed dormancy and promote germination [1], and several other hormones, including brassinosteroids, ethylene, and cytokinin, show the same effects in enhancing seed germination [2,3]. However, ABA maintains seed dormancy and inhibits seed germination, which are antagonistic effects against the hormones listed above. Many mutants of ABA biosynthesis genes are nondormant or show reduced dormancy, e.g., nced6 nced9 double mutants show reduced seed dormancy [4] and aba3-2 clearly shows a nondormant phenotype [5]. The disruption of ABA catabolism genes, such as CYP707A1, CYP707A2, and CYP707A3, exhibit enhanced seed dormancy [6,7].
Auxin is a critical phytohormone which mediates diverse developmental responses in plants, such as adventitious root initiation, apical dominance, vascular tissue formation, as well as flower and fruit development [6,7]. Previous studies also reported the involvement of auxin in maintaining seed dormancy and inhibiting seed germination. Liu et al. (2013) found that in Arabidopsis, exogenous application of indole-3-acetic acid (IAA) effectively enhanced seed dormancy in a dose-dependent manner [8]. There is no doubt that this process is closely related to auxin homeostasis mediated by auxin biosynthesis, degradation, transport, and conjugate modification [9], which also affects auxin signaling [10]. Consistently, it was also found that disruption of IAA biosynthesis genes YUC1 and YUC6 greatly enhanced seed germination [8]. The arf10arf16 double mutant also showed better germination than wilde type (WT) [8]. A few studies also clarified the elaborate signaling networks and the sophisticated crosstalk occurring between auxin and ABA in regulating a series of developmental processes. Brady et al. (2002) reported that ABI3 is involved in auxin signaling and regulates lateral root development [11]. Shuai et al. (2017) found that exogenous auxin application could repress soybean seed germination by decreasing the gibberellin/abscisic acid (GA/ABA) ratio [12]. A recent study reported that, to prevent the repressive effect of ABA on fruit growth, GA and auxin suppressed ABA accumulation via activation of the expression of a cytochrome P450 monooxygenase that catalyzes ABA catabolism during fruit ripening [13].
Glycosylation is an important way to buffer the homeostasis of phytohormones. Over the past decades, a few auxin-related UDP-glucosyltransferases (UGTs) were identified in a few plants to play crucial roles in regulating plant development and stress responses. For instance, iaglu was the first IAA glycosyltransferase identified in maize [14]. The homolog of iaglu was recently identified in rice, and was shown to be involved in catalyzing IAA glycosylation and regulating seed vigor [15]. In Arabidopsis, a few auxin-related UGTs were also identified, such as UGT84B1 in IAA catalysis, UGT74E2 activity toward indole-3-butyric acid (IBA), and UGT74D1 in IAA and IBA modification [16,17,18]. Recently, Chen (2020) found that UGT76F1 was involved in regulating auxin homeostasis by glycosylation of indole-3-pyruvic acid (IPyA), a major precursor of the auxin indole-3-acetic acid (IAA) biosynthesis, which mediates auxin-dependent hypocotyl elongation in Arabidopsis [19]. Interestingly, several auxin-related UGTs were found to be involved in regulating both plant development and stress responses. It was reported that UGT74E2 catalyzes the glycosylation of IBA and also participates in modulating plant architecture and water deficit stress tolerance [17]. Additionally, Arabidopsis UGT75D1 was found to affect seed germination under stress conditions, likely by regulating ARF16–ABI3 signaling [20].
In this study, in an attempt to generate transgenic rice tolerant to water deficit, we introduced UGT74E2 into rice. However, after analyzing the transgenic plants, we found that UGT74E2 caused effects in rice distinct from those observed in Arabidopsis. Overexpression of UGT74E2 promoted seed germination in the presence of IBA and ABA, and also under abiotic stress conditions. Further investigation indicated that the overexpression lines had lower levels of free IBA and ABA compared to wild-type plants. Auxin signaling pathway gene expression, such as for OsARF and OsGH3 genes and ABA signaling pathway genes OsABI3 and OsABI5, was substantially downregulated in germinating seeds of UGT74E2 overexpression lines. The regulation of seed germination and stress tolerance by UGT74E2 may occur due to modulation of IBA and ABA levels and signaling pathways. The distinct roles of UGT74E2 in rice implied that complex and different molecular regulation networks exist between Arabidopsis and rice.
2. Results
2.1. UGT74E2 Catalyzes IBA Glycosylation and Enhances Seed Germination in Transgenic Rice
To explore the effect of UGT74E2 in rice plant development, we generated transgenic rice lines overexpressing Arabidopsis UGT74E2. In total, we obtained 14 UGT74E2 transgenic lines. Based on the relatively high expression level of UGT74E2 assessed by RT-PCR, OE29 and OE30 were selected for further analysis (Figure 1A). We firstly evaluated the role of UGT74E2 in catalyzing substrate IBA in rice. Under nontreatment conditions, inner IBA-glucose ester (IBA-Glc) levels were very low and beyond detection in WT and UGT74E2-overexpressed plants (Figure 1B). When applying exogenous IBA to the plants, the level of IBA-Glc was strongly increased, and the relative contents of IBA-Glc in the OE29 and OE30 transgenic lines were much higher than that in WT (Figure 1B,C). The peak of IBA-Glc separated by HPLC analysis was further confirmed by LC-MS (Figure 1D). The biochemical assay verified the role of UGT74E2 in catalyzing IBA glycosylation in rice.
