Involvement of the Retinal Pigment Epithelium in the Development of Retinal Lattice Degeneration
Abstract
1. Introduction
2. Results
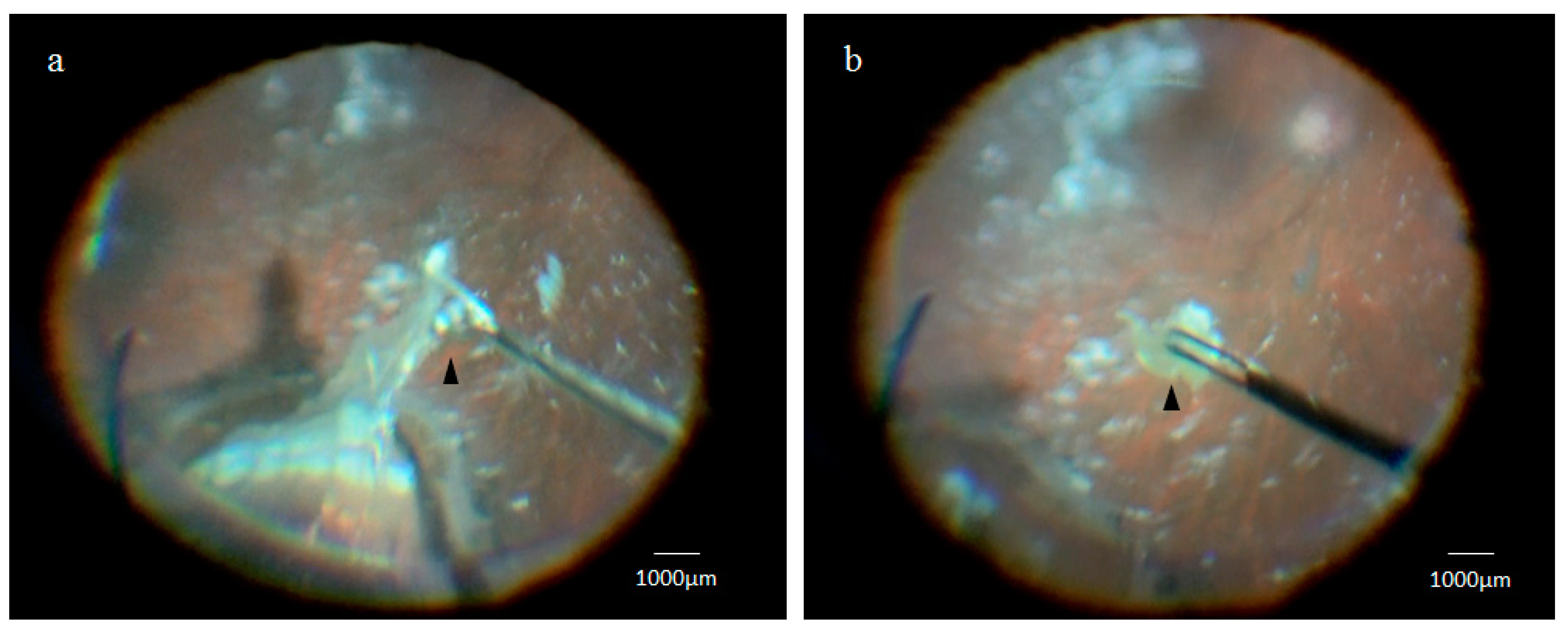
2.1. Collection of the Retinal Lattice Degeneration Specimens
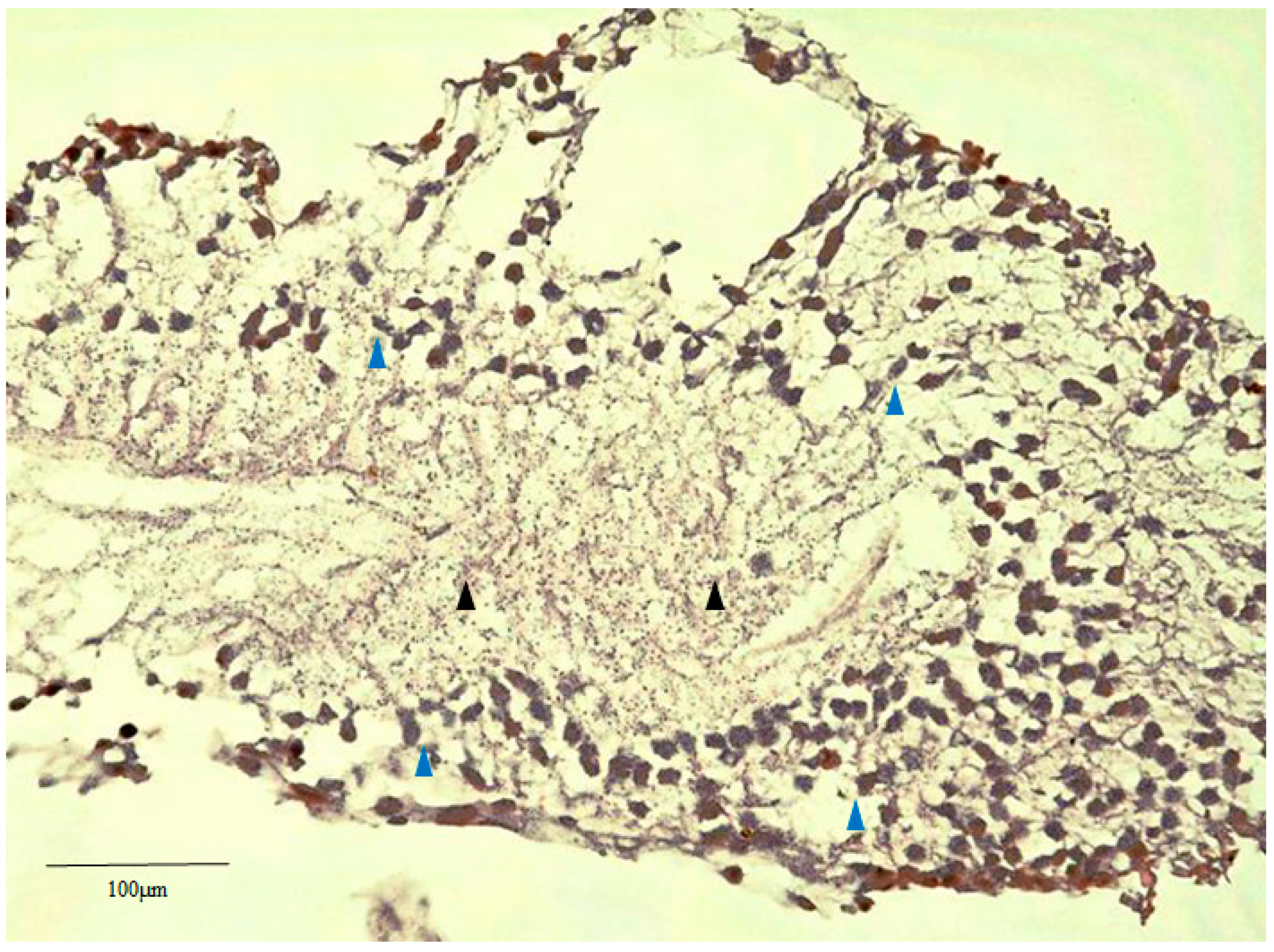
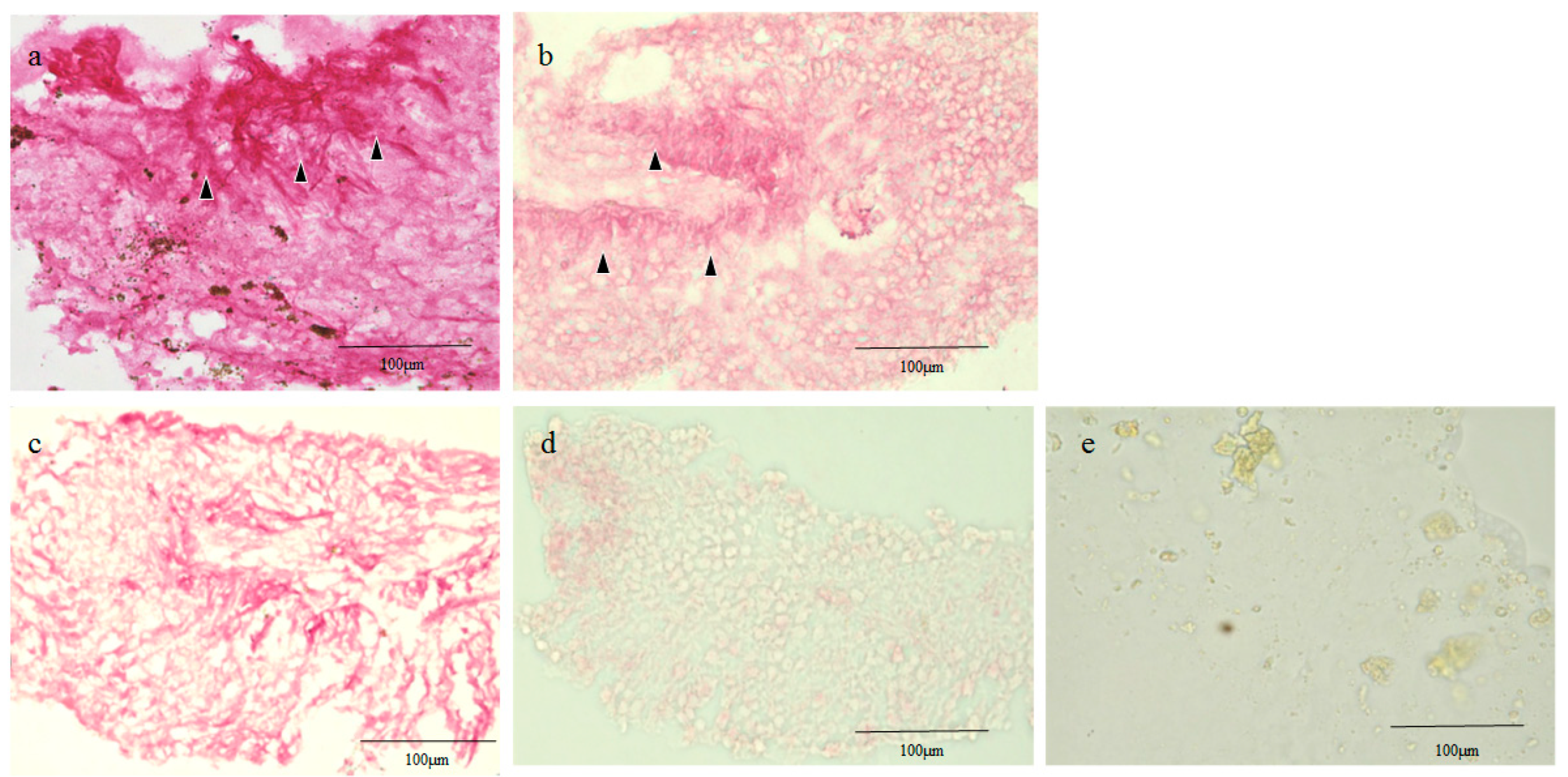
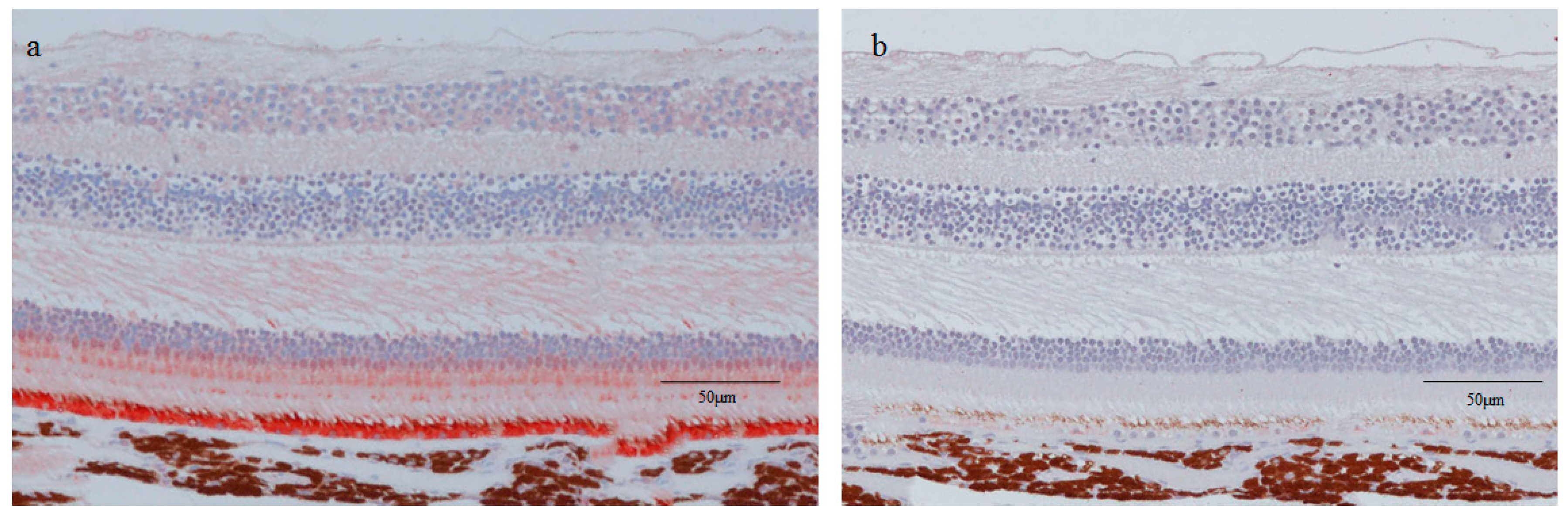
2.2. Hematoxylin Staining of Retinal Lattice Degeneration
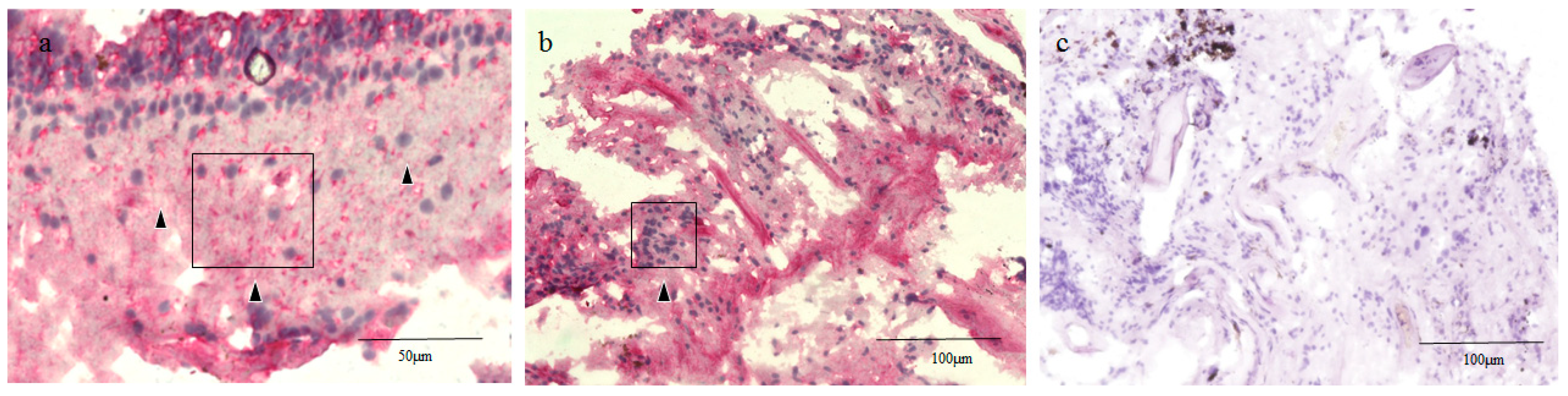
2.3. Glial Fibrillary Acidic Protein (GFAP), RPE65, and CK Immunostaining of the Human Retinal Lattice Degeneration Specimens
2.4. RPE65 and CK Immunostaining of the Normal Healthy Retina of the Monkey Eyes
3. Discussion
4. Subjects and Methods
4.1. Collection of the Retinal Lattice Degenerations Specimens
4.2. Hematoxylin Staining of Retinal Lattice Degeneration
4.3. Glial Fibrillary Acidic Protein (GFAP), RPE65, Pan-CK, and CK18 Immunostaining of the Human Retinal Lattice Degeneration Specimens
4.4. RPE65 and CK Immunostaining of the Normal Healthy Retina of Monkey Eyes
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RPE | Retinal pigment epithelium |
| GFAP | Glial fibrillary acidic protein |
| RPE65 | Retinal pigment epithelium-specific protein 65 kDa |
| CK | Cytokeratin |
| RT | Room temperature |
| PFA | Paraformaldehyde |
| PBS | Phosphate-buffered saline |
References
- Lewis, H. Peripheral retinal degenerations and the risk of retinal detachment. Am. J. Ophthalmol. 2003, 136, 155–160. [Google Scholar] [CrossRef]
- O’Malley, P.; Allen, R.A.; Straatsma, B.R.; O’Malley, C.C. Paving-stone degeneration of the retina. Arch. Ophthalmol. 1965, 73, 169–182. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, P.F.; Allen, R.A. Peripheral cystoid degeneration of the retina. Incidence and distribution in 1,000 autopsy eyes. Arch. Ophthalmol. 1967, 77, 769–776. [Google Scholar] [CrossRef]
