Altered Gap Junction Network Topography in Mouse Models for Human Hereditary Deafness
Abstract
:1. Introduction
2. Results
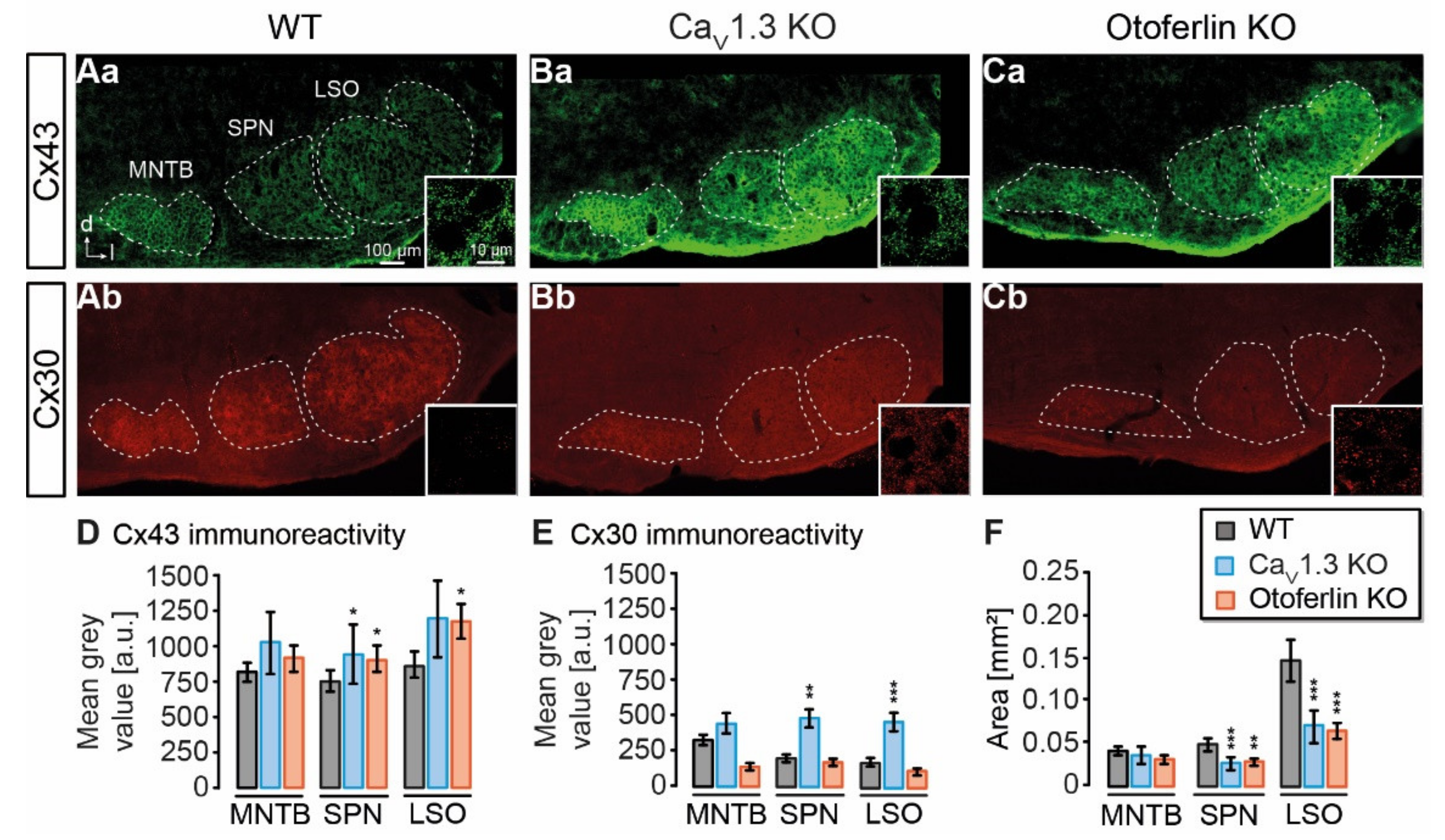
2.1. Expression of Cx43 and Cx30 in the Auditory Brainstem
2.2. Electrophysiological Properties of LSO Astrocytes
2.3. Unaltered LSO Astrocyte Network Properties
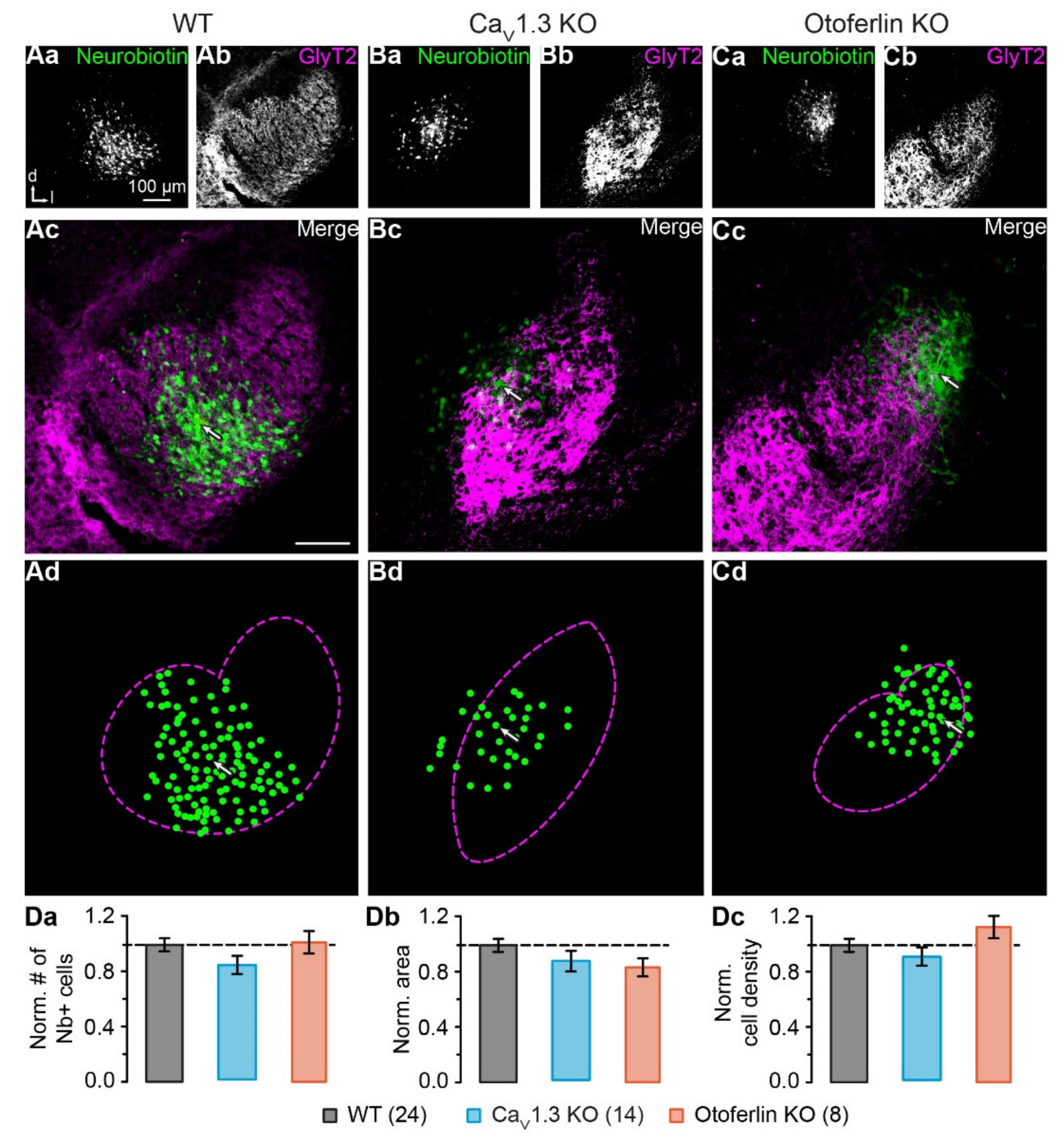
2.4. Disturbed LSO Astrocyte Network Topography
3. Discussion
3.1. Connexin Expression in KO Models
3.2. Activity-Dependent Alteration of Astrocyte Network Topography
3.3. Mechanism Underlying the Altered Network Topography
3.4. Signaling between Astrocytes and Neurons
3.5. Conclusion
4. Materials and Methods
4.1. Genotyping
4.2. Immunohistochemistry
4.3. Preparation of Acute Tissue Slices
4.4. Electrophysiology and Tracer Loading
4.5. Visualization of Coupled Cells
4.6. Analysis of Network Topography
4.7. Statistics
4.8. Additional Information
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSF | Artificial cerebrospinal fluid |
| AF | Alexa fluor |
| BSA | Bovine serum albumin |
| CaV | Voltage-activated calcium channel |
| Cx | Connexin |
| EDTA | Ethylenediaminetetraacetic acid |
| EGTA | Glycol-bis(2 aminoethylether)-N,N′,N′,N′-tetraacetic acid |
| GJ | Gap junction |
| GlyT | Glycine transporter |
| HEPES | N (2 hydroxyethyl)piperazine-N′ 2 ethanesulfonic acid |
| IC | Inferior colliculus |
| KO | Knock-out |
| LSO | Lateral superior olive |
| MNTB | Medial nucleus of the trapezoid body |
| NGS | Normal goat serum |
| nPA | Non-passive astrocyte |
| PA | Passive astrocyte |
| PBS | Phosphate buffered solution |
| PCR | Polymerase chain reaction |
| R | Ratio |
| RT | Room temperature |
| SOC | Superior olivary complex |
| SPN | Superior paraolivery nucleus |
| SR101 | Sulforhodamine 101 |
| Tris | Tris(hydroxymethyl)aminomethane |
| WT | Wild type |
References
- Augustin, V.; Bold, C.; Wadle, S.L.; Langer, J.; Jabs, R.; Philippot, C.; Weingarten, D.J.; Rose, C.R.; Steinhauser, C.; Stephan, J. Functional anisotropic panglial networks in the lateral superior olive. Glia 2016, 64, 1892–1911. [Google Scholar] [CrossRef] [PubMed]
