A Medium-Throughput System for In Vitro Oxidative Stress Assessment in IPEC-J2 Cells
Abstract
1. Introduction
2. Results
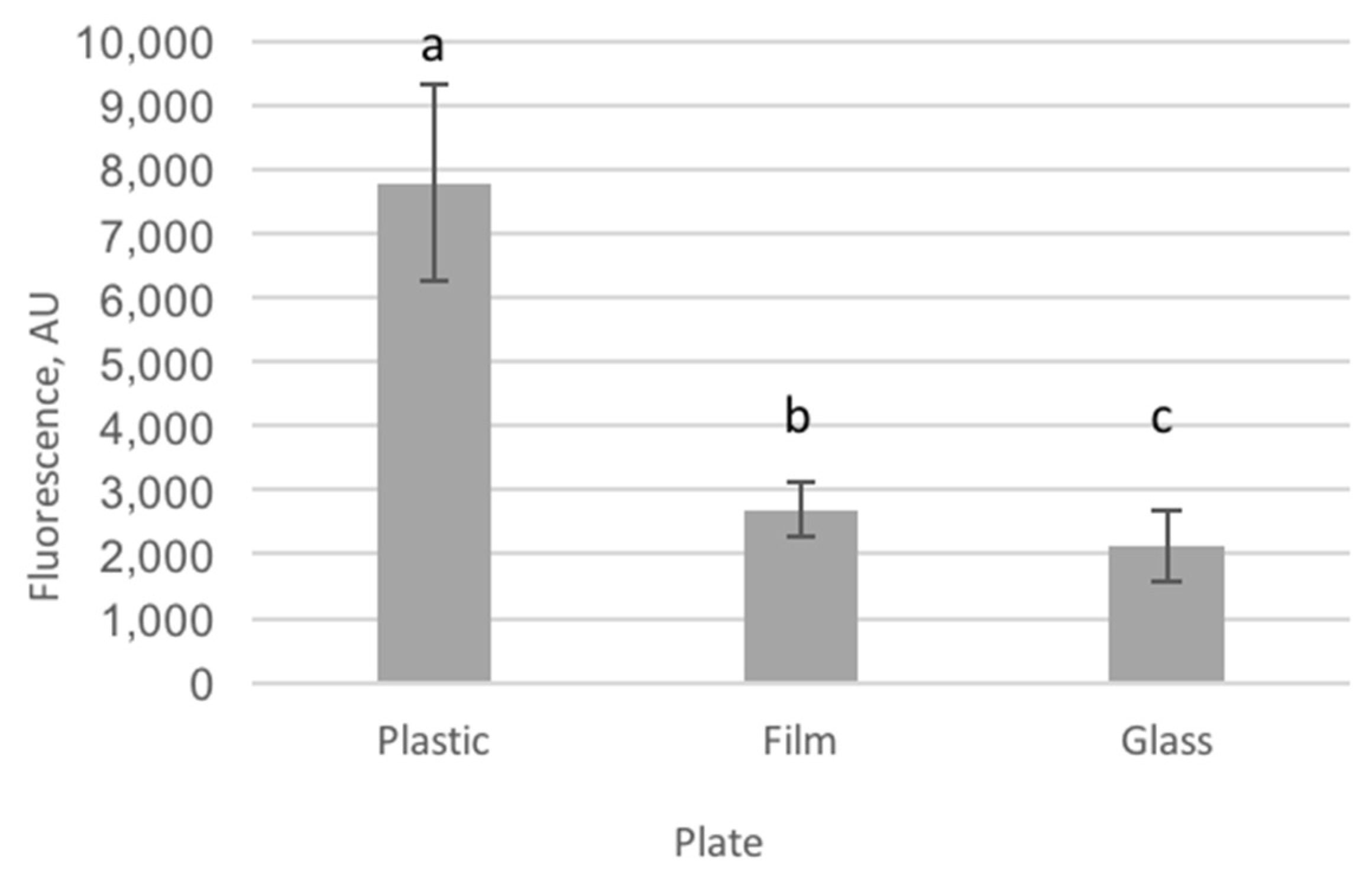
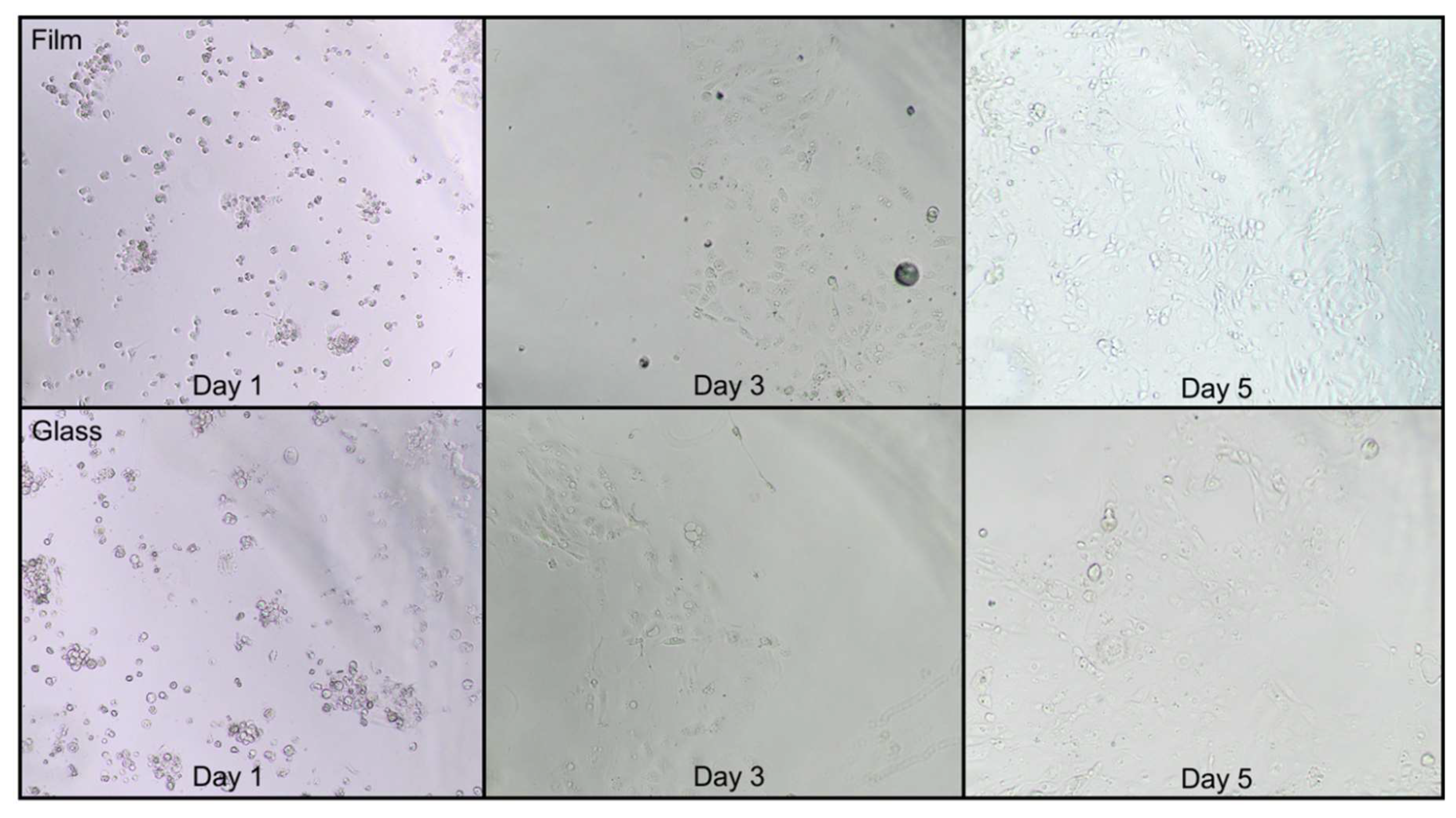
2.1. Imaging Plate Selection for Cell Culture
2.2. Intracellular Oxidative Stress
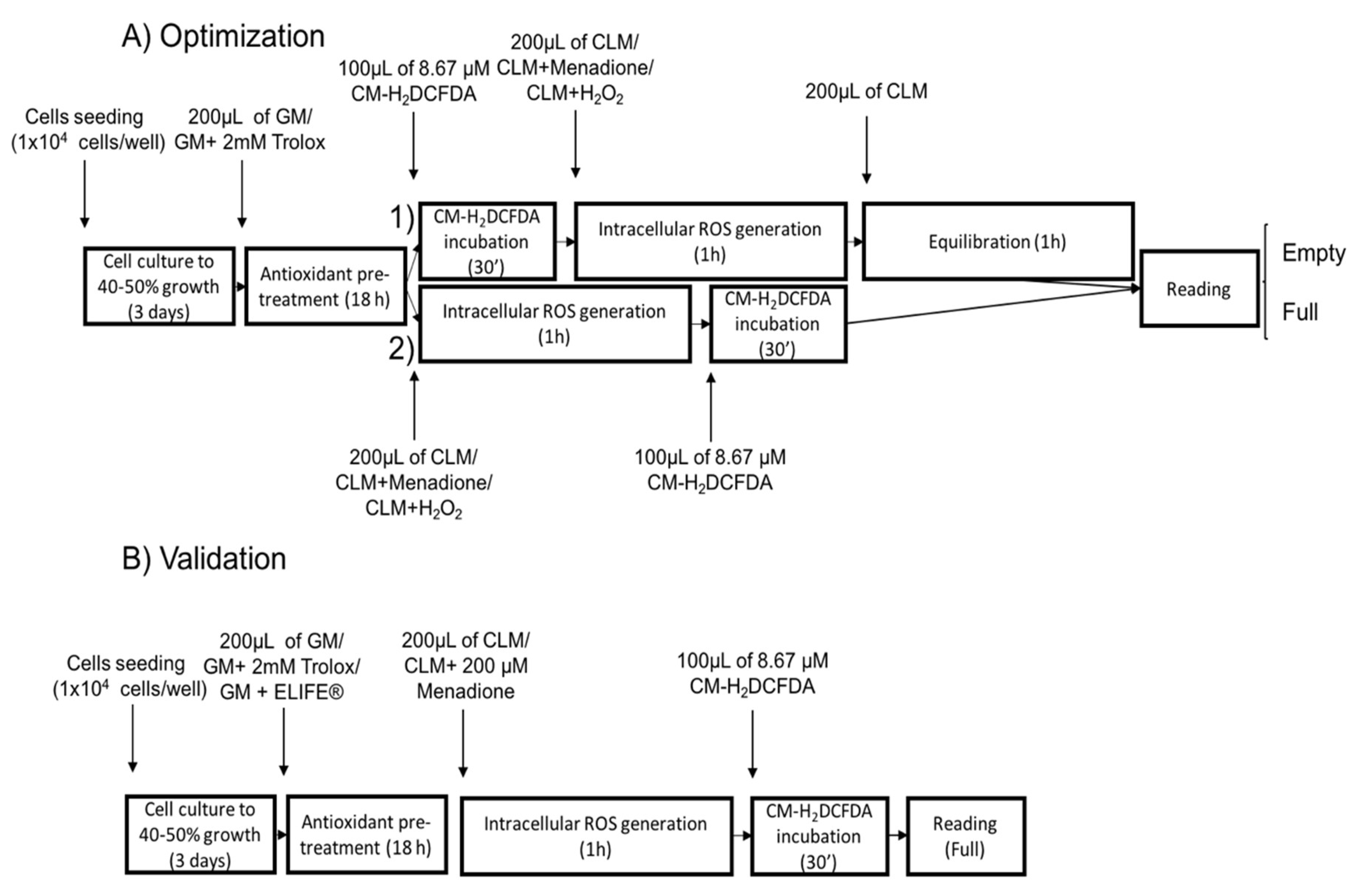
2.2.1. Plate Reading Conditions
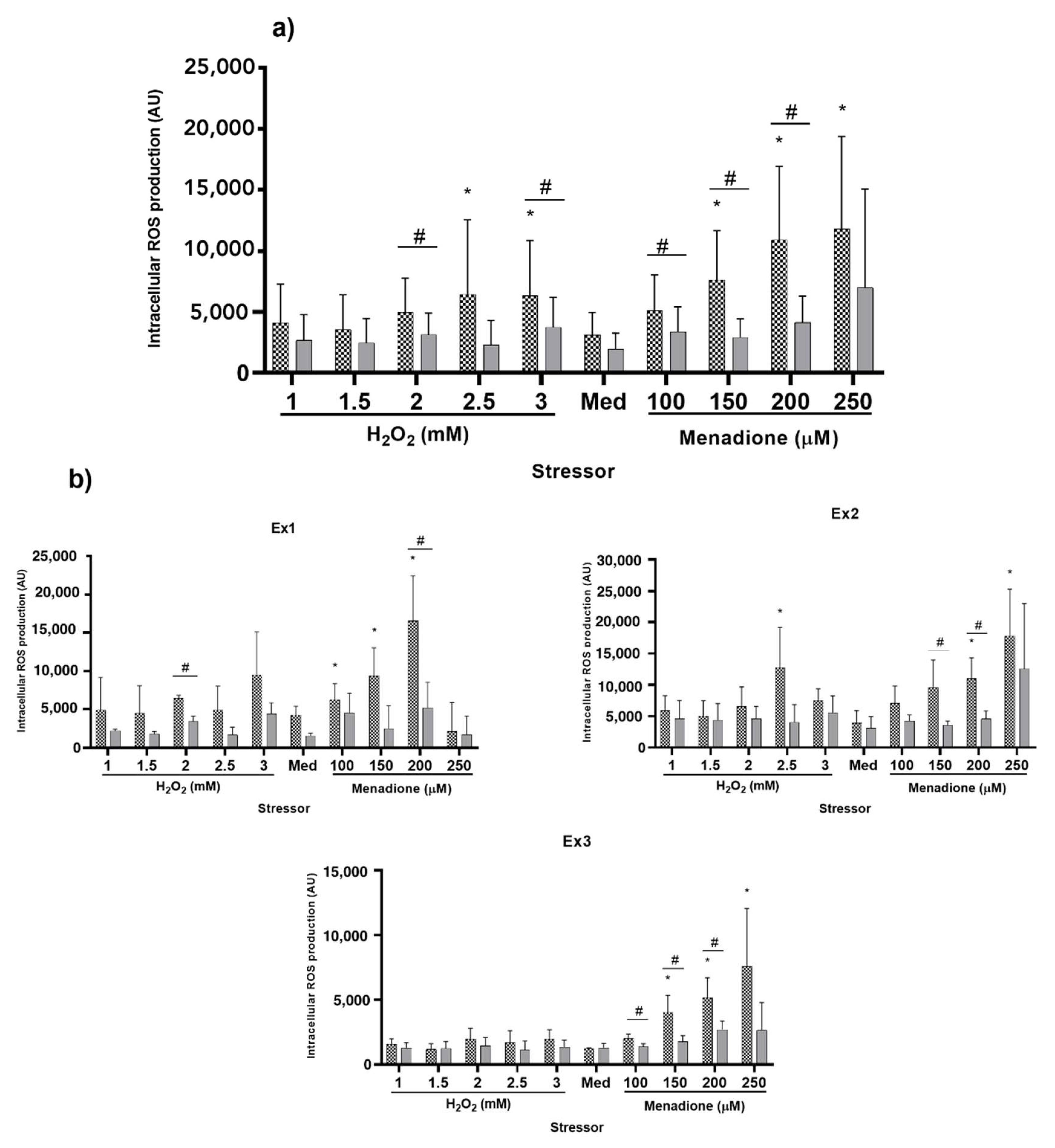
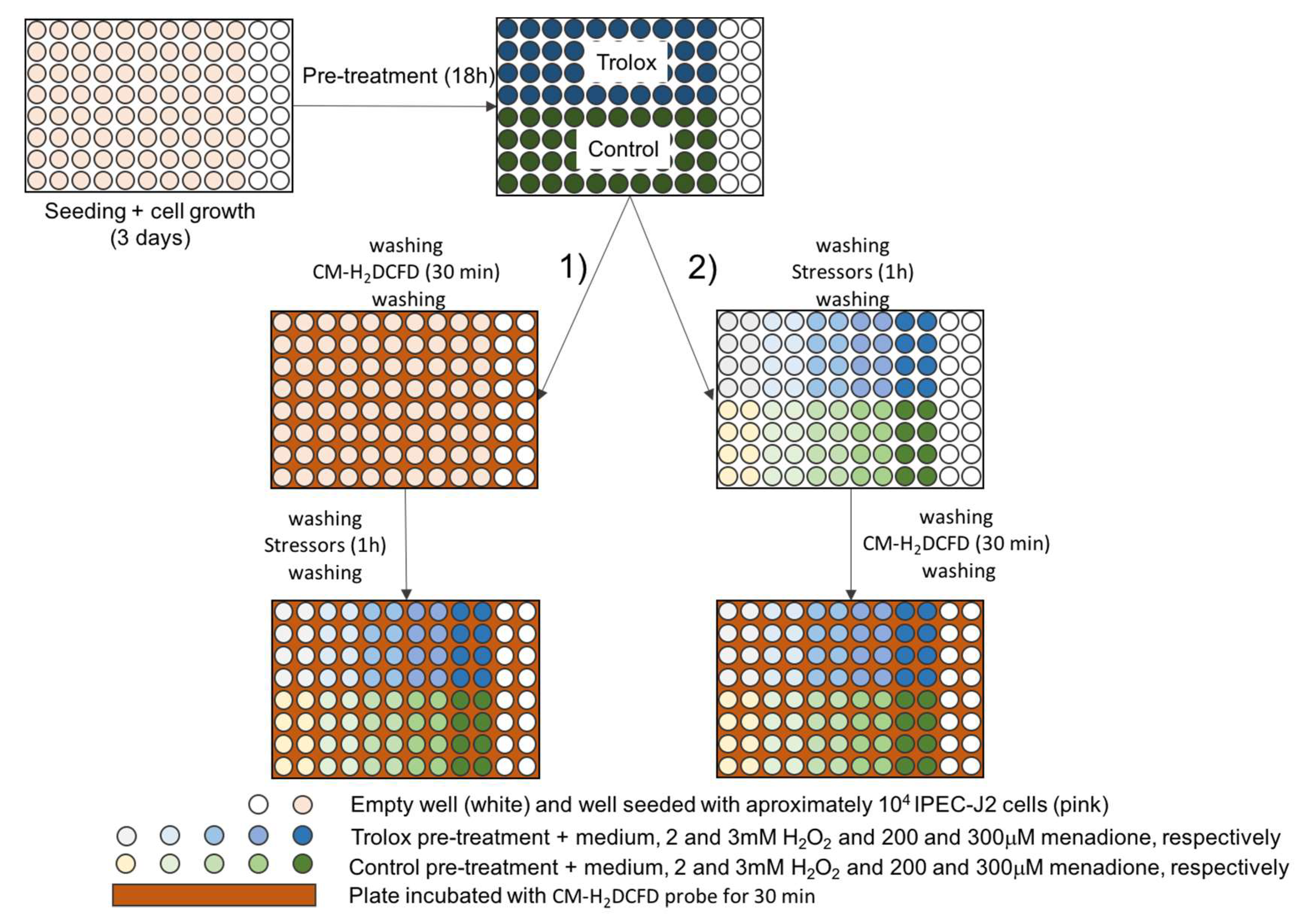
2.2.2. Experimental Setup
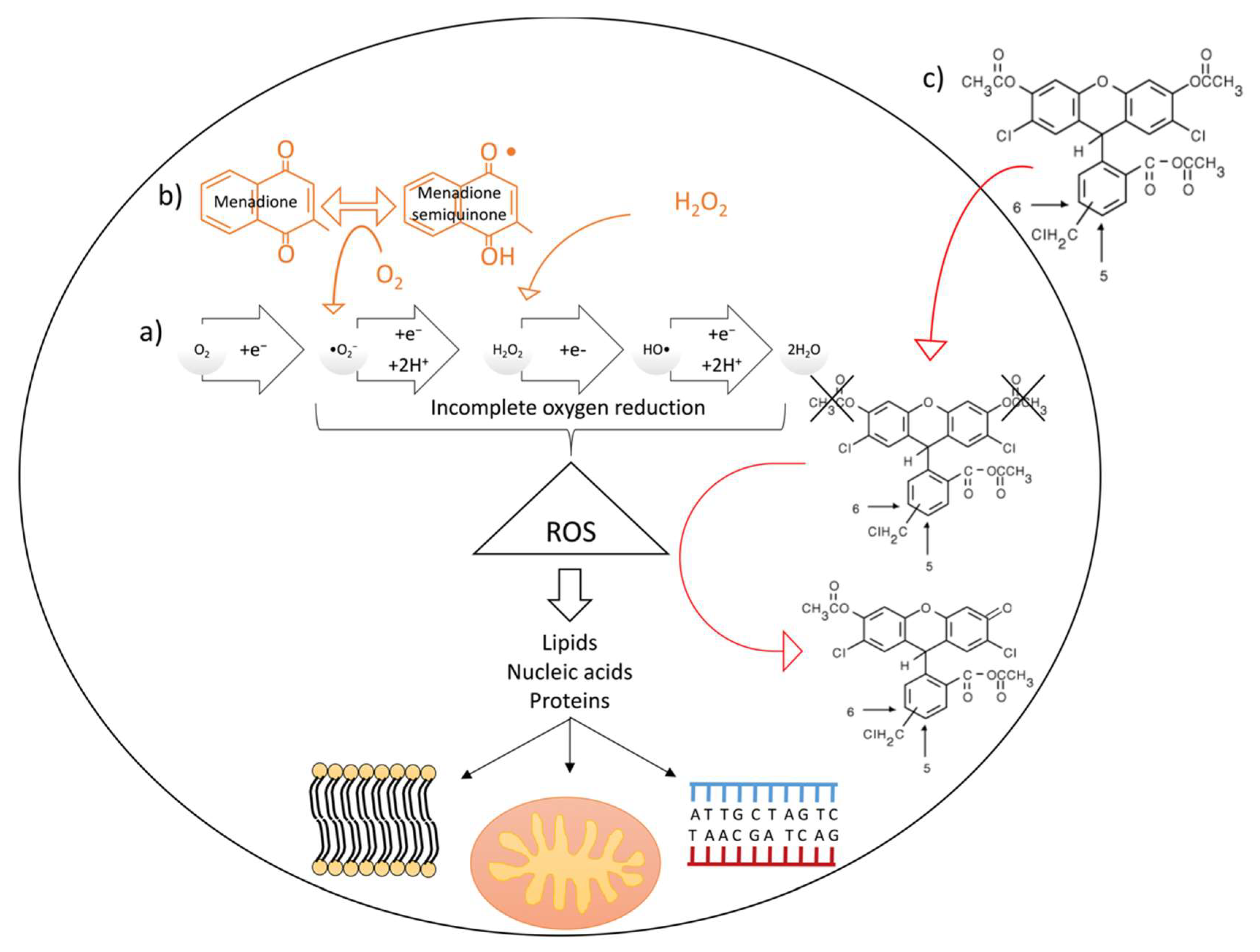
2.2.3. Oxidative Stress Assessment
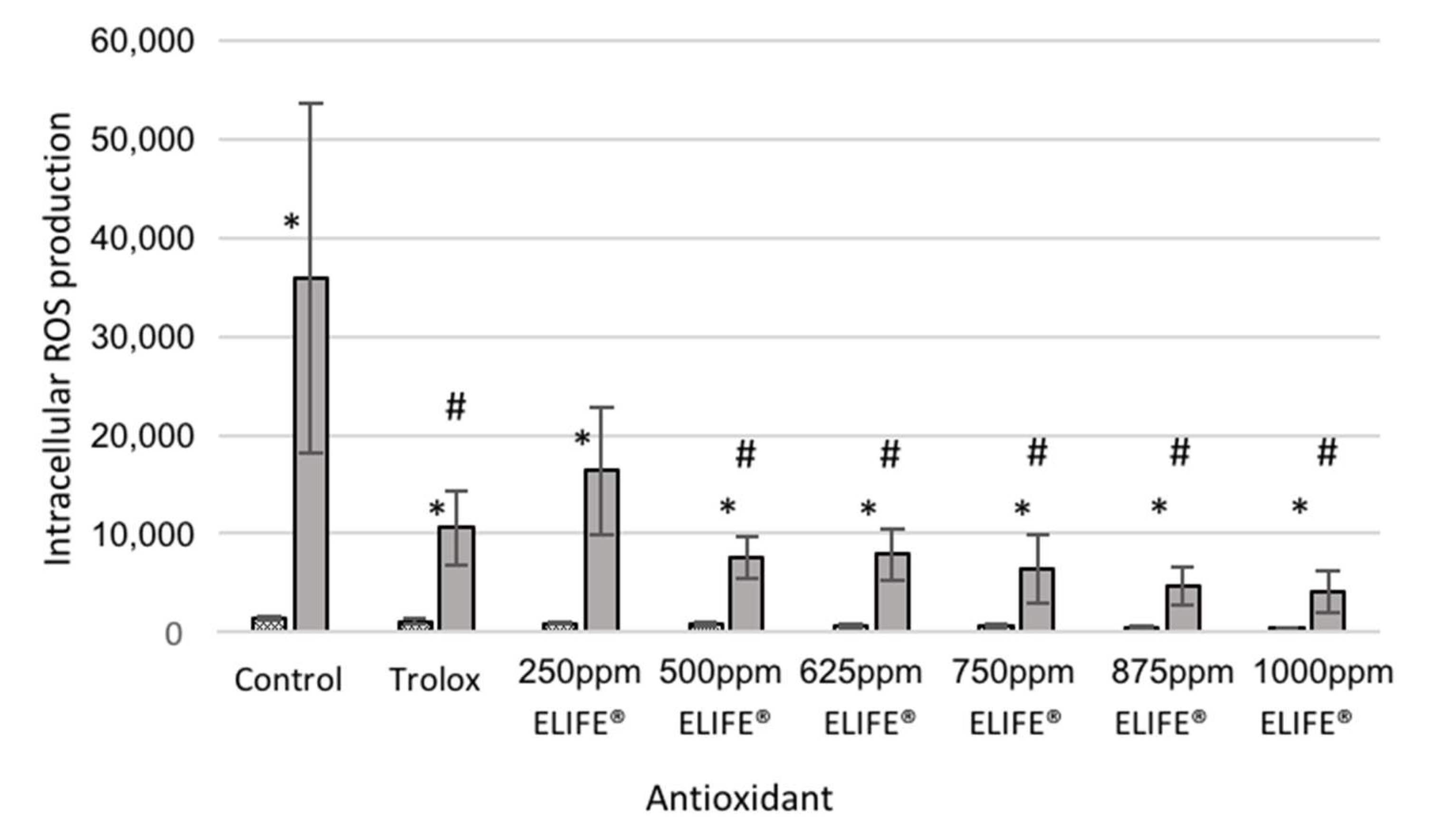
2.2.4. Validation of the System with ELIFE
3. Discussion
3.1. Imaging Plate Selection for Cell Culture
3.2. Intracellular Oxidative Stress
3.2.1. Plate Reading Conditions
3.2.2. Experimental Setup
3.2.3. Oxidative Stress Assessment
3.2.4. Validation of the System with ELIFE
4. Materials and Methods
4.1. Well Plate for In Vitro Anti-Oxidant Capacity
4.2. Cell Line and Culture Conditions
4.3. Antioxidant Pre-Treatment
4.4. Oxidative Stress Assessment
4.5. Cell Viability
4.6. Validation of the System with ELIFE
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forman, H.J.; Maiorino, M.; Ursini, F. Signaling Functions of Reactive Oxygen Species. Biochemistry 2010, 49, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Torres, A.; Jones-Carson, J.; Mastroeni, P.; Ischiropoulos, H.; Fang, F.C. Antimicrobial Actions of the Nadph Phagocyte Oxidase and Inducible Nitric Oxide Synthase in Experimental Salmonellosis. I. Effects on Microbial Killing by Activated Peritoneal Macrophages in vitro. J. Exp. Med. 2000, 192, 227–236. [Google Scholar] [CrossRef]