- Straatsma, B.R.; Zeegen, P.D.; Foos, R.Y.; Feman, S.S.; Shabo, A.L. Lattice degeneration of the retina. XXX Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 1974, 77, 619–649. [Google Scholar] [CrossRef]
- Byer, N.E. Lattice degeneration of the retina. Surv. Ophthalmol. 1979, 23, 213–248. [Google Scholar] [CrossRef]
- Semes, L.P. Lattice degeneration of the retina and retinal detachment. Optom. Clin. 1992, 2, 71–91. [Google Scholar]
- Bec, P.; Malecaze, F.; Arne, J.L.; Mathis, A. Lattice degeneration of the peripheral retina: Ultrastructural study. Ophthalmologica 1985, 191, 107–113. [Google Scholar] [CrossRef]
- Mata, N.L.; Moghrabi, W.N.; Lee, J.S.; Bui, T.V.; Radu, R.A.; Horwitz, J.; Travis, G.H. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem. 2004, 279, 635–643. [Google Scholar] [CrossRef]
- Labelle, P.; Reilly, C.M.; Naydan, D.K.; Labelle, A.L. Immunohistochemical characteristics of normal canine eyes. Vet. Pathol. 2012, 49, 860–869. [Google Scholar] [CrossRef]
- Murray, T.G.; Green, W.R.; Maumenee, I.H.; Kopits, S.E. Spondyloepiphyseal dysplasia congenita. Light and electron microscopic studies of the eye. Arch. Ophthalmol. 1985, 103, 407–411. [Google Scholar] [CrossRef]
- Del Monte, M.A.; Maumenee, I.H.; Green, W.R.; Kenyon, K.R. Histopathology of Sanfilippo’s syndrome. Arch. Ophthalmol. 1983, 101, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Jaissle, G.B.; May, C.A.; van de Pavert, S.A.; Wenzel, A.; Claes-May, E.; Giessl, A.; Szurman, P.; Wolfrum, U.; Wijnholds, J.; Fischer, M.D.; et al. Bone spicule pigment formation in retinitis pigmentosa: Insights from a mouse model. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Makita, S.; Sugiyama, S.; Hong, Y.J.; Yasuno, Y.; Elsner, A.E.; Tamiya, S.; Tsukahara, R.; Iwasaki, T.; Goto, H. Evaluation of intraretinal migration of retinal pigment epithelial cells in age-related macular degeneration using polarimetric imaging. Sci. Rep. 2017, 7, 3150. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Jung, J.J.; Curcio, C.A.; Balaratnasingam, C.; Gallego-Pinazo, R.; Dolz-Marco, R.; Freund, K.B.; Yannuzzi, L.A. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: Clinical and histologic study. Am. J. Ophthalmol. 2016, 164, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Vossmerbaeumer, U.; Kuehl, S.; Kern, S.; Kluter, H.; Jonas, J.B.; Bieback, K. Induction of retinal pigment epithelium properties in ciliary margin progenitor cells. Clin. Exp. Ophthalmol. 2008, 36, 358–366. [Google Scholar] [CrossRef]
- Bhutto, I.A.; Ogura, S.; Baldeosingh, R.; McLeod, D.S.; Lutty, G.A.; Edwards, M.M. An acute injury model for the phenotypic characteristics of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD143–AMD151. [Google Scholar] [CrossRef]
- Xu, G.Z.; Li, W.W.; Tso, M.O. Apoptosis in human retinal degenerations. Trans. Am. Ophthalmol. Soc. 1996, 94, 411–431. [Google Scholar]
- Straatsma, B.R.; Allen, R.A. Lattice degeneration of the retina. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1962, 66, 600–613. [Google Scholar]
- Tang, P.H.; Buhusi, M.C.; Ma, J.X.; Crouch, R.K. RPE65 is present in human green/red cones and promotes photopigment regeneration in an in vitro cone cell model. J. Neurosci. 2011, 31, 18618–18626. [Google Scholar] [CrossRef]
- Castanheira, P.; Torquetti, L.; Nehemy, M.B.; Goes, A.M. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq. Bras. Oftalmol. 2008, 71, 644–650. [Google Scholar] [CrossRef]