- Wadle, S.L.; Augustin, V.; Langer, J.; Jabs, R.; Philippot, C.; Weingarten, D.J.; Rose, C.R.; Steinhauser, C.; Stephan, J. Anisotropic Panglial Coupling Reflects Tonotopic Organization in the Inferior Colliculus. Front. Cell. Neurosci. 2018, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Tress, O.; Haas, B.; Karram, K.; Trotter, J.; Willecke, K.; Kettenmann, H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 2010, 58, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Moshrefi-Ravasdjani, B.; Hammel, E.L.; Kafitz, K.W.; Rose, C.R. Astrocyte Sodium Signalling and Panglial Spread of Sodium Signals in Brain White Matter. Neurochem. Res. 2017, 42, 2505–2518. [Google Scholar] [CrossRef] [PubMed]
- Griemsmann, S.; Hoft, S.P.; Bedner, P.; Zhang, J.; von Staden, E.; Beinhauer, A.; Degen, J.; Dublin, P.; Cope, D.W.; Richter, N.; et al. Characterization of Panglial Gap Junction Networks in the Thalamus, Neocortex, and Hippocampus Reveals a Unique Population of Glial Cells. Cereb. Cortex 2015, 25, 3420–3433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallraff, A.; Kohling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhauser, C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef] [Green Version]
- Houades, V.; Koulakoff, A.; Ezan, P.; Seif, I.; Giaume, C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J. Neurosci. 2008, 28, 5207–5217. [Google Scholar] [CrossRef]
- Claus, L.; Philippot, C.; Griemsmann, S.; Timmermann, A.; Jabs, R.; Henneberger, C.; Kettenmann, H.; Steinhauser, C. Barreloid Borders and Neuronal Activity Shape Panglial Gap Junction-Coupled Networks in the Mouse Thalamus. Cereb. Cortex 2018, 28, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Eitelmann, S.; Hirtz, J.J.; Stephan, J. A Vector-Based Method to Analyze the Topography of Glial Networks. Int. J. Mol. Sci. 2019, 20, 2821. [Google Scholar] [CrossRef] [Green Version]
- Kandler, K.; Clause, A.; Noh, J. Tonotopic reorganization of developing auditory brainstem circuits. Nat. Neurosci. 2009, 12, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.M.; Fex, J. Tonotopic organization in the inferior colliculus of the rat demonstrated with the 2-deoxyglucose method. Exp. Brain Res. 1986, 61, 506–512. [Google Scholar] [CrossRef]
- Merzenich, M.M.; Reid, M.D. Representation of the cochlea within the inferior colliculus of the cat. Brain Res. 1974, 77, 397–415. [Google Scholar] [CrossRef]
- Rietzel, H.J.; Friauf, E. Neuron types in the rat lateral superior olive and developmental changes in the complexity of their dendritic arbors. J. Comp. Neurol. 1998, 390, 20–40. [Google Scholar] [CrossRef]