- Rao, R.K.; Basuroy, S.; Rao, V.U.; Karnaky, K.J., Jr.; Gupta, A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002, 368, 471. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2012; pp. 729–737. [Google Scholar]
- Herring, T.A.; Cuppett, S.L.; Zempleni, J. Genomic implications of H2O2 for cell proliferation and growth of Caco-2 cells. Dig. Dis. Sci. 2007, 52, 3005–3015. [Google Scholar] [CrossRef][Green Version]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Phull, P.S.; Green, C.J.; Jacyna, M.R. A radical view of the stomach: The role of oxygen-derived free radicals and anti-oxidants in gastroduodenal disease. Eur. J. Gastroenterol. Hepatol. 1995, 7, 265–274. [Google Scholar]
- Bélanger, S.; Lavoie, J.-C.; Chessex, P. Influence of bilirubin on the antioxidant capacity of plasma in newborn infants. Neonatology 1997, 71, 233–238. [Google Scholar] [CrossRef]
- Cao, J.; Guo, F.; Zhang, L.; Dong, B.; Gong, L. Effects of dietary Selenomethionine supplementation on growth performance, antioxidant status, plasma selenium concentration, and immune function in weaning pigs. J. Anim. Sci. Biotechnol. 2014, 5, 46. [Google Scholar] [CrossRef]
- Chauhan, S.; Celi, P.; Leury, B.; Clarke, I.; Dunshea, F. Dietary antioxidants at supranutritional doses improve oxidative status and reduce the negative effects of heat stress in sheep. J. Anim. Sci. 2014, 92, 3364–3374. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Hu, L.; Suo, Y.; Zhang, L.; Jin, F.; Feng, X.; Teng, N.