Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation
Abstract
:1. Introduction
2. Results
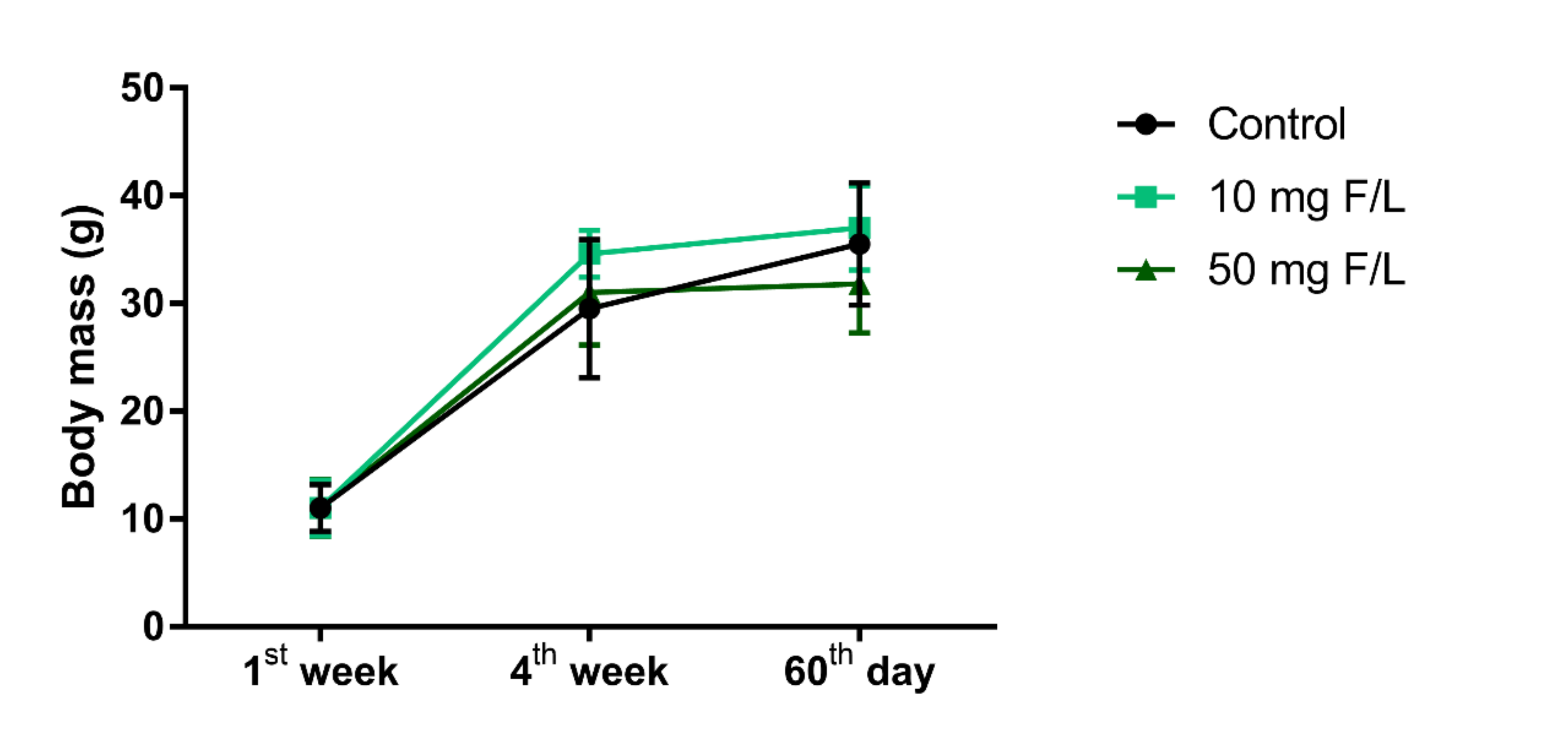
2.1. Prolonged Exposure to NaF Did Not Impair Weight Gain
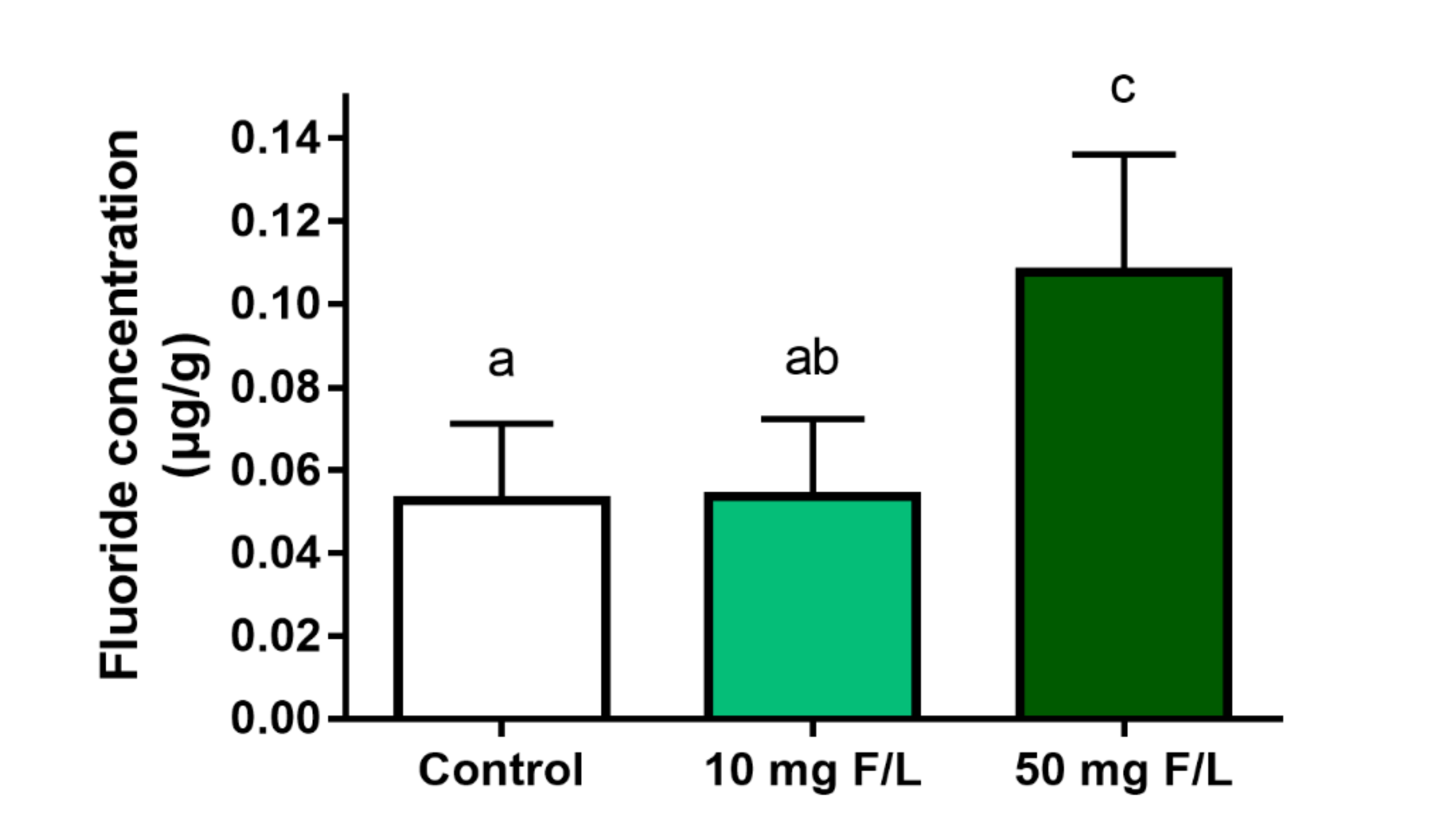
2.2. Long-Term Exposure to NaF Increased Cerebellum Fluoride Levels
2.3. Exposure to Fluoride Modulated the Cerebellar Oxidative Biochemistry Balance in Mice
2.4. Prolonged Exposure to Fluoride Significantly Modulated the Cerebellar Proteome of Mice
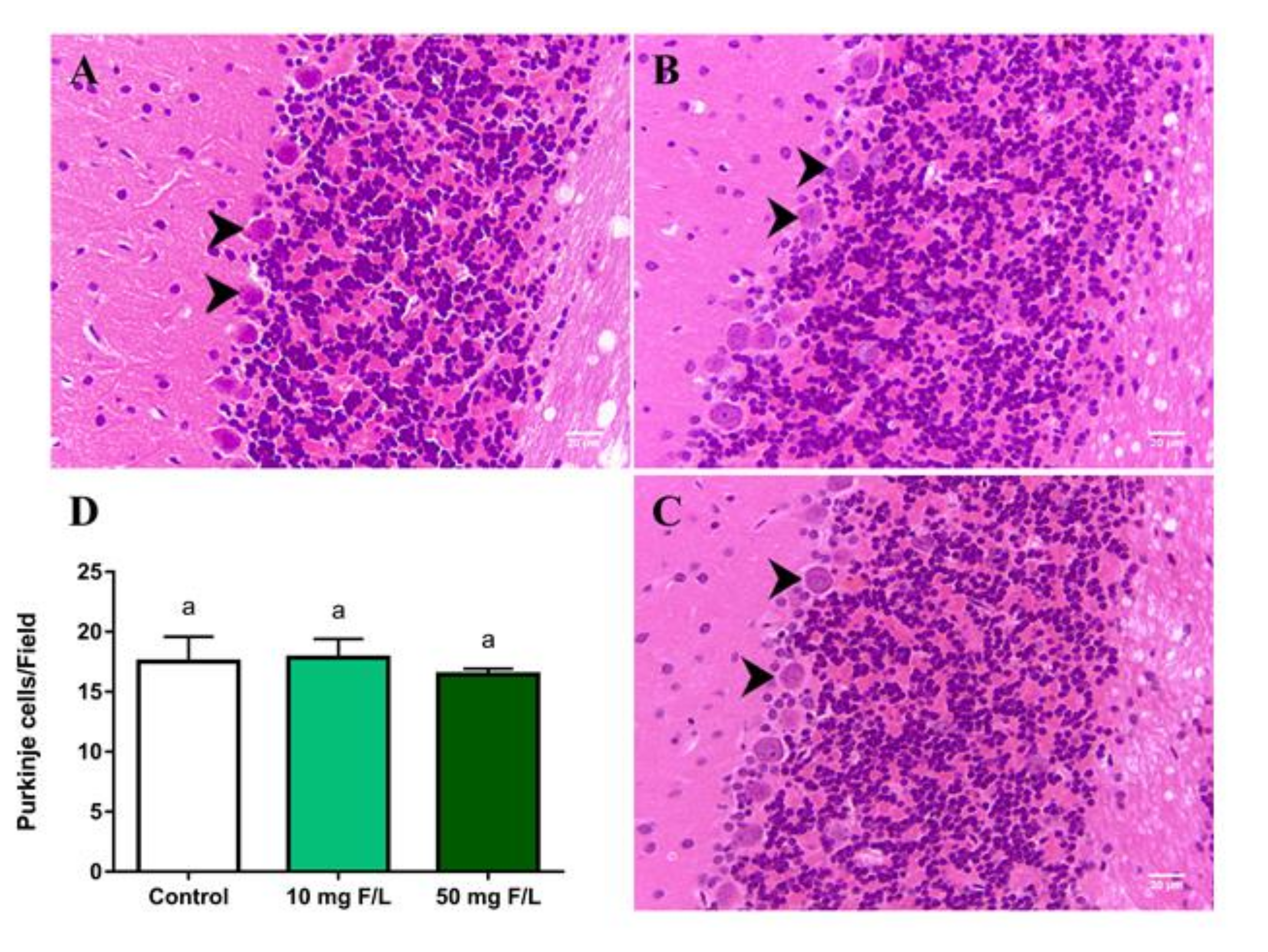
2.5. Long-Term Exposure to NaF Did Not Change the Density of Purkinje Cells
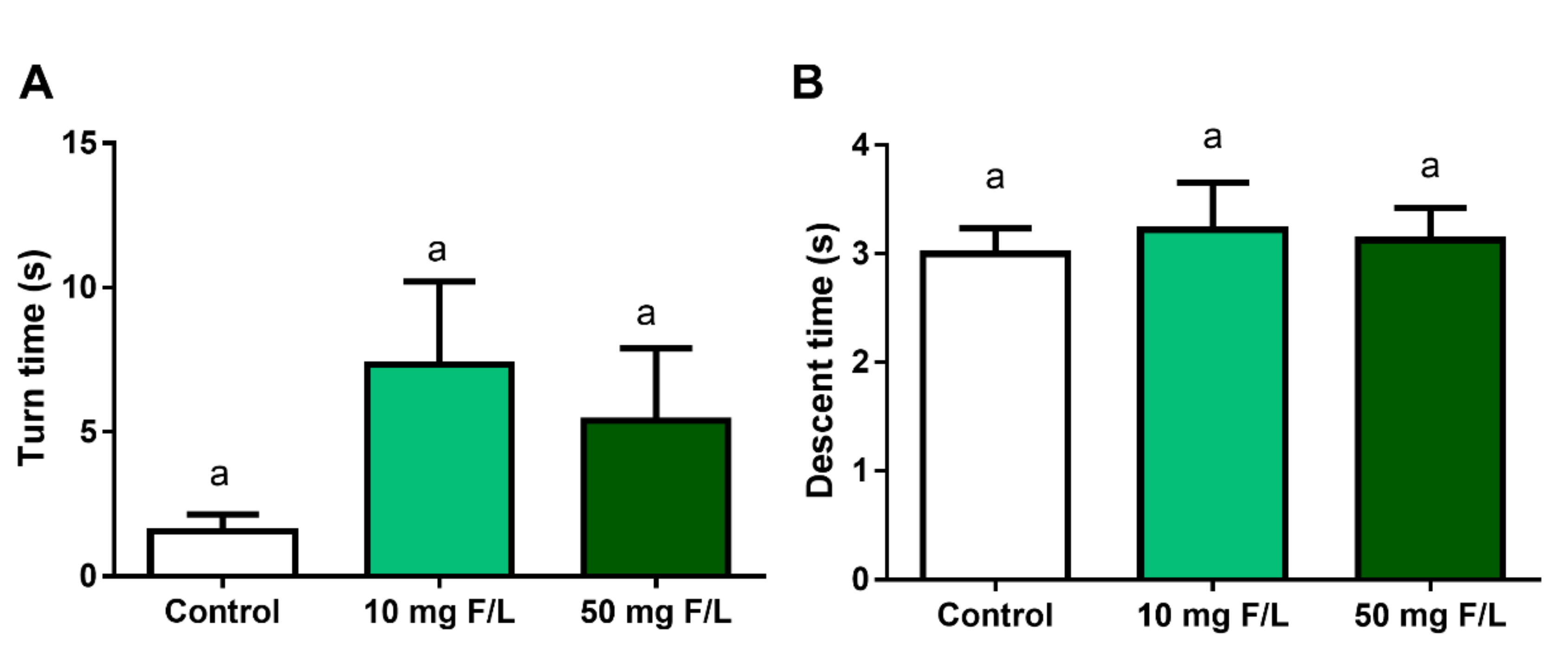
2.6. Chronic Exposure to NaF at 10 and 50 mg F/L Altered Functional Locomotion Parameters, Both Horizontal and Vertical, and Exploration
2.7. Chronic Exposure to NaF Did Not Impaired Motor Coordination and Animal Balance
2.8. Exposure to NaF, at Both Concentrations, Did Not Change Locomotor Activity
2.9. There Was No Change in Motor Balance in Animals Exposed to NaF
3. Discussion
4. Materials and Methods
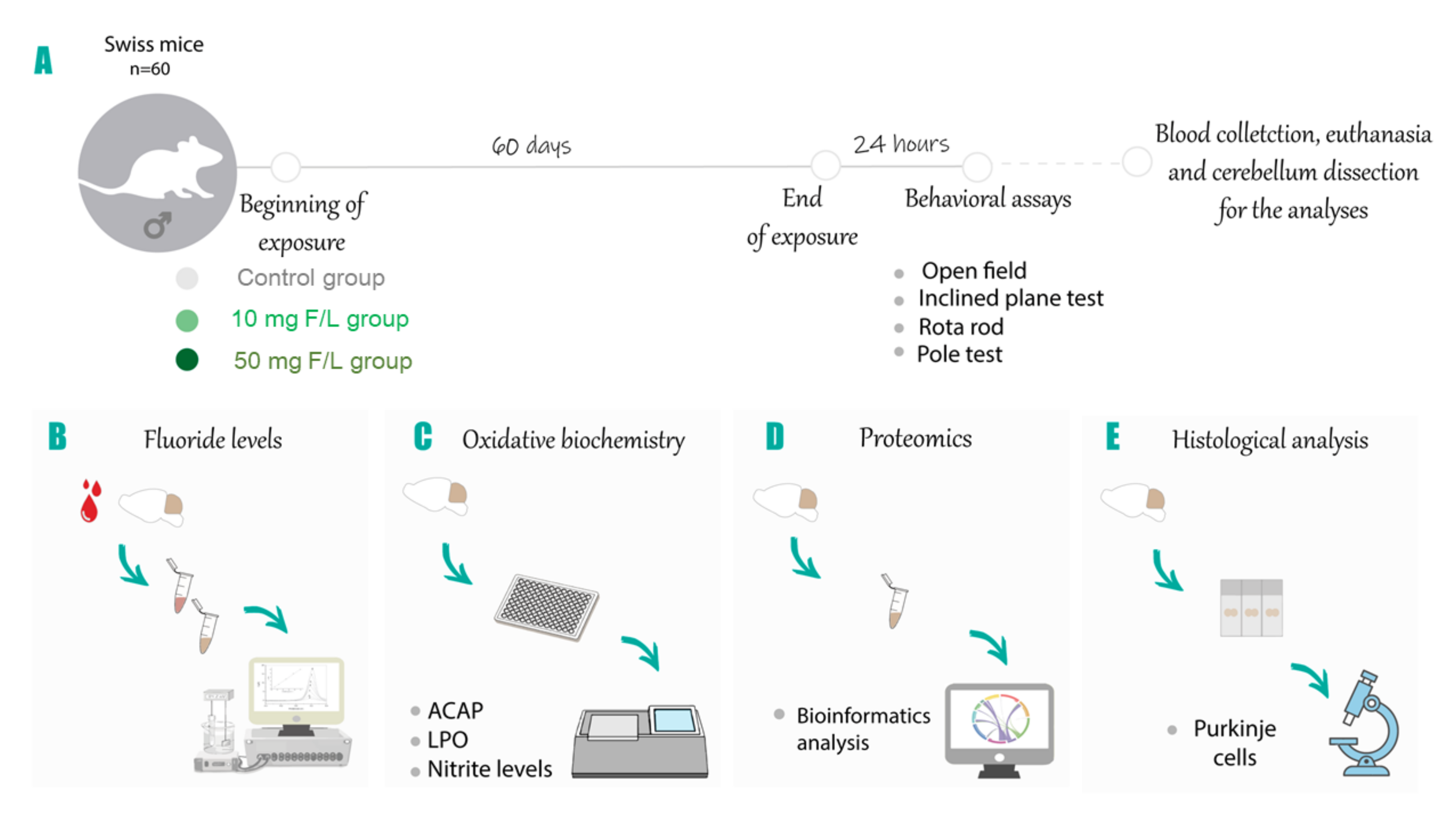
4.1. Experimental Animals and Exposure Protocol
4.2. Behavioral Assessment
4.2.1. Open Field
4.2.2. Inclined Plane
4.2.3. Pole Test
4.2.4. Rotarod
4.3. Fluoride Levels Analysis
4.4. Oxidative Biochemitry Analysis
4.4.1. Antioxidant Capacity against Peroxyl Radicals (ACAP)
4.4.2. Determination of Lipid Peroxidation (LPO)
4.4.3. Determination of Nitrite Levels (NO)
4.5. Characterization of the Proteomic Profile
4.5.1. Protein Extraction
4.5.2. Mass Spectrometry
4.6. Perfusion and Histological Processing
Quantitative Analysis of Purkinje Neurons
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Abbreviations
| NaF | Sodium Fluoride |
| ACAP | Antioxidant Capacity Against Peroxyl Radicals |
| MDA | Lipid Peroxidation |
| NO | Nitrite |
| CNS | Central Nervous System |
| HF | Hydrogen Fluoride |
| MAPK | Mitogen-Activated Protein Kinases |
| AP 1 | Activator Protein 1 |
| NF-B | Nuclear Factor Kappa B |
| ROS | Reactive Oxygen Species |
| HSP | Heat Shock Proteins |
| SOD | Superoxide Dismutase |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.-P.; Lu, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular mechanisms of fluoride toxicity. Chem. Interactions 2010, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 38, pp. 104–108. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- AFTSDR; Service UPH. Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2003. [Google Scholar]
- McDonagh, M.S.; Whiting, P.; Wilson, P.M.; Sutton, A.J.; Chestnutt, I.; Cooper, J.; Misso, K.; Bradley, M.; Treasure, E.; Kleijnen, J. Systematic review of water fluoridation. BMJ 2000, 321, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, C.A.N.; Leite, A.L.; Peres-Buzalaf, C.; Carvalho, J.G.; Whitford, G.M.; Everett, E.T.; Siqueira, W.L.; Buzalaf, M.A.R. Bone Response to Fluoride Exposure Is Influenced by Genetics. PLoS ONE 2014, 9, e114343. [Google Scholar] [CrossRef]
- Da Silva Pereira, H.A.; de Lima, A.L.; Charone, S.; Lobo, J.G.V.M.; Cestari, T.M.; Peres-Buzalaf, C.; Buzalaf, M.A.R. Proteomic analysis of liver in rats chronically exposed to fluoride. PLoS ONE 2013, 8, e75343. [Google Scholar]