- Balagholi, S.; Alizadeh, S.; Bagheri, A.; Amizadeh, Y.; Kanavi, M.R. The effects of platelet gel on cultured human retinal pigment epithelial (hRPE) cells. Bosn. J. Basic Med. Sci. 2017, 17, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Akrami, H.; Soheili, Z.S.; Sadeghizadeh, M.; Khalooghi, K.; Ahmadieh, H.; Kanavi, M.R.; Samiei, S.; Pakravesh, J. Evaluation of RPE65, CRALBP, VEGF, CD68, and tyrosinase gene expression in human retinal pigment epithelial cells cultured on amniotic membrane. Biochem. Genet. 2011, 49, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Sasai, Y.; Kawasaki, H.; Amemiya, K.; Ooto, S.; Kitada, M.; Suemori, H.; Nakatsuji, N.; Ide, C.; Honda, Y.; et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Muniz, A.; Betts, B.S.; Trevino, A.R.; Buddavarapu, K.; Roman, R.; Ma, J.X.; Tsin, A.T.C. Evidence for two retinoid cycles in the cone-dominated chicken eye. Biochemistry 2009, 48, 6854–6863. [Google Scholar] [CrossRef]
- Lupien, C.; Brenner, M.; Guérin, S.L.; Salesse, C. Expression of glial fibrillary acidic protein in primary cultures of human Müller cells. Exp. Eye Res. 2004, 79, 423–429. [Google Scholar] [CrossRef]
- Soler, M.V.C.; Gallo, J.E.; Dodds, R.A.; Suburo, A.M. A mouse model of proliferative vitreoretinopathy induced by dispase. Exp. Eye Res. 2002, 75, 491–504. [Google Scholar] [CrossRef]
- Feist, R.M., Jr.; King, J.F.; Morris, R.; Witherspoon, C.D.; Guidry, C. Myofibroblast and extracellular matrix origins in proliferative vitreoretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 347–357. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Kannan, R.; Felszeghy, S.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. The regulation of NFE2 L2 (NRF2) signaling and epithelial-to-mesenchymal transition in age-related macular degeneration pathology. Int. J. Mol. Sci. 2019, 20, 5800. [Google Scholar] [CrossRef]
- Casaroli-Marano, R.P.; Pagan, R.; Vilaró, S. Epithelial-mesenchymal transition in proliferative vitreoretinopathy: Intermediate filament protein expression in retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2062–2072. [Google Scholar]