- Malmierca, M.S.; Blackstad, T.W.; Osen, K.K. Computer-assisted 3-D reconstructions of Golgi-impregnated neurons in the cortical regions of the inferior colliculus of rat. Hear. Res. 2011, 274, 13–26. [Google Scholar] [CrossRef]
- Sanes, D.H.; Song, J.; Tyson, J. Refinement of dendritic arbors along the tonotopic axis of the gerbil lateral superior olive. Brain Res. Dev. Brain Res. 1992, 67, 47–55. [Google Scholar] [CrossRef]
- Bal, R.; Green, G.G.; Rees, A.; Sanders, D.J. Firing patterns of inferior colliculus neurons-histology and mechanism to change firing patterns in rat brain slices. Neurosci. Lett. 2002, 317, 42–46. [Google Scholar] [CrossRef]
- Ghirardini, E.; Wadle, S.L.; Augustin, V.; Becker, J.; Brill, S.; Hammerich, J.; Seifert, G.; Stephan, J. Expression of functional inhibitory neurotransmitter transporters GlyT1, GAT-1, and GAT-3 by astrocytes of inferior colliculus and hippocampus. Mol. Brain 2018, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Kandler, K.; Gillespie, D.C. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci. 2005, 28, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Friauf, E.; Krächan, E.G.; Müller, N.I.C. Lateral superior olive. In The Oxford Handbook of the Auditory Brainstem; Kandler, K., Ed.; Oxford University Press: New York, NY, USA, 2019; pp. 328–394. [Google Scholar] [CrossRef]
- Kotak, V.C.; Korada, S.; Schwartz, I.R.; Sanes, D.H. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J. Neurosci. 1998, 18, 4646–4655. [Google Scholar] [CrossRef] [Green Version]
- Nabekura, J.; Katsurabayashi, S.; Kakazu, Y.; Shibata, S.; Matsubara, A.; Jinno, S.; Mizoguchi, Y.; Sasaki, A.; Ishibashi, H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat. Neurosci. 2004, 7, 17–23. [Google Scholar] [CrossRef]
- Fischer, A.U.; Muller, N.I.C.; Deller, T.; Del Turco, D.; Fisch, J.O.; Griesemer, D.; Kattler, K.; Maraslioglu, A.; Roemer, V.; Xu-Friedman, M.A.; et al. GABA is a modulator, rather than a classical transmitter, in the medial nucleus of the trapezoid body-lateral superior olive sound localization circuit. J. Physiol. 2019, 597, 2269–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.; Kandler, K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat. Neurosci. 2003, 6, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, N.X.; Yi, E.; Gale, J.E.; Glowatzki, E.; Bergles, D.E. The origin of spontaneous activity in the developing auditory system. Nature 2007, 450, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sanes, D.H.; Takacs, C. Activity-dependent refinement of inhibitory connections. Eur. J. Neurosci. 1993, 5, 570–574. [Google Scholar] [CrossRef]