- Carvalho, J.G.; Leite, A.L.; Peres-Buzalaf, C.; Salvato, F.; Labate, C.A.; Everett, E.T.; Whitford, G.M.; Buzalaf, M.A.R. Renal Proteome in Mice with Different Susceptibilities to Fluorosis. PLoS ONE 2013, 8, e53261. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, C.A.; Leite, A.L.; Silva, T.L.; Dos Santos, L.D.; Nogueira, F.C.; De Oliveira, R.C.; Palma, M.S.; Domont, G.B.; Buzalaf, M.A. Proteomic analysis of kidney in rats chronically exposed to fluoride. Chem. Interactions 2009, 180, 305–311. [Google Scholar] [CrossRef]
- Dionizio, A.S.; Melo, C.G.S.; Sabino-Arias, I.T.; Ventura, T.M.S.; Leite, A.L.; Souza, S.R.G.; Santos, E.X.; Heubel, A.D.; Souza, J.G.; Perles, J.V.C.M.; et al. Chronic treatment with fluoride affects the jejunum: Insights from proteomics and enteric innervation analysis. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Melo, C.G.D.S.; Perles, J.V.C.M.; Zanoni, J.N.; De Souza, S.R.G.; Santos, E.X.; Leite, A.L.; Heubel, A.D.; E Souza, C.O.; De Souza, J.G.; Buzalaf, M.A.R. Enteric innervation combined with proteomics for the evaluation of the effects of chronic fluoride exposure on the duodenum of rats. Sci. Rep. 2017, 7, 1070. [Google Scholar] [CrossRef]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential fluoride toxicity from oral medicaments: A review. Iran J. Basic Med. Sci. 2017, 20, 841–848. [Google Scholar]
- Bashash, M.; Thomas, D.; Hu, H.; Martinez-Mier, E.A.; Sanchez, B.N.; Basu, N.; Peterson, P.E.; Ettinger, A.S.; Wright, R.; Zhang, Z.; et al. Prenatal fluoride exposure and cognitive outcomes in children at 4 and 6–12 years of age in Mexico. J. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental Fluoride Neurotoxicity: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozsvath, D.L. Fluoride and environmental health: A review. Rev. Environ. Sci. Bio/Technology 2008, 8, 59–79. [Google Scholar] [CrossRef]
- Cury, J.; Ricomini-Filho, A.P.; Berti, F.L.P.; Tabchoury, C.P.M. Systemic Effects (Risks) of Water Fluoridation. Braz. Dent. J. 2019, 30, 421–428. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, S.; Gao, J. Analysis of MBP, NSE, F-content and CHE activity in brain tissue of rats with chronic fluorosis. J. Chin. J. Endemiol. 2002, 21, 358. [Google Scholar]
- Ma, J.; Liu, F.; Liu, P.; Dong, Y.-Y.; Chu, Z.; Hou, T.; Dang, Y. Impact of early developmental fluoride exposure on the peripheral pain sensitivity in mice. Int. J. Dev. Neurosci. 2015, 47, 165–171. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.-E.; Favorov, O.; Tommerdahl, M.; Whitsel, B.; Lee, C.J. Fluoride Induces a Volume Reduction in CA1 Hippocampal Slices Via MAP Kinase Pathway Through Volume Regulated Anion Channels. Exp. Neurobiol. 2016, 25, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustina, F.; Sofro, Z.M.; Partadiredja, G. Subchronic Administration of High-Dose Sodium Fluoride Causes Deficits in Cerebellar Purkinje Cells But Not Motor Coordination of Rats. Boil. Trace Element Res. 2018, 188, 424–433. [Google Scholar] [CrossRef]
- Al-Hayani, A.; Elshal, E.B.; Aal, I.; Al-Shammer, E. Does vitamin E protect against sodium fluoride toxicity on the cerebellar cortex of albino rats. J. Middle East J. Sci. Res. 2013, 16, 1019–1026. [Google Scholar]