- Grisanti, S.; Guidry, C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Investig. Ophthalmol. Vis. Sci. 1995, 36, 391–405. [Google Scholar]
- Palma-Nicolás, J.P.; López-Colomé, A.M. Thrombin induces Slug-mediated E-cadherin transcriptional repression and the parallel up-regulation of N-cadherin by a transcription-independent mechanism in RPE cells. J. Cell Physiol. 2013, 228, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wei, O.; Liu, O.; Ren, C.; Liu, J.; Cai, W.; Zhu, M.; Jin, H.; He, M.; Yu, J. Effects of bradykinin on TGF β1 induced epithelial mesenchymal transition in ARPE 19 cells. Mol. Med. Rep. 2018, 17, 5878–5886. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bueno, I.; Rodriguez de la Rua, E.; Hileeto, D.; Parrado, M.L.; Regueiro-Purriños, M.; Sala-Puigdollers, A.; Srivastava, G.K.; Gonzolo-Orden, J.M.; Pastor, J.C. Histology and immunochemistry evaluation of autologous translocation of retinal pigment epithelium-choroid graft in porcine eyes. Acta. Ophthalmol. 2013, 91, e125–e132. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, U.; Kivelä, T.; Tarkkanen, A. Cytoskeleton in normal and reactive human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3178–3186. [Google Scholar]
- Martínez-Navarrete, G.C.; Angulo, A.; Martín-Nieto, J.; Cuenca, N. Gradual morphogenesis of retinal neurons in the peripheral retinal margin of adult monkeys and humans. J. Comp. Neurol. 2008, 511, 557–580. [Google Scholar] [CrossRef]
- Guo, X.Y.; Jin, L.F.; Ji, S.H.; Ji, W.Z. Rhesus monkey (macaca mulatta) Muller cells exhibit retinal stem/progenitor cell features in vitro. Dongwuxue Yanjiu 2011, 32, 611–616. (In Chinese) [Google Scholar] [CrossRef]
- Giannelli, S.G.; Demontis, G.C.; Pertile, G.; Rama, P.; Broccoli, V. Adult human Müller glia cells are a highly efficient source of rod photoreceptors. Stem Cells 2011, 29, 344–356. [Google Scholar] [CrossRef]