- Muller, N.I.C.; Sonntag, M.; Maraslioglu, A.; Hirtz, J.J.; Friauf, E. Topographic map refinement and synaptic strengthening of a sound localization circuit require spontaneous peripheral activity. J. Physiol. 2019, 597, 5469–5493. [Google Scholar] [CrossRef]
- Clause, A.; Kim, G.; Sonntag, M.; Weisz, C.J.; Vetter, D.E.; Rubsamen, R.; Kandler, K. The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement. Neuron 2014, 82, 822–835. [Google Scholar] [CrossRef] [Green Version]
- Clause, A.; Lauer, A.M.; Kandler, K. Mice Lacking the Alpha9 Subunit of the Nicotinic Acetylcholine Receptor Exhibit Deficits in Frequency Difference Limens and Sound Localization. Front. Cell. Neurosci. 2017, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.Y.; Ahmed, Z.M.; Riazuddin, S.; Bhinder, M.A.; Shahzad, M.; Husnain, T.; Riazuddin, S.; Griffith, A.J.; Friedman, T.B. Identities and frequencies of mutations of the otoferlin gene (OTOF) causing DFNB9 deafness in Pakistan. Clin. Genet. 2009, 75, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Duman, D.; Sirmaci, A.; Cengiz, F.B.; Ozdag, H.; Tekin, M. Screening of 38 genes identifies mutations in 62% of families with nonsyndromic deafness in Turkey. Genet. Test. Mol. Biomark. 2011, 15, 29–33. [Google Scholar] [CrossRef]
- Baig, S.M.; Koschak, A.; Lieb, A.; Gebhart, M.; Dafinger, C.; Nurnberg, G.; Ali, A.; Ahmad, I.; Sinnegger-Brauns, M.J.; Brandt, N.; et al. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat. Neurosci. 2011, 14, 77–84. [Google Scholar] [CrossRef]
- Iwasa, Y.; Nishio, S.Y.; Yoshimura, H.; Kanda, Y.; Kumakawa, K.; Abe, S.; Naito, Y.; Nagai, K.; Usami, S. OTOF mutation screening in Japanese severe to profound recessive hearing loss patients. BMC Med. Genet. 2013, 14, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platzer, J.; Engel, J.; Schrott-Fischer, A.; Stephan, K.; Bova, S.; Chen, H.; Zheng, H.; Striessnig, J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 2000, 102, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Longo-Guess, C.; Gagnon, L.H.; Bergstrom, D.E.; Johnson, K.R. A missense mutation in the conserved C2B domain