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poultry Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, E.; Laliena, A.; Vallejo, D.; Tuñón, M.; Rodríguez-López, J.; Rodríguez-Pérez, R.; García-Fernández, M. Effects of a low-fat diet with antioxidant supplementation on biochemical markers of multiple sclerosis long-term care residents. Nutr. Hosp. 2013, 28, 2229–2235. [Google Scholar] [PubMed]
- Xiong, X.; Yang, H.; Wang, X.; Hu, Q.; Liu, C.; Wu, X.; Deng, D.; Hou, Y.; Nyachoti, C.; Xiao, D. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015, 93, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Feairheller, D.L.; Park, J.Y.; Sturgeon, K.M.; Williamson, S.T.; Diaz, K.M.; Veerabhadrappa, P.; Brown, M.D. Racial Differences in Oxidative Stress and Inflammation: In Vitro and In Vivo. Clin. Transl. Sci. 2011, 4, 32–37. [Google Scholar] [CrossRef]
- Kvietys, P.R.; Granger, D.N. Endothelial cell monolayers as a tool for studying microvascular pathophysiology. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G1189–G1199. [Google Scholar] [CrossRef]
- Vergauwen, H.; Prims, S.; Degroote, J.; Wang, W.; Casteleyn, C.; van Cruchten, S.; de Smet, S.; Michiels, J.; van Ginneken, C. In Vitro Investigation of Six Antioxidants for Pig Diets. Antioxidants 2016, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, H.; Tambuyzer, B.; Jennes, K.; Degroote, J.; Wang, W.; De Smet, S.; Michiels, J.; Van Ginneken, C. Trolox and ascorbic acid reduce direct and indirect oxidative stress in the IPEC-J2 cells, an in vitro model for the porcine gastrointestinal tract. PLoS ONE 2015, 10, e0120485. [Google Scholar] [CrossRef]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 cell-based quantitative antioxidant activity assay for antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef]