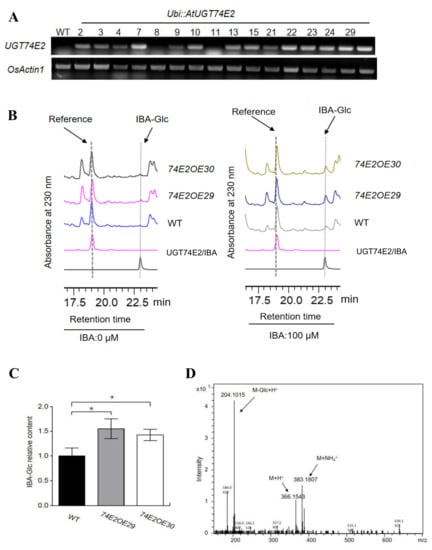
Figure 1.
UGT74E2 catalyzes indole-3-butyric acid (IBA) glycosylation in rice. (A) RT-PCR analysis of the mRNA levels of UGT74E2 in transgenic rice plants. (B) HPLC profiling of IBA glucose conjugates (IBA-Glc) extracted from seven-day-old wild type (WT) and UGT74E2-overexpressed lines after 0 μM IBA or 100 μM IBA treatment for 12 h. (C) Relative IBA-Glc contents in UGT74E2 transgenic rice plants (* p < 0.05). (D) LC-MS confirmation of IBA-glucose ester under positive ion mode.
2.2. UGT74E2 Promotes Seed Germination and Shoot Growth in the Presence of IBA and ABA
In analyzing the biological roles of UGT74E2 in rice, we firstly noticed that overexpression of UGT74E2 resulted in faster seed germination, which also accelerated subsequent seedling growth (Figure 2A,B). This result suggests that UGT74E2 might play important roles in the regulation of seed germination and postgermination growth by glycosylating IBA in rice. Additionally, to see whether the auxin signaling pathway was affected, we detected some crucial genes involved in the auxin signaling pathway in seven-day-old seedlings, including OsARF1, OsARF4, OsARF5, OsARF7, OsARF8 [21], the transcription repressor OsIAA1 [7], as well as auxin amido synthetase genes OsGH3-1, OsGH3-2, OsGH3-5, OsGH3-10, and OsGH3-13 (Figure 2D), which help to maintain auxin homeostasis [22]. qRT-PCR results showed that all of these genes were significantly downregulated in the overexpression plants, demonstrating that auxin signaling and homeostasis was perturbed in rice upon UGT74E2 overexpression (Figure 2C).
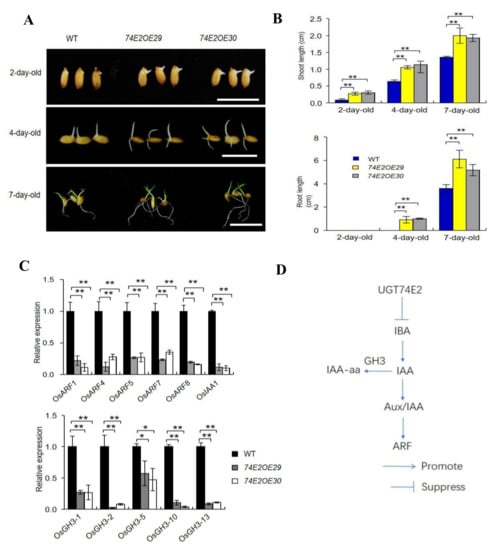
Figure 2.
UGT74E2 affected the seed germination and postgermination growth of the transgenic plants. (A) Seed germination and postgermination growth of UGT74E2 transgenic plants. Here, at least 30 seeds were observed for each line (bars = 3 cm). (B) Shoot and root lengths of UGT74E2 overexpression lines for 2, 4, and 7 days. Bars represent standard deviation of at least 10 seedlings. (C) Expression of auxin signaling pathway genes in seven-day-old UGT74E2-overexpressed rice plants compared to WT. Transcript levels were normalized to the mRNA levels of OsActin. Data shown are means ± SD. Student’s t-test was performed (* p < 0.05, ** p < 0.01). Experiments were conducted for three biological replicates. (D) Location of GH3, IAA, and ARF genes in the auxin biosynthesis and signaling pathway.
Previous studies reported that auxin induces seed dormancy and represses seed germination [8,12]. In our study, we treated the WT, OE29, and OE30 seeds with IBA, and observed that UGT74E2 promoted seed germination and shoot growth in OE29 and OE30 lines in the presence of IBA (Figure 3A–C). In comparison, WT was much more delayed in seed germination and seedling growth. Abscisic acid (ABA), as the major endogenous factor, is also well studied regarding seed germination repression and postgermination growth [23]. Thus, to see whether UGT74E2-overexpressed lines respond to ABA, we also subjected the WT, OE29, and OE30 seeds to ABA treatment (Figure 3A–C). Seed germination and shoot growth of OE29 and OE30 were observed to be more accelerated than in WT (Figure 3A–C), meaning that they are not sensitive to ABA. In a recent study, He et al. (2020) examined the interaction between IAA and ABA, and found that exogenous IAA enhanced ABA-mediated reduction of seed vigor in rice [15]. Accordingly, we also observed that the combination of IBA and ABA suppressed seed germination more strongly than a single hormone (Figure 3A–C). In the presence of both hormones, seed germination and shoot growth were obviously promoted by UGT74E2 overexpression compared to WT. These observations imply that the effect of exogenous IBA and ABA on seed germination in OE29 and OE30 could be alleviated by UGT74E2.
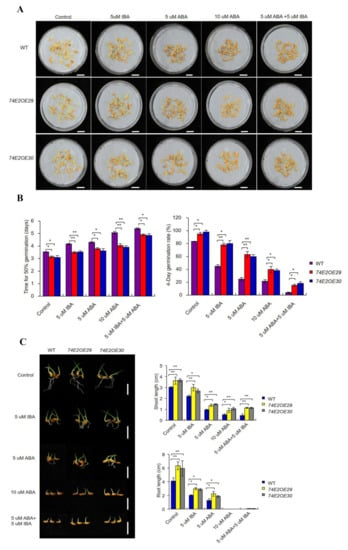
Figure 3.