of otoferlin causes deafness in a new mouse model of DFNB9. Hear. Res. 2007, 234, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirtz, J.J.; Boesen, M.; Braun, N.; Deitmer, J.W.; Kramer, F.; Lohr, C.; Muller, B.; Nothwang, H.G.; Striessnig, J.; Lohrke, S.; et al. Cav1.3 calcium channels are required for normal development of the auditory brainstem. J. Neurosci. 2011, 31, 8280–8294. [Google Scholar] [CrossRef]
- Hirtz, J.J.; Braun, N.; Griesemer, D.; Hannes, C.; Janz, K.; Lohrke, S.; Muller, B.; Friauf, E. Synaptic refinement of an inhibitory topographic map in the auditory brainstem requires functional Cav1.3 calcium channels. J. Neurosci. 2012, 32, 14602–14616. [Google Scholar] [CrossRef]
- Stephan, J.; Friauf, E. Functional analysis of the inhibitory neurotransmitter transporters GlyT1, GAT-1, and GAT-3 in astrocytes of the lateral superior olive. Glia 2014, 62, 1992–2003. [Google Scholar] [CrossRef]
- Kafitz, K.W.; Meier, S.D.; Stephan, J.; Rose, C.R. Developmental profile and properties of sulforhodamine 101--Labeled glial cells in acute brain slices of rat hippocampus. J. Neurosci. Methods 2008, 169, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Schools, G.P.; Kimelberg, H.K. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: Mature astrocytes are electrophysiologically passive. J. Neurophysiol. 2006, 95, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Langer, J.; Stephan, J.; Theis, M.; Rose, C.R. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 2012, 60, 239–252. [Google Scholar] [CrossRef]
- Schools, G.P.; Zhou, M.; Kimelberg, H.K. Development of gap junctions in hippocampal astrocytes: Evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J. Neurophysiol. 2006, 96, 1383–1392. [Google Scholar] [CrossRef]
- Kiyoshi, C.M.; Du, Y.; Zhong, S.; Wang, W.; Taylor, A.T.; Xiong, B.; Ma, B.; Terman, D.; Zhou, M. Syncytial isopotentiality: A system-wide electrical feature of astrocytic networks in the brain. Glia 2018, 66, 2756–2769. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, P.; Schroder, W.; Traub, O.; Steinhauser, C.; Dermietzel, R.; Willecke, K. Late onset and increasing expression of the gap junction protein connexin30 in adult murine brain and long-term cultured astrocytes. Glia 1999, 25, 111–119. [Google Scholar] [CrossRef]