- Eccles, J.C. Physiology of Motor Control in Man. Ster. Funct. Neurosurg. 1981, 44, 5–15. [Google Scholar] [CrossRef]
- Lamont, M.G.; Weber, J.T. The role of calcium in synaptic plasticity and motor learning in the cerebellar cortex. Neurosci. Biobehav. Rev. 2012, 36, 1153–1162. [Google Scholar] [CrossRef]
- Valenzuela, C.F.; Lindquist, B.; Zamudio-Bulcock, P.A. A Review of Synaptic Plasticity at Purkinje Neurons with a Focus on Ethanol-Induced Cerebellar Dysfunction. Int. Rev. Neurobiol. 2010, 91, 339–372. [Google Scholar] [CrossRef]
- Manto, M.; Mariën, P. Schmahmann’s syndrome - identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias 2015, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Dunipace, A.; Brizendine, E.; Zhang, W.; Wilson, M.; Miller, L.; Katz, B.; Warrick, J.; Stookey, G. Effect of Aging on Animal Response to Chronic Fluoride Exposure. J. Dent. Res. 1995, 74, 358–368. [Google Scholar] [CrossRef]
- Angmar-Mansson, B.; Whitford, G. Enamel Fluorosis Related to Plasma F Levels in the Rat. Caries Res. 1984, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Whitford, G.M. The metabolism and toxicity of fluoride. Monogr. Oral Sci. 1996, 16, 1–153. [Google Scholar]
- Dec, K.; Lukomska, A.; Maciejewska, D.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Chlubek, D.; Wasik, A.; Gutowska, I. The Influence of Fluorine on the Disturbances of Homeostasis in the Central Nervous System. Boil. Trace Element Res. 2016, 177, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Buzalaf, M.A.R.; Whitford, G.M. Fluoride metabolism. In Fluoride and the Oral Environment; Karger Publishers: Basel, Switzerlnad, 2011; pp. 20–36. [Google Scholar]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; A Lennon, M.; Petersen, P.E.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and Oral Health. Community Dent Heal. 2016, 33, 69–99. [Google Scholar]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940. [Google Scholar] [CrossRef]
- Karube, H.; Nishitai, G.; Inageda, K.; Kurosu, H.; Matsuoka, M. NaF Activates MAPKs and Induces Apoptosis in Odontoblast-like Cells. J. Dent. Res. 2009, 88, 461–465. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Xia, T.; He, P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-κB in primary cultured rat hippocampal neurons. Toxicol. Lett. 2008, 179, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Chi, H.S.; Chen, J.; Besten, P.K.D. JNK/c-Jun signaling pathway mediates the fluoride-induced down-regulation of MMP-20 in vitro. Matrix Boil. 2007, 26, 633–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, T.T.; Pereira, H.A.B.S.; Dionizio, A.; Sanchez, C.D.C.; Carvalho, T.D.S.; Fernandes, M.D.S.; Buzalaf, M.A.R. Changes in energy metabolism induced by fluoride: Insights from inside the mitochondria. Chemosphere 2019, 236, 124357. [Google Scholar] [CrossRef] [PubMed]
- Iano, F.G.; Ferreira, M.C.; Quaggio, G.B.; Fernandes, M.S.; De Oliveira, R.C.; Ximenes, V.F.; Buzalaf, M.A.R. Effects of chronic fluoride intake on the antioxidant systems of the liver and kidney in rats. J. Fluor. Chem. 2014, 168, 212–217. [Google Scholar] [CrossRef]