UGT74E2-overexpressed plants are not sensitive to IBA and abscisic acid (ABA) treatment. (A) Seed germination of WT and UGT74E2 transgenic rice under IBA and ABA treatments for eight days. Here, at least 30 seeds were observed for each line (bars = 3 cm). (B) Time to 50% germination and four-day germination rates were calculated in the presence of IBA and ABA. (C) Shoot and root lengths of UGT74E2-overexpressed lines in the presence of IBA and ABA (scale bars = 3 cm). Experiments were conducted for three biological replicates. Bars represent standard deviation of at least 10 seedlings. Student’s t-test was performed (* p < 0.05, ** p < 0.01).
2.3. UGT74E2 Overexpression Affects Auxin and ABA Response
Glycosyltransferases can regulate auxin and ABA homeostasis in plants [24,25]. To further understand UGT74E2 function, the levels of IBA and ABA were assessed after 24 h of seed imbibition. IBA and ABA levels were significantly decreased in germinating seeds in UGT74E2-overexpressed lines compared to in WT plants (Figure 4A,B). Furthermore, ABA signaling pathway gene expression of ABI3 and ABI5 was also detected by qRT-PCR after seed imbibition for 24, 36, and 48 h, respectively (Figure 4C,D). Consistently, the relative mRNA levels of ABI3 and ABI5 were found to be dramatically downregulated in the UGT74E2-overexpressed lines compared to wild type, largely contributing to break seed dormancy and promote seed germination in UGT4E2-overexpressed lines. Since GA is also a key regulator in seed germination and plays antagonistic roles with ABA [26], we also evaluated the expression of several genes involved in GA biosynthesis in imbibed seeds, including GA3ox2, OsGA20ox1, OsGA20ox2, and OsKO2. The results showed that all four genes were upregulated in UGT74E2-overexpressed lines (Figure S1), thereby enhancing GA biosynthesis. This finding, in another aspect, supported our above observation that ABA levels and signaling declined upon UGT74E2 overexpression.
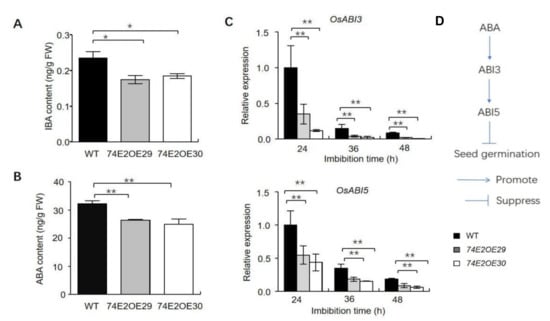
Figure 4.
UGT74E2 altered IBA and ABA contents in germinating rice seeds. (A) The contents of IBA in germinating WT and UGT74E2 transgenic rice seeds after being imbibed for 24 h. (B) The contents of ABA in germinating WT and UGT74E2 transgenic rice seeds after being imbibed for 24 h. (C) Expression of OsABI3 and OsABI5 in germinating seeds in WT and UGT74E2 transgenic rice. Gene expression was normalized to the reference gene OsActin. For each experiment, three replicates were done; data are presented as means ± SD. Student’s t-test for each genotype was performed (* p < 0.05, ** p < 0.01). (D) Role of ABI3 and ABI5 genes in the ABA signaling pathway during seed germination.
2.4. UGT74E2 Overexpression in Rice Showed Enhanced Seed Germination and Shoot Elongation under Abiotic Stress
ABA and auxin play important roles in plant responses to drought and salt stresses. To study the function of UGT74E2 in abiotic stress responses, we treated OE29 and OE30 with NaCl and polyethylene glycol (PEG), respectively, and recorded the seed germination rates after eight days (Figure 5A). Under nontreatment conditions, the germination rates of UGT74E2OE seeds were higher than those of wild type. Upon exposure to 200 mM NaCl, 15% PEG, and 150 mM mannitol, the germination of WT seeds was largely inhibited, while OE29 and OE30 seeds were less affected. After six days, nearly 80% of UGT74E2OE seeds germinated under 200 mM NaCl treatment, and 40% of WT. When subjected to 15% PEG, approximately 80% UGT74E2OE seeds germinated but only 60% WT seeds germinated. In the presence of 150 mM mannitol, the germination rates of UGT74E2OE lines were approximately 90%, while less than 80% WT seeds germinated. On the tenth day, we measured shoot and root elongation under different conditions and found that the shoots and roots of the UGT74E2OE plants were longer than those of WT plants under NaCl, PEG. and mannitol treatments (Figure 5B), suggesting that overexpression of UGT74E2 enhanced seed germination and postgermination growth in rice under drought and salt stresses. IBA and ABA level were further measured in WT and UGT74E2-overexpressed lines, with both hormones found to be significantly increased after stress treatment, indicating that they are important players involved in stress responses. In comparison with WT, IBA and ABA were observed to accumulate less in the UGT74E2-overexpressed lines under both control and stress conditions (Figure 5C,D), further demonstrating that UGT74E2 reduces IBA levels by glycosylation and also influences ABA levels.
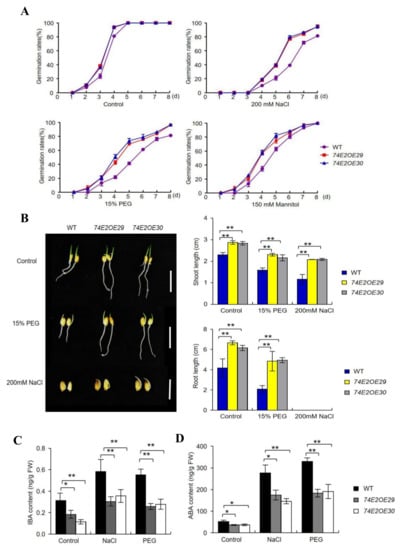
Figure 5.