- Amemiya, K.; Haruta, M.; Takahashi, M.; Kosaka, M.; Eguchi, G. Adult human retinal pigment epithelial cells capable of differentiating into neurons. Biochem. Biophys. Res. Commun. 2004, 316, 1–5. [Google Scholar] [CrossRef]
- Kuznetsova, A.V.; Grirorian, É.N.; Aleksandrova, M.A. Adult human retinal pigment epithelial cells—A potential source of cells for regeneration retina. Tsitologiia 2011, 53, 505–512. (In Russian) [Google Scholar]
- Ikeda, T.; Nakamura, K.; Oku, H.; Horie, T.; Kida, T.; Takai, S. Immunohistological study of monkey foveal retina. Sci. Rep. 2019, 9, 5258. [Google Scholar] [CrossRef]
- Robinson, M.R.; Streeten, B.W. The surface morphology of retinal breaks and retinal lattice degeneration. A scanning electron microscopic study. Ophthalmology 1986, 93, 237–246. [Google Scholar] [CrossRef]
- Ulbrich, S.; Friedrichs, J.; Valtink, M.; Murovski, S.; Franz, C.M.; Müller, D.J.; Funk, R.H.W.; Engelmann, K. Retinal pigment epithelium cell alignment on nanostructured collagen matrices. Cells Tissues Organs 2011, 194, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Bastiaans, J.; van Meurs, J.C.; van Holten-Neelen, C.; Nagtzaam, N.M.; van Hagen, P.M.; Chambers, R.C.; Hooijkaas, H.; Dik, W.A. Thrombin induces epithelial-mesenchymal transition and collagen production by retinal pigment epithelial cells via autocrine PDGF-receptor signaling. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8306–8314. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Vigier, S.; Labour, M.N.; Jorgensen, C.; Belamie, E.; Noël, D. Induction of mesenchymal stem cell differentiation and cartilage formation by cross-linker-free collagen microspheres. Eur. Cell Mater. 