- Nagy, J.I.; Patel, D.; Ochalski, P.A.; Stelmack, G.L. Connexin30 in rodent, cat and human brain: Selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 1999, 88, 447–468. [Google Scholar] [CrossRef]
- Felix, L.; Stephan, J.; Rose, C.R. Astrocytes of the early postnatal brain. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Ghezali, G.; Calvo, C.F.; Pillet, L.E.; Llense, F.; Ezan, P.; Pannasch, U.; Bemelmans, A.P.; Etienne Manneville, S.; Rouach, N. Connexin 30 controls astroglial polarization during postnatal brain development. Development 2018, 145, dev155275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Minge, D.; Griemsmann, S.; Herde, M.K.; Steinhauser, C.; Henneberger, C. Spatial properties of astrocyte gap junction coupling in the rat hippocampus. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golub, J.S.; Tong, L.; Ngyuen, T.B.; Hume, C.R.; Palmiter, R.D.; Rubel, E.W.; Stone, J.S. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J. Neurosci. 2012, 32, 15093–15105. [Google Scholar] [CrossRef] [Green Version]
- Korn, M.J.; Koppel, S.J.; Cramer, K.S. Astrocyte-secreted factors modulate a gradient of primary dendritic arbors in nucleus laminaris of the avian auditory brainstem. PLoS ONE 2011, 6, e27383. [Google Scholar] [CrossRef] [Green Version]
- Korn, M.J.; Koppel, S.J.; Li, L.H.; Mehta, D.; Mehta, S.B.; Seidl, A.H.; Cramer, K.S. Astrocyte-secreted factors modulate the developmental distribution of inhibitory synapses in nucleus laminaris of the avian auditory brainstem. J. Comp. Neurol. 2012, 520, 1262–1277. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.H.; Long, L.; Tang, Y.C.; Hu, H.T.; Tang, F.R. Ca(v)1.2, Ca(v)1.3, and Ca(v)2.1 in the mouse hippocampus during and after pilocarpine-induced status epilepticus. Hippocampus 2007, 17, 235–251. [Google Scholar] [CrossRef]
- Schug, N.; Braig, C.; Zimmermann, U.; Engel, J.; Winter, H.; Ruth, P.; Blin, N.; Pfister, M.; Kalbacher, H.; Knipper, M. Differential expression of otoferlin in brain, vestibular system, immature and mature cochlea of the rat. Eur. J. Neurosci. 2006, 24, 3372–3380. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.; Haack, N.; Kafitz, K.W.; Durry, S.; Koch, D.; Hochstrate, P.; Seifert, G.; Steinhauser, C.; Rose, C.R. Kir4.1 channels mediate a depolarization of hippocampal astrocytes under hyperammonemic conditions in situ. Glia 2012, 60, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Pusch, M.; Neher, E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers. Arch. 1988, 411, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H. The Bonferroni and Šidák corrections for multiple comparisons. In Encyclopedia of Measurement and Statistics; Salkind, N., Ed.; Sage Publications: Thousand Oaks, CA, USA, 2007; pp. 103–107. [Google Scholar]