- Shuhua, X.; Ziyou, L.; Ling, Y.; Fei, W.; Sun, G. A Role of Fluoride on Free Radical Generation and Oxidative Stress in BV-2 Microglia Cells. Mediat. Inflamm. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhang, W.; Xue, X.; Zhang, Y.; Niu, R.; Li, X.; Li, B.; Wang, X.; Wang, J. Fluoride decreased the sperm ATP of mice through inhabiting mitochondrial respiration. Chemosphere 2016, 144, 1012–1017. [Google Scholar] [CrossRef]
- Waugh, D.T. Fluoride Exposure Induces Inhibition of Sodium-and Potassium-Activated Adenosine Triphosphatase (Na+, K+-ATPase) Enzyme Activity: Molecular Mechanisms and Implications for Public Health. Int. J. Environ. Res. Public Health 2019, 16, 1427. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Vega, J.A.; Sánchez-Gutiérrez, M.; Del Razo, L.M. Decreased in vitro fertility in male rats exposed to fluoride-induced oxidative stress damage and mitochondrial transmembrane potential loss. Toxicol. Appl. Pharmacol. 2008, 230, 352–357. [Google Scholar] [CrossRef]
- Tian, X.-L.; Feng, J.; Dong, N.-S.; Lyu, Y.; Wei, C.-L.; Li, B.; Ma, Y.-Q.; Xie, J.-X.; Qiu, Y.-L.; Song, G.-H.; et al. Subchronic exposure to arsenite and fluoride from gestation to puberty induces oxidative stress and disrupts ultrastructure in the kidneys of rat offspring. Sci. Total. Environ. 2019, 686, 1229–1237. [Google Scholar] [CrossRef]
- Dec, K.; Lukomska, A.; Baranowska-Bosiacka, I.; Pilutin, A.; Maciejewska, D.; Skonieczna-Żydecka, K.; Derkacz, R.; Goschorska, M.; Wasik, A.; Rębacz-Maron, E.; et al. Pre-and postnatal exposition to fluorides induce changes in rats liver morphology by impairment of antioxidant defense mechanisms and COX induction. Chemosphere 2018, 211, 112–119. [Google Scholar] [CrossRef]
- Dec, K.; Lukomska, A.; Skonieczna-Żydecka, K.; Jakubczyk, K.; Tarnowski, M.; Lubkowska, A.; Baranowska-Bosiacka, I.; Styburski, D.; Skórka-Majewicz, M.; Maciejewska, D.; et al. Chronic Exposure to Fluoride Affects GSH Level and NOX4 Expression in Rat Model of This Element of Neurotoxicity. Biomol. 2020, 10, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, M.; Basha, P.M. Effect of Maternal Exposure of Fluoride on Biometals and Oxidative Stress Parameters in Developing CNS of Rat. Biol. Trace Element Res. 2009, 133, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.A.; Cole, M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Zhang, J.; Zhang, Z. Effects of Fluoride on Synaptic Membrane Fluidity and PSD-95 Expression Level in Rat Hippocampus. Biol. Trace Element Res. 2010, 139, 197–203. [Google Scholar] [CrossRef]
- Hüttemann, M.; Pecina, P.; Rainbolt, M.; Sanderson, T.H.; Kagan, V.E.; Samavati, L.; Doan, J.W.; Lee, I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 2011, 11, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Ow, Y.-L.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar] [CrossRef]
- Neupane, P.; Bhuju, S.; Thapa, N.; Bhattarai, H.K. ATP Synthase: Structure, Function and Inhibition. Biomol. Concepts 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Winklhofer, K.F.; Haass, C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2010, 1802, 29–44. [Google Scholar] [CrossRef]