UGT74E2 transgenic lines are not sensitive to salt and drought stresses during the germination and postgermination stagea. (A) Germination rates of WT and UGT74E2-overexpressed lines under NaCl, polyethylene glycol (PEG), and mannitol treatment. (B) Shoot and root lengths of UGT74E2-overexpressed lines under PEG and NaCl treatment (scale bars = 3 cm). (C) IBA and (D) ABA contents in seven-day-old rice after 200 mM NaCl and 15% PEG treatment for 12 h. Data are means ± SD. For each experiment, three biological replicates were done. Student’s t-test was performed (* p < 0.05, ** p < 0.01).
2.5. Established UGT74E2-Overexpressed Seedlings Are Sensitive to Abiotic Stresses
Next, we tested the growth of established seedlings under drought and salt stresses. The WT, OE29, and OE30 lines were kept under normal conditions in nutrient solution for three weeks to grow, then treated with 200 mM NaCl and 15% PEG, respectively, for one more week. Interestingly, the UGT74E2-overexpressed lines showed sensitivity to NaCl and PEG treatments. OE29 and OE30 were observed to turn yellow and became more withered compared to WT seedlings (Figure 6A); the survival rates of OE29, OE30, and WT were 28.3%, 26.6%, and 78.3%, respectively, under NaCl treatment, and 35.6%, 36.8%, and 81.5%, respectively, under PEG treatment (Figure 6B), demonstrating that the stress response regulated by UGT74E2 is highly dependent on ABA. Undoubtedly, the reduced ABA levels in the UGT74E2-overexpressed lines (Figure 5D) largely accounted for the sensitive phenotype under abiotic stress conditions.
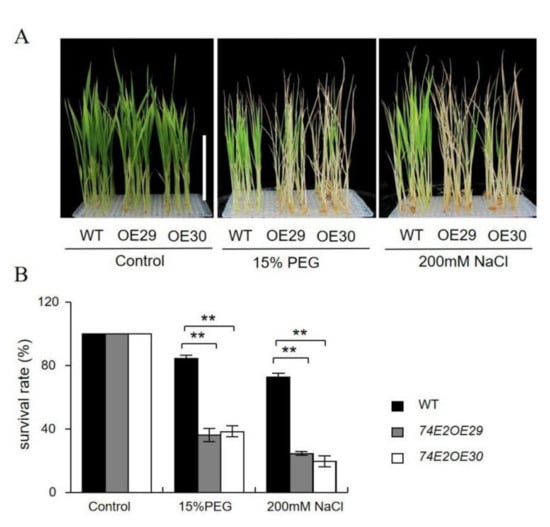
Figure 6.
UGT74E2-overexpressed lines are less tolerant to polyethylene glycol (PEG) and NaCl during the seedling stage. The growth (A) and survival rates (B) of UGT74E2 transgenic rice plants upon exposure to 200 mM NaCl and 15% PEG for seven days (scale bars = 10 cm). For each experiment, three biological replicates were performed. Student’s t-test was performed (** p < 0.01).
2.6. Overexpression of UGT74E2 Leads to Increased Reactive Oxygen Species Accumulation
Abiotic stress tends to result in overproduction of ROS and causes oxidative damage to the plants. IBA levels could also affect ROS accumulation in plants [12]. Thus, we performed nitroblue tetrazoliun (NBT) and diaminobenzidine (DAB) staining on the three-week-old, healthy WT and UGT74E2OE plants after they were exposed to NaCl and PEG treatments. Under nontreatment conditions, no staining was observed for the three lines. When treated with NaCl and PEG, all rice leaves were stained, and the color in OE29 and OE30 was much stronger than that in WT plants (Figure 7A), indicating that ROS accumulates more in UGT74E2-overexpressed plants. To explain the process at the molecular level, we also investigated the expression of antioxidant enzyme encoding genes, including OsSODB, OsAPXO2, OsCAT-A, and OsCAT-B. Consistently, upon exposure to NaCl and PEG conditions, the expression levels of these genes were more upregulated in WT than in the UGT74E2-overexpressed plants (Figure 7B), indicating that UGT74E2 overexpression in rice repressed antioxidant gene expression, thereby reducing ROS scavenging capacity.
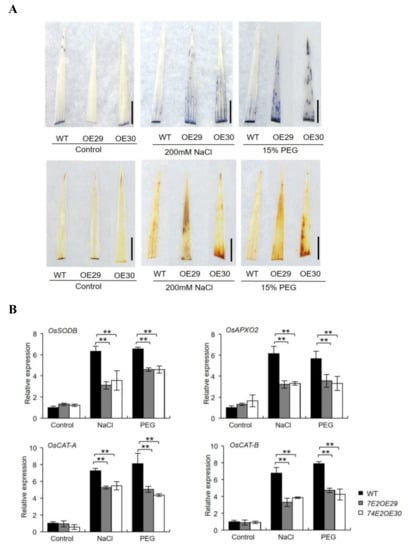
Figure 7.
Accumulation of ROS under NaCl and PEG treatments. (A) Two-week-old seedlings were subjected to nitroblue tetrazoliun (NBT) and diaminobenzidine (DAB) staining after treatment with NaCl and polyethylene glycol (PEG) for 24 h. (scale bars = 1 cm) (B) Genes encoding antioxidant enzymes are downregulated upon UGT74E2 overexpression under abiotic stress. For RNA extraction, seven-day-old rice plants were subjected to 200 mM NaCl and 15% PEG treatment for 12 h. Gene expression was normalized to the reference gene OsActin. For each experiment, three replicates were done; data are presented as means ± SD. Student’s t-test for each genotype was performed (** p < 0.01).