| Geno-Type | H2O | 5× PCR Buffer | Forward Primer | Reverse Primer | Taq Poly-Merase | PCR Protocol | Amplicon Size (bp) |
|---|---|---|---|---|---|---|---|
| WT | 7.7 μL | 4.0 μL | 2.0 μL, 5 pmol/μL, 5′-GCA AAC TAT GCA AGA GGC ACC AGA-3′ | 2.0 μL, 5 pmol/μL, 5′-TAC TTC CAT TCC ACT ATA CTA ATG CAG GCT-3′ | 0.3 μL | 2 min 92 °C; 20 s 52 °C; 30 s 72 °C; 30 cycles (20 s 92 °C; 20 s 52 °C; 30 s 72 °C); 7 min 72 °C; 15 °C cool down | 300 |
| CaV1.3 KO | 7.9 μL | 4.0 μL | 2.0 μL, 5 pmol/μL, 5′-TTC CAT TTG TCA CGT CCT GCA CCA-3′ | 2.0 μL, 5 pmol/μL, 5′-TAC TTC CAT TCC ACT ATA CTA ATG CAG GCT-3′ | 0.1 μL | 2 min 92 °C; 20 s 52 °C; 30 s 72 °C; 43 cycles (25 s 92 °C; 20 s 52 °C; 30 s 72 °C); 7 min 72 °C; 15 °C cool down | 450 |
| Otoferlin KO | 7.9 μL | 4.0 μL | 0.5 μL, 10 pmol/μL, 5′-TAC TGC CCA CAT GAG CTT TG-3′ | 0.5 μL, 10 pmol/μL, 5′-CAG AGG AAT CCA GCT GAA GG-3′ | 0.1 μL | 2 min 95 °C; 30 s 95 °C; 34 cycles (20 s 57 °C; 30 s 72 °C); 5 min 72 °C; 15 °C cool down | 186/163 (WT), 349 (KO) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eitelmann, S.; Petersilie, L.; Rose, C.R.; Stephan, J. Altered Gap Junction Network Topography in Mouse Models for Human Hereditary Deafness. Int. J. Mol. Sci. 2020, 21, 7376. https://doi.org/10.3390/ijms21197376
Eitelmann S, Petersilie L, Rose CR, Stephan J. Altered Gap Junction Network Topography in Mouse Models for Human Hereditary Deafness. International Journal of Molecular Sciences. 2020; 21(19):7376. https://doi.org/10.3390/ijms21197376
Chicago/Turabian StyleEitelmann, Sara, Laura Petersilie, Christine R. Rose, and Jonathan Stephan. 2020. "Altered Gap Junction Network Topography in Mouse Models for Human Hereditary Deafness" International Journal of Molecular Sciences 21, no. 19: 7376. https://doi.org/10.3390/ijms21197376
APA StyleEitelmann, S., Petersilie, L., Rose, C. R., & Stephan, J. (2020). Altered Gap Junction Network Topography in Mouse Models for Human Hereditary Deafness. International Journal of Molecular Sciences, 21(19), 7376. https://doi.org/10.3390/ijms21197376