- Boveris, A.; Navarro, A. Brain mitochondrial dysfunction in aging. IUBMB Life 2008, 60, 308–314. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X. Heat Shock Protein Reports on Proteome Stress. Biotechnol. J. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxidants Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Elpers, C.; Reunert, J.; McCormick, M.L.; Mohr, J.; Biskup, S.; Schwartz, O.; Rust, S.; Grüneberg, M.; Seelhöfer, A.; et al. SOD1 deficiency: A novel syndrome distinct from amyotrophic lateral sclerosis. Brain 2019, 142, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.R.; Igoudjil, A.; Magrané, J.; Li, Y.; Hansen, J.M.; Manfredi, G.; Glass, J.D. SOD1 targeted to the mitochondrial intermembrane space prevents motor neuropathy in the Sod1 knockout mouse. Brain 2010, 134, 196–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, J.F. The multiple roles of Purkinje cells in sensori-motor calibration: To predict, teach and command. Curr. Opin. Neurobiol. 2011, 21, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Lamarão-Vieira, K.; Pamplona-Santos, D.; Nascimento, P.C.; Corrêa, M.G.; Bittencourt, L.O.; Dos Santos, S.M.; Cartágenes, S.C.; Fernandes, L.M.P.; Monteiro, M.C.; Maia, C.S.F.; et al. Physical Exercise Attenuates Oxidative Stress and Morphofunctional Cerebellar Damages Induced by the Ethanol Binge Drinking Paradigm from Adolescence to Adulthood in Rats. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Valenzuela, C.F.; Jotty, K. Mini-Review: Effects of Ethanol on GABAA Receptor-Mediated Neurotransmission in the Cerebellar Cortex—Recent Advances. Cerebellum 2015, 14, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yamaguchi, K.; Nagao, S.; Yamazaki, T. Long-Term Depression as a Model of Cerebellar Plasticity. In Progress in Brain Research; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 210, pp. 1–30. [Google Scholar]
- Mapelli, L.; Pagani, M.; Garrido, J.A.; D’Angelo, E.U. Integrated plasticity at inhibitory and excitatory synapses in the cerebellar circuit. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, E.U. The Organization of Plasticity in the Cerebellar Cortex: From Synapses to Control. In Progress in Brain Research; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 210, pp. 31–58. [Google Scholar]
- Murphy, M.; Rick, J.; Milgram, N.; Ivy, G. A Simple and Rapid Test of Sensorimotor Function in the Aged Rat. Neurobiol. Learn. Mem. 1995, 64, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Fredericks, C.M. Disorders of the cerebellum and its connections. In Pathophysiology of the Motor Systems: Principles and Clinical Presentations; FA Davis: Philadelphia, PA, USA, 1996; pp. 445–466. [Google Scholar]
- Miranda, G.H.N.; Gomes, B.A.Q.; Bittencourt, L.O.; Aragão, W.A.B.; Nogueira, L.; Dionizio, A.S.; Buzalaf, M.A.R.; Monteiro, M.C.; Lima, R.R. Chronic Exposure to Sodium Fluoride Triggers Oxidative Biochemistry Misbalance in Mice: Effects on Peripheral Blood Circulation. Oxidative Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Brenes, J.C.; Padilla, M.; Fornaguera, J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav. Brain Res. 2009, 197, 125–137. [Google Scholar] [CrossRef]