2.7. Expression Analysis of Stress-Responsive Genes under Drought and Salt Stresses
To explore the possible molecular pathways of UGT74E2 involved in regulating stress responses in rice, we evaluated the mRNA levels of four stress-responsive genes with real-time PCR, such as OsHKT1, OsDREB1A, OsDREB1F, OsCOIN, OsMYB4, and OsDREB2A. As shown in Figure 8, under normal conditions, no significant differences were observed. After treatment with NaCl and PEG, the expression of these stress-related genes was greatly elevated, while the upregulation of these genes in wild type was more prominent than in UGT74E2OE lines, implying that overexpression of UGT74E2 could decrease the expression of stress-responsive genes when facing stress conditions, which may contribute to the reduced stress tolerance of the plant.
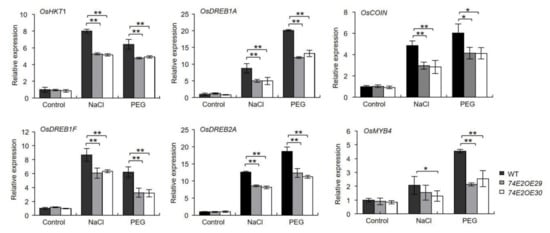
Figure 8.
Expression of stress-responsive genes is downregulated upon UGT74E2 overexpression under abiotic stress. For RNA extraction, seven-day-old rice plants were subjected to 200 mM NaCl and 15% PEG (polyethylene glycol) treatment for 12 h. Gene expression was normalized to the reference gene OsActin. For each experiment, three replicates were done; data are presented as means ± SD. Student’s t-test for each genotype was performed (* p < 0.05, ** p < 0.01)
3. Discussion
Faster seed germination is an index of seed vigor, which also accelerates seedling establishment and growth. In this study, we transferred Arabidopsis UGT74E2 into rice and investigated its role and mechanism in regulating rice seed germination and stress responses. We found that UGT74E2 overexpression accelerated rice seed germination (Figure 2A). This effect was also observed in the presence of IBA and ABA (Figure 3A), and also under abiotic stress conditions (Figure 5A). Our findings were highly consistent with a recent study [15] reporting that knockout of a rice IAA glycosyltransferase gene IAGLU led to lower seed vigor. The iaglu mutant is sensitive to exogenous application of IBA and ABA, and both IBA and ABA contents were increased in the iaglu mutant, further inducing the expression of ARF genes and ABI genes and leading to lower seed germination and slower postgermination growth [15]. A former study also showed that UGT75D1, a glycosyltransferase preferring auxin indole-3-butyric acid, plays positive roles in regulating seed germination and seedling greening under abiotic stress by modulating ARF16–ABI3 signaling [20]. Accordingly, we also found that both IBA and ABA levels declined in UGT74E2-overexpressed plants (Figure 4A,B), and their signaling pathway were also affected, which was supported by the downregulation of auxin signaling/biosynthesis pathway genes, such as ARF, IAA, and GH3 genes (Figure 2C), as well as ABA signaling pathway genes, such as ABI3 and ABI5 (Figure 4C). All these data accounted for the fast seed germination of the UGT74E2-overexpressed lines. This process, modulated by UGT74E2, highlighted the crosstalk between the IBA and ABA signaling pathways. Both IBA and ABA contents decreased in UGT74E2-overexpressed plants, indicating that UGT74E2 may be involved in modulating homeostasis of the two hormones. Auxin was previously reported to repress seed germination by decreasing the GA/ABA ratio in soybean [27]. Accordingly, we found that the GA/ABA ratio increased upon UGT74E2 overexpression (Figure 4 and Figure S1), which accelerated seed germination. This also implies close crosstalk between ABA and GA in UGT74E2-overexpressed lines.
In this study, we observed that the UGT74E2-overexpressed lines exhibited enhanced germination and postgermination growth under abiotic stress conditions (Figure 5A,B), however, when exposing the established seedlings to PEG and NaCl, they showed sensitivity to these treatments (Figure 6A,B). These findings indicate that the UGE74E2-mediated stress response is highly dependent on the ABA signaling pathway. Similar effects were also observed for many other genes involved in the ABA-dependent pathway, such as DREB transcription factors [28,29], the cucumber ATAF1 gene [30], the Arabidopsis UGT76C2 gene, etc. [31]. When the germinating seeds were exposed to water deficit conditions such as drought and salinity, ABA was quickly synthesized and induced ABI3 and ABI5 expression, which then inhibited seed germination and arrested postgermination growth [32,33]. The process is actually a protective mechanism to avoid harm to young seedlings [34,35]. In UGT74E2-overexpressed lines, endogenous ABA levels were decreased compared to WT plants (Figure 4B), therefore, the overexpression (OE) lines exhibited enhanced germination and postgermination growth in response to drought and salinity (Figure 5A,B). However, after transition into the seedling stage, an opposite response was observed for the UGT74E2-overexpressed lines, which showed stress sensitivity to PEG and NaCl treatment in comparison to WT (Figure 6A,B). It is well known that ABA accumulates in response to drought and salt stresses and plays critical roles in responding to stress; the reduced ABA levels observed in our study undoubtedly weakened the stress response in UGT74E2-overexpressed lines (Figure 5D). Supportive to this view, genes contributing to ABA production play positive roles in plant adaptations to abiotic stresses. For instance, overexpression of OsNCED3, a key rate-limiting enzyme in rice enhances abiotic stress tolerance in rice, whereas knockout of this gene results in a stress-sensitive phenotype [34]. HAT1 and HAT3, two class II HD-ZIP transcription factors, were identified as negative regulators of ABA biosynthesis. Plants overexpressing HAT1 exhibit ABA-insensitive phenotype and are less tolerant to drought stress, while hat1hat3 double mutants show enhanced tolerance to drought stress [35].