- Halliwell, B. Cell culture, oxidative stress, and antioxidants: Avoiding pitfalls. Biomed. J. 2014, 37, 99. [Google Scholar] [CrossRef]
- Girard-Lalancette, K.; Pichette, A.; Legault, J. Sensitive cell-based assay using DCFH oxidation for the determination of pro-and antioxidant properties of compounds and mixtures: Analysis of fruit and vegetable juices. Food Chem. 2009, 115, 720–726. [Google Scholar] [CrossRef]
- Jensen, G.S. Cell-Based Antioxidant Protection Assay. U.S. Patent 8,465,988, 18 June 2013. [Google Scholar]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Liu, C.-S.; Glahn, R.P.; Liu, R.H. Assessment of carotenoid bioavailability of whole foods using a Caco-2 cell culture model coupled with an in vitro digestion. J. Agri. Food Chem. 2004, 52, 4330–4337. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, R.; Barrabés, S.; Sarrats, A.; Rudd, P.M.; de Llorens, R. Altered glycosylation in tumours focused to cancer diagnosis. Dis. Markers 2008, 25, 207–218. [Google Scholar] [CrossRef]
- Roura, E.; Koopmans, S.-J.; Lallès, J.-P.; Le Huerou-Luron, I.; de Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef] [PubMed]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef] [PubMed]
- Støy, A.C.F.; Heegaard, P.M.; Sangild, P.T.; Østergaard, M.V.; Skovgaard, K. Gene expression analysis of the IPEC-J2 cell line: A simple model for the inflammation-sensitive preterm intestine. Isrn Genom. 2013, 2013, 980651. [Google Scholar] [CrossRef][Green Version]