- Dixon, L.K.; DeFries, J.C. Development of open-field behavior in mice: Effects of age and experience. Dev. Psychobiol. 1968, 1, 100–107. [Google Scholar] [CrossRef]
- Maia, C.D.S.F.; Lucena, G.M.R.D.S.; Corrêa, P.B.F.; Serra, R.B.; Matos, R.W.D.M.; Menezes, F.D.C.; Dos Santos, S.N.; De Sousa, J.B.; Da Costa, E.T.; Ferreira, V.M.M. Interference of ethanol and methylmercury in the developing central nervous system. NeuroToxicology 2009, 30, 23–30. [Google Scholar] [CrossRef]
- Oliveira, G.B.; Fontes, E.D.A.; De Carvalho, S.; Da Silva, J.B.; Fernandes, L.M.P.; Oliveira, M.C.S.P.; Prediger, R.D.; Gomes-Leal, W.; Lima, R.R.; Maia, C.S.F. Minocycline mitigates motor impairments and cortical neuronal loss induced by focal ischemia in rats chronically exposed to ethanol during adolescence. Brain Res. 2014, 1561, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.M.P.; Lopes, K.S.; Santana, L.N.S.; Fontes-Júnior, E.A.; Ribeiro, C.H.M.A.; Silva, M.C.F.; Paraense, R.S.D.O.; Crespo-López, M.E.; Gomes, A.R.Q.; Lima, R.R.; et al. Repeated Cycles of Binge-Like Ethanol Intake in Adolescent Female Rats Induce Motor Function Impairment and Oxidative Damage in Motor Cortex and Liver, but Not in Blood. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.B.R.; Cunha, P.A.; Ribera, P.C.; Barros, M.A.; Cartágenes, S.C.; Fernandes, L.M.P.; Teixeira, F.B.; Fontes-Junior, E.A.; Prediger, R.D.; Lima, R.R.; et al. Heavy Chronic Ethanol Exposure From Adolescence to Adulthood Induces Cerebellar Neuronal Loss and Motor Function Damage in Female Rats. Front. Behav. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.B.; Santana, L.N.D.S.; Bezerra, F.R.; De Carvalho, S.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; Maia, C.S.F.; Lima, R.R. Chronic Ethanol Exposure during Adolescence in Rats Induces Motor Impairments and Cerebral Cortex Damage Associated with Oxidative Stress. PLoS ONE 2014, 9, e101074. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, N.; Hirose, Y.; Ohara, S.; Ono, T.; Watanabe, Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res. Commun. Chem. Pathol. Pharmacol. 1985, 50. [Google Scholar]
- Antzoulatos, E.; Jakowec, M.W.; Petzinger, G.M.; Wood, R.I. Sex differences in motor behavior in the MPTP mouse model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2010, 95, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.N.; Pinheiro, A.M.; Belém-Filho, I.J.A.; Fernandes, L.M.P.; Cartágenes, S.C.; Ribera, P.C.; Fontes-Júnior, E.A.; Crespo-López, M.E.; Monteiro, M.C.; Lima, M.O.; et al. Unravelling motor behaviour hallmarks in intoxicated adolescents: Methylmercury subtoxic-dose exposure and binge ethanol intake paradigm in rats. Environ. Sci. Pollut. Res. 2018, 25, 21937–21948. [Google Scholar] [CrossRef]
- Fontes-Júnior, E.A.; Maia, C.S.F.; Fernandes, L.M.P.; Gomes-Leal, W.; Costa-Malaquias, A.; Lima, R.R.; Prediger, R.D.; Crespo-López, M.E. Chronic Alcohol Intoxication and Cortical Ischemia: Study of Their Comorbidity and the Protective Effects of Minocycline. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Taves, D.R. Separation of fluoride by rapid diffusion using hexamethyldisiloxane. Talanta 1968, 15, 969–974. [Google Scholar] [CrossRef]