Inner auxin levels were reported to be closely related to plant stress adaptations [36]. Park et al. (2013) reported that the transgenic potato overexpressing AtYUC6 exhibited high auxin levels and reduced ROS accumulation, thus enhancing the drought-tolerant phenotype [36]. Shi et al. (2014) found that the iaaM-OX transgenic lines with higher endogenous indole-3-acetic acid (IAA) levels exhibited enhanced drought stress resistance, while yuc1yuc2yuc6 triple mutants with lower endogenous IAA levels showed decreased stress resistance in comparison to nontreated WT plants [37]. Moreover, exogenous application of IAA was also shown to reduce H2O2 and O2- levels in response to abiotic stresses, as well as lead to higher activities of antioxidant enzymes to cope with abiotic stresses [30]. In agreement with this point, we found that the UGT74E2-overexpressed plants showed significantly higher ROS production under drought and salt treatment (Figure 7A), suggesting that ROS accumulation might be enhanced via endogenous IBA level reduction and elevated expression of antioxidant enzyme genes upon UGT74E2 overexpression (Figure 7B). In addition, auxin was reported to induce the expression of many abiotic stress-related genes, such as RAB18, RD22, RD29A, RD29B, DREB2A, and DREB2B, thereby contributing to improved abiotic stress resistance [30]. Consistently, in our study, UGT74E2 overexpression repressed some abiotic stress-responsive genes in rice, including OsHKT1, OsDREB1A, OsDREB1F, OsCOIN, OsMYB4, and OsDREB2A (Figure 8), which, to some degree, explained the sensitivity of UGT74E2OE seedlings under confronting stress.
Previous research reported that UGT74E2OE lines exhibited increased IBA-glc as well as free IBA content, with the explanation being that increasing IBA-glucosyltransferase activity could induce IBA synthesis [17]. However, our study found that overexpression of UGT74E2 in rice led to decreased free IBA (Figure 4A) and increased IBA-glc levels (Figure 1B,C). The distinct effects caused by UGT74E2 in rice and Arabidopsis underlined that the complex regulation of auxin homeostasis exists in different plants, and UGT74E2 is likely to be involved in different cues in rice and Arabidopsis. However, our observations were consistent with a few former studies. Liu et al. (2019) reported that ectopic expression of OsIAGT1 led to declined endogenous IAA content and increased levels of IAA-Glc [38]. The induction of UGT74D1 increased the level of OxIAA-Glc and decreased OxIAA remarkably [18]. We also analyzed the expression of auxin signaling pathway genes in UGT74E2-overexpressed rice. In line with the reduced IBA content, expression of auxin signaling pathway genes was reduced in OE29 and OE30 rice plants (Figure 2C). These results further confirmed that UGT74E2 negatively affects auxin levels and signaling in rice, which is different from what is observed in Arabidopsis.
In addition, it was also reported that overexpression of UGT74E2 in Arabidopsis enhances stress responses [17]. However, we found that UGT74E2-overexpressed plants are sensitive to salt and drought stress at the seeding stage. These observations demonstrate that a gene can acquire new functions or cause distinct effects via heterologous expression in other species. Some literature also supports this view. For instance, Quilis et al. (2008) found that transgenic rice overexpressing Arabidopsis NPR1 showed sensitivity to drought and salt stress [39]. However, overexpression of AtNPR1 in tobacco enhanced oxidative stress tolerance in transgenic plants [40]. AtBBX21 could promote seed germination and seedling photomorphogenesis in Arabidopsis [41,42]. Heterologous expression of AtBBX21 in potato was more robust, resulted in more tuber production, and showed higher rates of photosynthesis [43]. Moreover, AtBBX32, as a member of the B-box gene family, could regulate light signal transduction in Arabidopsis [44,45], while increasing grain yield in soybean [45]. The novel roles of the gene acquired by heterologous expression imply complex molecular regulation networks in different plant species. Heterologous expression is worth further exploration, as novel and valuable traits might be generated by introducing this gene into other plants.
4. Materials and Methods
4.1. Cloning and Plasmid Construction of UGT74E2
To generate UGT74E2-overexpressed lines, a full-length UGT74E2 cDNA coding sequence was cloned from Arabidopsis thaliana into a pUN1301 binary vector, which was driven by a ubiquitin promoter. Rice transformation was performed by Biorun biological company (Wuhan, China, http://www.biorun.net/).
4.2. Seed Germination
Thirty seeds per replicate of wild type (WT) and UGT74E2-overexpressed lines were imbibed in 9-cm diameter Petri dishes with 10 mL distilled water at 28 °C for 8 days. Seed germination of wild type and UGT74E2-overexpressed lines was also conducted under various concentrations of IBA, ABA, salt, and PEG treatments. The germination rates of the seeds were tested. Three replications were performed.
4.3. Plant Materials and Stress Treatment
The rice seeds were surface sterilized in 75% ethanol (v/v) for 2 min, then 0.1% mercuric chloride solution for 3 min, and finally washed 3–4 times with deionized water. For salt treatment in nutrient solution, plants were grown in nutrient solution for three weeks at 28 °C, followed by treatment with 200 mM saline or water (control plants) for one more week. At least 60 plants per line were assayed. After salt treatment, the percentage of surviving plants was determined. For drought stress treatment in nutrient solution, plants were grown in nutrient solution for three weeks under normal conditions. The plants were treated with 15% PEG for one more week. Plant phenotypes were observed and photographed with a digital camera (Alpha 6400, Sony, Japan).
4.4. HPLC and LC-MS Analysis
Reverse-phase high performance liquid chromatography (HPLC) was carried out on a Shimadzu HPLC system (Shimadzu, Japan). Samples of 20 µL each were loaded using an autosampler SIL-20A (Prominence SIL-20A) onto a 5 μm C18 column (150 mm × 4.6 mm; Welch, Ultimate, Shanghai). A linear gradient with increasing acetonitrile (solvent A) against double-distilled H2O (solvent B) at a flow rate of 1 mL/min over 40 min was used. Both solutions contained 0.1% H3PO4. Each peak on the chromatogram was monitored between 190 nm and 430 nm.