- Vergauwen, H. The IPEC-J2 cell line. In The Impact of Food Bioactives on Health; Springer: Berlin/Heidelberg, Germany, 2015; pp. 125–134. [Google Scholar]
- Zakrzewski, S.S.; Richter, J.F.; Krug, S.M.; Jebautzke, B.; Lee, I.-F.M.; Rieger, J.; Sachtleben, M.; Bondzio, A.; Schulzke, J.D.; Fromm, M. Improved cell line IPEC-J2, characterized as a model for porcine jejunal epithelium. PLoS ONE 2013, 8, e79643. [Google Scholar] [CrossRef]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef]
- Holmdahl, R.; Sareila, O.; Pizzolla, A.; Winter, S.; Hagert, C.; Jaakkola, N.; Kelkka, T.; Olsson, L.M.; Wing, K.; Backdahl, L. Hydrogen peroxide as an immunological transmitter regulating autoreactive T cells. Antioxid. Redox Signal. 2013, 18, 1463–1474. [Google Scholar] [CrossRef]
- van der Vliet, A.; Janssen-Heininger, Y.M. Hydrogen peroxide as a damage signal in tissue injury and inflammation: Murderer, mediator, or messenger? J. Cell. Biochem. 2014, 115, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Matzno, S.; Yamaguchi, Y.; Akiyoshi, T.; Nakabayashi, T.; Matsuyama, K. An attempt to evaluate the effect of vitamin K3 using as an enhancer of anticancer agents. Biol. Pharm. Bull. 2008, 31, 1270–1273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baran, I.; Ganea, C.; Scordino, A.; Musumeci, F.; Barresi, V.; Tudisco, S.; Privitera, S.; Grasso, R.; Condorelli, D.F.; Ursu, I. Effects of menadione, hydrogen peroxide, and quercetin on apoptosis and delayed luminescence of human leukemia Jurkat T-cells. Cell Biochem. Biophys. 