- Teixeira, F.B.; De Oliveira, A.C.A.; Leão, L.K.D.R.; Fagundes, N.C.F.; Fernandes, R.M.; Fernandes, L.M.P.; Da Silva, M.C.F.; Amado, L.L.; Sagica, F.; De Oliveira, E.H.C.; et al. Exposure to Inorganic Mercury Causes Oxidative Stress, Cell Death, and Functional Deficits in the Motor Cortex. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Amado, L.L.; Garcia, M.L.; Ramos, P.; Freitas, R.F.; Zafalon, B.; Ferreira, J.L.R.; Yunes, J.S.; Monserrat, J. A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: Application to evaluate microcystins toxicity. Sci. Total. Environ. 2009, 407, 2115–2123. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Leão, L.K.D.R.; Bittencourt, L.O.; Oliveira, A.C.; Nascimento, P.C.; Miranda, G.H.N.; Ferreira, R.O.; Nabiça, M.; Dantas, K.D.G.F.; Dionizio, A.; Cartágenes, S.; et al. Long-Term Lead Exposure Since Adolescence Causes Proteomic and Morphological Alterations in the Cerebellum Associated with Motor Deficits in Adult Rats. Int. J. Mol. Sci. 2020, 21, 3571. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Bittencourt, L.O.; Nascimento, P.C.; Ferreira, R.O.; Aragão, W.A.B.; Silva, M.C.F.; Gomes-Leal, W.; Fernandes, M.S.; Dionizio, A.; Buzalaf, M.R.; et al. Spinal cord neurodegeneration after inorganic mercury long-term exposure in adult rats: Ultrastructural, proteomic and biochemical damages associated with reduced neuronal density. Ecotoxicol. Environ. Saf. 2020, 191, 110159. [Google Scholar] [CrossRef]
- Bittencourt, L.O.; Dionizio, A.; Nascimento, P.C.; Puty, B.; Leão, L.K.D.R.; Luz, D.A.; Da Silva, M.C.F.; Amado, L.L.; Leite, A.L.; Buzalaf, M.R.; et al. Proteomic approach underlying the hippocampal neurodegeneration caused by low doses of methylmercury after long-term exposure in adult rats. Met. 2019, 11, 390–403. [Google Scholar] [CrossRef]
- Leite, A.L.; Lobo, J.G.V.M.; Pereira, H.A.B.D.S.; Fernandes, M.S.; Martini, T.; Zucki, F.; Sumida, D.H.; Rigalli, A.; Buzalaf, M.A.R. Proteomic Analysis of Gastrocnemius Muscle in Rats with Streptozotocin-Induced Diabetes and Chronically Exposed to Fluoride. PLoS ONE 2014, 9, e106646. [Google Scholar] [CrossRef] [Green Version]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.R.; Santana, L.N.S.; Fernandes, R.M.; Nascimento, E.M.; Oliveira, A.C.; Fernandes, L.M.P.; Dos Santos, E.M.N.; Tavares, P.A.N.; Dos Santos, I.R.; Gimarães-Santos, A.; et al. Neurodegeneration and Glial Response after Acute Striatal Stroke: Histological Basis for Neuroprotective Studies. Oxidative Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef]




| Groups | Biological Processes (% of Genes) |
|---|---|