For liquid chromatography–mass spectrometry (LC-MS) analysis (Shimadzu, Japan), the methods and mobile phases were similar to HPLC conditions, except that 0.1% acetic acid was used instead of 0.1% trifluoroacetic acid. The mass spectrometer was operated in positive electrospray ionization mode with 50 eV and a probe voltage of 5.0 kV. The dry heater was set to 180 °C. Data acquisition and analysis were performed with Xcalibur software (v. 2.0.6) [46].
4.5. Determination of IBA and ABA Concentrations
To determine the IBA and ABA concentrations, 0.1 g fresh leaves of different lines were weighed and 900 μL PBS (pH 7.2–7.4) was added, followed by homogenization and centrifugation for 20 min at 3000× rpm. The supernatant was then collected. To quantify ABA, the plant hormone abscisic acid (ABA) ELISA Kit (MM-3274201, http://www.mmbio.cn/) was used, and the concentrations of ABA in the samples were determined using a microplate reader (Tecan, InfiniteTMM200 PRO, Sursee, Switzerland). To quantify IBA, the plant hormone IBA Kit (MM-3585201, http://www.mmbio.cn/) was used, and the concentrations of IBA in the samples were determined using a microplate reader (Tecan, Infinite TMM200 PRO).
4.6. Detection of H2O2 and Superoxide by Diaminobenzidine (DAB) and Nitrobluetetrazolium (NBT) Staining
Three-week-old plants were treated with 200 mM NaCl and 15% PEG for 24 h. The plant leaves of each line were soaked in DAB and NBT stains for 12 h. The samples were decolorized using 95% ethanol for 1 h, and then decolorized using 75% ethanol at 70 °C. The methods of H2O2 detection and DAB staining were described by Du et al. [47]. The method of NBT staining was described by Wang et al. [48].
4.7. qRT-PCR
To measure the expression of relative genes by real-time PCR (qRT-PCR), the rice leaves were sampled after PEG and NaCl treatment and frozen immediately in liquid nitrogen. Total RNA was extracted from two-week-old rice plants by Trizol reagent and cDNA was obtained by using Primescript RT reagent kit. Real-time PCR reactions were performed using the Bio-Rad real-time thermal cycling system. SYBR-Green was used to detect gene expression abundances. Data were analyzed using the Bio-Rad CFX Manager software. The gene ubiquitin was used as the inner control. Primer information is included in Supplementary Table S1. The experiments were repeated three times and the results were analyzed.
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/1422-0067/21/19/7239/s1. Figure S1. Expression of GA biosynthesis pathway genes in germinating seeds imbibed for 36 h. Table S1. Sequences of primers used in this study.
Author Contributions
Conceptualization, T.W., C.Z., and Y.L; data curation, T.W. and Y.L.; formal analysis, G.D.; funding acquisition, S.J. and B.H.; investigation, T.W. and C.Z.; methodology, T.W., P.L., and T.M.; supervision, B.H.; writing—original draft, T.W. and Y.L.; writing—review and editing, T.C., B.H., and Y.L. All authors read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 31970290 to B.H., approval date: 09 October 2019), the Key R&D Program Projects in Shandong Province (No. 2018GNC110019 to B.H., approval date: 10 January 2017), and the Open Research Fund from the Key Lab of Plant Development and Environment Adaptation Biology, Ministry of Education of China (to S.J., approval date: 03 May 2019).
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
| ABA | Abscisic acid |
| DAB | Diaminobenzidine |
| IBA | Indole-butyric acid |
| HPLC | High Performance Liquid Chromatography |
| NBT | Nitroblue tetrazoliun |
| OE | Overexpression |
| PEG | Polyethylene glycol |
| WT | Wild type |
References
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.; Wu, Y. Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Aoki, H.; Koiwai, H.; Kamiya, Y.; Nambara, E.; Koshiba, T. Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol. 2004, 45, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Luo, J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Wang, X.; Hagen, G.; Guilfoyle, T.J. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 2018, 69, 417–435. [Google Scholar] [CrossRef]
- Brady, S.M.; Sarkar, S.F.; Bonetta, D.; McCourt, P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 2003, 34, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci. Rep. 2017, 7, 12620. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, 11542–11550. [Google Scholar] [CrossRef] [PubMed]
- Szerszen, J.; Szczyglowski, K.; Bandurski, R. iaglu, a gene from Zea mays involved in conjugation of growth hormone indole-3-acetic acid. Science 1994, 265, 1699–1701. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Yang, B.; Sun, S.; Peng, L.; Wang, Z. Indole-3-acetate beta-glucosyltransferase OsIAGLU regulates seed vigour through mediating crosstalk between auxin and abscisic acid in rice. Plant Biotechnol. J. 2020, 18, 1933–1945. [Google Scholar] [CrossRef]
- Jackson, R.G.; Kowalczyk, M.; Li, Y.; Higgins, G.; Ross, J.; Sandberg, G.; Bowles, D.J. Over-expression of an Arabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: Phenotypic characterisation of transgenic lines. Plant J. 2002, 32, 573–583. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef]
- Tanaka, K.; Hayashi, K.; Natsume, M.; Kamiya, Y.; Sakakibara, H.; Kawaide, H.; Kasahara, H. UGT74D1 catalyzes the glucosylation of 2-Oxindole-3-Acetic acid in the auxin metabolic pathway in Arabidopsis. Plant Cell Physiol. 2014, 55, 218–228. [Google Scholar] [CrossRef]