2010, 58, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Lord, T.; Kuczera, L.; Koppers, A.J.; Naumovski, N.; Connaughton, H.; Baker, M.A.; De Iuliis, G.N. On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology 2013, 1, 192–205. [Google Scholar] [CrossRef]
- Bladen, C.L.; Kozlowski, D.J.; Dynan, W.S. Effects of low-dose ionizing radiation and menadione, an inducer of oxidative stress, alone and in combination in a vertebrate embryo model. Radiat. Res. 2012, 178, 499–503. [Google Scholar] [CrossRef]
- Dunning, S.; Hannivoort, R.A.; de Boer, J.F.; Buist-Homan, M.; Faber, K.N.; Moshage, H. Superoxide anions and hydrogen peroxide inhibit proliferation of activated rat stellate cells and induce different modes of cell death. Liver Int. 2009, 29, 922–932. [Google Scholar] [CrossRef]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef]
- Zaccaria, M.; Ludovici, M.; Sanzani, S.M.; Ippolito, A.; Cigliano, R.A.; Sanseverino, W.; Scarpari, M.; Scala, V.; Fanelli, C.; Reverberi, M. Menadione-Induced Oxidative Stress Re-Shapes the Oxylipin Profile of Aspergillus flavus and Its Lifestyle. Toxins 2015, 7, 4315–4329. [Google Scholar] [CrossRef]
- Badarinath, A.; Rao, K.M.; Chetty, C.M.S.; Ramkanth, S.; Rajan, T.; Gnanaprakash, K. A review on in-vitro antioxidant methods: Comparisions, correlations and considerations. Int. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Rey, A.; Segura, J.; Olivares, A.; Cerisuelo, A.; Piñeiro, C.; López-Bote, C. Effect of micellized natural (D-α-tocopherol) vs. synthetic (DL-α-tocopheryl acetate) vitamin E supplementation given to turkeys on oxidative status and breast meat quality characteristics. Poultry Sci. 2015, 94, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Mohamed, A.; Dröse, S.; Brandt, U.; Fleming, I.; Brandes, R.P. Analysis of dichlorodihydrofluorescein and dihydrocalcein as probes for the detection of intracellular reactive oxygen species. Free Radic. Res. 2004, 38, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Forkink, M.; Smeitink, J.A.; Brock, R.; Willems, P.H.; Koopman, W.J. Detection and manipulation of mitochondrial reactive oxygen species in mammalian cells. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, X.; Wang, X.; Xu, C.; Guo, Q.; Zhu, L.; Zhu, S.; Xu, J. Pre-protective effect of lipoic acid on injury induced by H2O2 in IPEC-J2 cells. Mol. Cell. Biochem. 2013, 378, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cordina, N.M.; Sayyadi, N.; Parker, L.M.; Everest-Dass, A.; Brown, L.J.; Packer, N.H. Reduced background autofluorescence for cell imaging using nanodiamonds and lanthanide chelates. Sci. Rep. 2018, 8, 4521. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.; Berthier, E.; Beebe, D.J. Assessment of enhanced autofluorescence and impact on cell microscopy for microfabricated thermoplastic devices. Anal. Chem. 2012, 85, 44–49. [Google Scholar] [CrossRef]
- Eppendorf Cell Imaging Plates. Available online: https://online-shop.eppendorf.be/BE-en/Cell-Culture-and-Imaging-Consumables-110320/Cell-Imaging-Consumables-120210/Eppendorf-Cell-Imaging-Plates-PF-19458.html (accessed on 30 September 2020).