2014, 28, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Ponsioen, T.L.; van Luyn, M.J.; van der Worp, R.J.; van Meurs, J.C.; Hooymans, J.M.; Los, L.I. Collagen distribution in the human vitreoretinal interface. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4089–4095. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; McLeod, D.; Henson, D.B.; Bishop, P.N. Age-dependent changes in the basal retinovitreous adhesion. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J.; Balazs, E.A. Morphology and ultrastructure of human vitreous fibers. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1867–1871. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, H.; Fukumoto, M.; Sato, T.; Horie, T.; Kida, T.; Oku, H.; Nakamura, K.; Jin, D.; Takai, S.; Ikeda, T. Involvement of the Retinal Pigment Epithelium in the Development of Retinal Lattice Degeneration. Int. J. Mol. Sci. 2020, 21, 7347. https://doi.org/10.3390/ijms21197347
Mizuno H, Fukumoto M, Sato T, Horie T, Kida T, Oku H, Nakamura K, Jin D, Takai S, Ikeda T. Involvement of the Retinal Pigment Epithelium in the Development of Retinal Lattice Degeneration. International Journal of Molecular Sciences. 2020; 21(19):7347. https://doi.org/10.3390/ijms21197347
Chicago/Turabian StyleMizuno, Hiroshi, Masanori Fukumoto, Takaki Sato, Taeko Horie, Teruyo Kida, Hidehiro Oku, Kimitoshi Nakamura, Denan Jin, Shinji Takai, and Tsunehiko Ikeda. 2020. "Involvement of the Retinal Pigment Epithelium in the Development of Retinal Lattice Degeneration" International Journal of Molecular Sciences 21, no. 19: 7347. https://doi.org/10.3390/ijms21197347
APA StyleMizuno, H., Fukumoto, M., Sato, T., Horie, T., Kida, T., Oku, H., Nakamura, K., Jin, D., Takai, S., & Ikeda, T. (2020). Involvement of the Retinal Pigment Epithelium in the Development of Retinal Lattice Degeneration. International Journal of Molecular Sciences, 21(19), 7347. https://doi.org/10.3390/ijms21197347