- Chen, L.; Huang, X.; Zhao, S.; Xiao, D.; Xiao, L.; Tong, J.; Wang, W.; Lia, Y.; Ding, Z.; Hou, B. IPyA glucosylation mediates light and temperature signaling to regulate auxin-dependent hypocotylelongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 6910–6917. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, S.; Jiang, X.; Dong, R.; Li, P.; Li, Y.; Hou, B. Ectopic expression of UGT75D1, a glycosyltransferase preferring indole-3-butyric acid, modulates cotyledon development and stress tolerance in seed germination of Arabidopsis thaliana. Plant Mol. Biol. 2016, 90, 77–93. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Kaur, N.; Tyagi, A.K.; Khurana, J.P. The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct. Integr. Genomic. 2006, 6, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Priest, D.M.; Ambrose, S.J.; Vaistij, F.E.; Elias, L.; Higgins, G.S.; Ross, A.R.S.; Abrams, S.R.; Bowles, D.J. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 2006, 46, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, H.; Leung, D.W.M.; He, Z.; Zhang, J.; Peng, X.; Liu, E. Overexpression of OsIAAGLU reveals a role for IAA–glucose conjugation in modulating rice plant architecture. Plant Cell Rep. 2019, 38, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, Q.; Guo, J.; Wang, P.; Song, P.; Jia, Q.; Zhang, X.; Kudla, J.; Zhang, W.; Zhang, Q. Peroxisomal CuAOζ and its product H2O2 regulate the distribution of auxin and IBA-dependent lateral root development in Arabidopsis. J. Exp. Bot. 2017, 68, 4851–4867. [Google Scholar] [CrossRef] [PubMed]
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W.J.J. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under stress: Involvement of auxin and cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta. 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Cao, Y.; Qi, C.; Li, S.; Liu, L.; Wang, G.; Mao, A.; Ren, S.; Guo, Y. CsATAF1 positively regulates drought stress tolerance by an ABA-dependent pathway and by promoting ROS scavenging in cucumber. Plant Cell Physiol. 2018, 59, 930–945. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Dong, R.; Hou, B. AtUGT76C2, an Arabidopsis cytokinin glycosyltransferase is involved in drought stress adaptation. Plant Sci. 2015, 236, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, L.; Mongrand, S.; Chua, N. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 2001, 98, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, L.; Mongrand, S.; McLachlin, D.T.; Chait, B.T.; Chua, N. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 2002, 32, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in Rice. Front. Plant Sci. 2018, 6, 162. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, D.; Zhou, H.; Zheng, T.; Yin, Y.; Lin, H. Transcription factor HAT1 is a substrate of SnRK2.3 kinase and negatively regulates ABA synthesis and signaling in Arabidopsis responding to drought. PLoS Genet. 2018, 14, e1007336. [Google Scholar] [CrossRef]
- Park, H.C.; Cha, J.; Yun, D. Roles of YUCCAs in auxin biosynthesis and drought stress responses in plants. Plant Signal. Behav. 2013, 8, e24495. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol. Bioch. 2013, 82, 209–217. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, T.; Xiao, D.; Zhao, S.; Lin, J.; Wang, T.; Li, Y.; Hou, B. OsIAGT1 is a glucosyltransferase gene involved in the glucose conjugation of auxins in rice. Rice 2019, 12, 92. [Google Scholar] [CrossRef]
- Quilis, J.; Peñas, G.; Messeguer, J.; Brugidou, C.; Segundo, B.S. The Arabidopsis AtNPR1 inversely modulates defense responses against fungal, bacterial or viral pathogens while conferring hypersensitivity to abiotic stresses in transgenic rice. Mol. Plant Microbe. Interact. 2008, 21, 1215–1231. [Google Scholar] [CrossRef]
- Srinivasan, T.; Kumar, K.R.R.; Meur, P.B.K.G. Heterologous expression of Arabidopsis NPR1 (AtNPR1) enhances oxidative stress tolerance in transgenic tobacco plants. Biotechnol. Lett. 2009, 31, 1343–1351. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, Y.; Li, J.; Lin, F.; Holm, M.; Deng, X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 2016, 113, 7655–7660. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, Y.; Li, J.; Holm, M.; Deng, X.W. The B-Box domain protein BBX21 promotes photomorphogenesis. Plant Physiol. 2018, 176, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Crocco, C.D.; Ocampo, G.G.; Ploschuk, E.L.; Mantese, A.; Bottoa, J.F. Heterologous expression of AtBBX21 enhances the rate of photosynthesis and alleviates photoinhibition in solanum tuberosum. Plant Physiol. 2018, 177, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Holtan, H.E.; Bandong, S.; Marion, C.M.; Adam, L.; Tiwari, S.; Shen, Y.; Maloof, J.N.; Maszle, D.R.; Ohto, M.; Preuss, S.; et al. BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol. 2011, 156, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Preuss, S.B.; Meister, R.; Xu, Q.; Urwin, C.P.; Tripodi, F.A.; Screen, S.E.; Anil, V.S.; Zhu, S.; Morrell, J.A.; Liu, G.; et al. Expression of the Arabidopsis thaliana BBX32 gene in soybean increases grain yield. PLoS ONE 2018, 7, e30717. [Google Scholar] [CrossRef] [PubMed]
- Heidler, J.; Valek, L.; Wittig, I.; Tegeder, I. Redox-proteomes of human NOS1-transduced versus MOCK SH-SY5Y neuroblastoma cells under full nutrition, serum-free starvation, and rapamycin treatment. Data Brief 2018, 21, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, P.; Chen, J.; Song, C. Comprehensive functional Aanalysis of the catalase gene family in Arabidopsis thaliana. J. Interg. Plant Biol. 2008, 50, 1318–1326. [Google Scholar] [CrossRef]
- Wang, C.; Jing, R.; Mao, X.; Chang, X.; Li, A. TaABC1, a member of the activity ofbc1complex protein kinase family from common wheat, confers enhanced tolerance to abiotic stresses in Arabidopsis. J. Exp. Bot. 2010, 62, 1299–1311. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).