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.M.; Szczepanowska, J.; Koopman, W.J.; Wieckowski, M.R. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta BBA Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Marjanovič, I.; Kandušer, M.; Miklavčič, D.; Keber, M.M.; Pavlin, M. Comparison of flow cytometry, fluorescence microscopy and spectrofluorometry for analysis of gene electrotransfer efficiency. J. Membr. Biol. 2014, 247, 1259–1267. [Google Scholar] [CrossRef]
- Criddle, D.N.; Gillies, S.; Baumgartner-Wilson, H.K.; Jaffar, M.; Chinje, E.C.; Passmore, S.; Chvanov, M.; Barrow, S.; Gerasimenko, O.V.; Tepikin, A.V. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J. Biol. Chem. 2006, 281, 40485–40492. [Google Scholar] [CrossRef]
- Shen, J.; Khan, N.; Lewis, L.D.; Armand, R.; Grinberg, O.; Demidenko, E.; Swartz, H. Oxygen consumption rates and oxygen concentration in Molt-4 cells and their mtDNA depleted (ρ0) mutants. Biophys. J. 2003, 84, 1291–1298. [Google Scholar] [CrossRef]
- Scott, J.W.; Cort, W.M.; Harley, H.; Parrish, D.R.; Saucy, G. 6-Hydroxychroman-2-carboxylic acids: Novel antioxidants. J. Am. Oil Chem. Soc. 1974, 51, 200–203. [Google Scholar] [CrossRef]
- Moss, J.I.; Pontes, E.; Hansen, P.J. Insulin-like growth factor-1 protects preimplantation embryos from anti-developmental actions of menadione. Arch. Toxicol. 2009, 83, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Ruebhart, D.R.; Wickramasinghe, W.; Cock, I.E. Protective efficacy of the antioxidants vitamin E and Trolox against Microcystis aeruginosa and microcystin-LR in Artemia franciscana nauplii. J. Toxicol. Environ. Health Part A 2009, 72, 1567–1575. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.; Li, L.; Qiao, S.; Zhang, G.; Li, D. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef]
- Ertel, A.; Verghese, A.; Byers, S.W.; Ochs, M.; Tozeren, A. Pathway-specific differences between tumor cell lines and normal and tumor tissue cells. Mol. Cancer 2006, 5, 55. [Google Scholar] [CrossRef]
- Frandsen, S.K.; McNeil, A.K.; Novak, I.; McNeil, P.L.; Gehl, J. Difference in membrane repair capacity between cancer cell lines and a normal cell line. J. Membr. Biol. 2016, 249, 569–576. [Google Scholar] [CrossRef]
- Nossol, C.; Barta-Böszörményi, A.; Kahlert, S.; Zuschratter, W.; Faber-Zuschratter, H.; Reinhardt, N.; Ponsuksili, S.; Wimmers, K.; Diesing, A.-K.; Rothkötter, H.-J. Comparing two intestinal porcine epithelial cell lines (IPECs): Morphological differentiation, function and metabolism. PLoS ONE 2015, 10, e0132323. [Google Scholar] [CrossRef]
- Lazic, S.E.; Clarke-Williams, C.J.; Munafò, M.R. What exactly is ‘N’in cell culture and animal experiments? PLoS Biol. 2018, 16, e2005282. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Barraza-Garza, G.; Castillo-Michel, H.; de la Rosa, L.A.; Martinez-Martinez, A.; Pérez-León, J.A.; Cotte, M.; Alvarez-Parrilla, E. Infrared spectroscopy as a tool to study the antioxidant activity of polyphenolic compounds in isolated rat enterocytes. Oxid. Med. Cell. Longev. 2016, 2016, 9245150. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols-induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Corsi, R.; Mosti, G.; Cavezzi, A.; Urso, S.U.; Dimitrova, G.; Fioroni, E.; Colucci, R.; Quinzi, V. A Polyphenol-Based Multicomponent Nutraceutical in Dysmetabolism and Oxidative Stress: Results from a Pilot Study. J. Diet. Suppl. 2018, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and performance: A systematic review and meta-analysis. Sports Med. 2017, 47, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuso, M.; Van Cruchten, S.; Van Ginneken, C. A Medium-Throughput System for In Vitro Oxidative Stress Assessment in IPEC-J2 Cells. Int. J. Mol. Sci. 2020, 21, 7263. https://doi.org/10.3390/ijms21197263
Ayuso M, Van Cruchten S, Van Ginneken C. A Medium-Throughput System for In Vitro Oxidative Stress Assessment in IPEC-J2 Cells. International Journal of Molecular Sciences. 2020; 21(19):7263. https://doi.org/10.3390/ijms21197263
Chicago/Turabian StyleAyuso, Miriam, Steven Van Cruchten, and Chris Van Ginneken. 2020. "A Medium-Throughput System for In Vitro Oxidative Stress Assessment in IPEC-J2 Cells" International Journal of Molecular Sciences 21, no. 19: 7263. https://doi.org/10.3390/ijms21197263
APA StyleAyuso, M., Van Cruchten, S., & Van Ginneken, C. (2020). A Medium-Throughput System for In Vitro Oxidative Stress Assessment in IPEC-J2 Cells. International Journal of Molecular Sciences, 21(19), 7263. https://doi.org/10.3390/ijms21197263