| 10 mg F/L vs. Control | Axon guidance (14.9%) Regulation of dendrite morphogenesis (9.6%) Positive regulation of DNA-binding transcription factor activity (8.8%) Regulation of axon extension (6.1%) Positive regulation of JNK cascade (4.4%) Regulation of alternative mRNA splicing, via spliceosome (4.4%) Acetyl-CoA biosynthetic process from pyruvate (3.5%) Activation of cysteine-type endopeptidase activity involved in apoptotic process (3.5%) Central nervous system neuron axogenesis (3.5%) Cysteine-type endopeptidase inhibitor activity involved in apoptotic process (3.5%) Hypothalamus gonadotrophin-releasing hormone neuron development (3.5%) mRNA splice site selection (3.5%) negative regulation of mRNA splicing, via spliceosome (3.5%) Regulation of calcium ion transport into cytosol (3.5%) Dendrite extension (2.6%) Glycolytic process through fructose-6-phosphate (2.6%) Ionotropic glutamate receptor activity (2.6%) Mitochondrial ATP synthesis coupled proton transport (2.6%) Nuclear receptor activity (2.6%) Positive regulation of ATP biosynthetic process (2.6%) Positive regulation of sprouting angiogenesis (2.6%) Potassium: proton exchanging ATPase activity (2.6%) Regulation of glycolytic process (2.6%) |
| 50 mg F/L vs. Control | Axon guidance (13.6%) Regulation of axogenesis (13.6%) Regulation of release of sequestered calcium ion into cytosol (9.1%) Dendritic spine morphogenesis (7.3%) Activation of cysteine-type endopeptidase activity involved in apoptotic process (4.5%) Regulation of alternative mRNA splicing, via spliceosome (4.5%) Cysteine-type endopeptidase inhibitor activity involved in apoptotic process (4.5%) Dendrite extension (4.5%) Negative regulation of mRNA splicing, via spliceosome (4.5%) mRNA splice site selection (3.6%) Barbed-end actin filament capping (3.6%) Central nervous system neuron axogenesis (3.6%) Mitochondrial ATP synthesis coupled proton transport (3.6%) Pyruvate dehydrogenase (NAD+) activity (3.6%) Ionotropic glutamate receptor activity (3.6%) Canonical glycolysis (3.6%) Auditory receptor cell morphogenesis (2.7%) Regulation of glycolytic process (2.7%) Potassium: proton exchanging ATPase activity (2.7%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, G.O.; Martins Ferreira, M.K.; Davis, L.; Bittencourt, L.O.; Bragança Aragão, W.A.; Dionizio, A.; Rabelo Buzalaf, M.A.; Crespo-Lopez, M.E.; Maia, C.S.F.; Lima, R.R. Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation. Int. J. Mol. Sci. 2020, 21, 7297. https://doi.org/10.3390/ijms21197297
Lopes GO, Martins Ferreira MK, Davis L, Bittencourt LO, Bragança Aragão WA, Dionizio A, Rabelo Buzalaf MA, Crespo-Lopez ME, Maia CSF, Lima RR. Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation. International Journal of Molecular Sciences. 2020; 21(19):7297. https://doi.org/10.3390/ijms21197297
Chicago/Turabian StyleLopes, Géssica Oliveira, Maria Karolina Martins Ferreira, Lodinikki Davis, Leonardo Oliveira Bittencourt, Walessa Alana Bragança Aragão, Aline Dionizio, Marília Afonso Rabelo Buzalaf, Maria Elena Crespo-Lopez, Cristiane Socorro Ferraz Maia, and Rafael Rodrigues Lima. 2020. "Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation" International Journal of Molecular Sciences 21, no. 19: 7297. https://doi.org/10.3390/ijms21197297
APA StyleLopes, G. O., Martins Ferreira, M. K., Davis, L., Bittencourt, L. O., Bragança Aragão, W. A., Dionizio, A., Rabelo Buzalaf, M. A., Crespo-Lopez, M. E., Maia, C. S. F., & Lima, R. R. (2020). Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation. International Journal of Molecular Sciences, 21(19), 7297. https://doi.org/10.3390/ijms21197297









