Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke
Abstract
1. Introduction
2. Results
2.1. Characterization of Thrombemboli
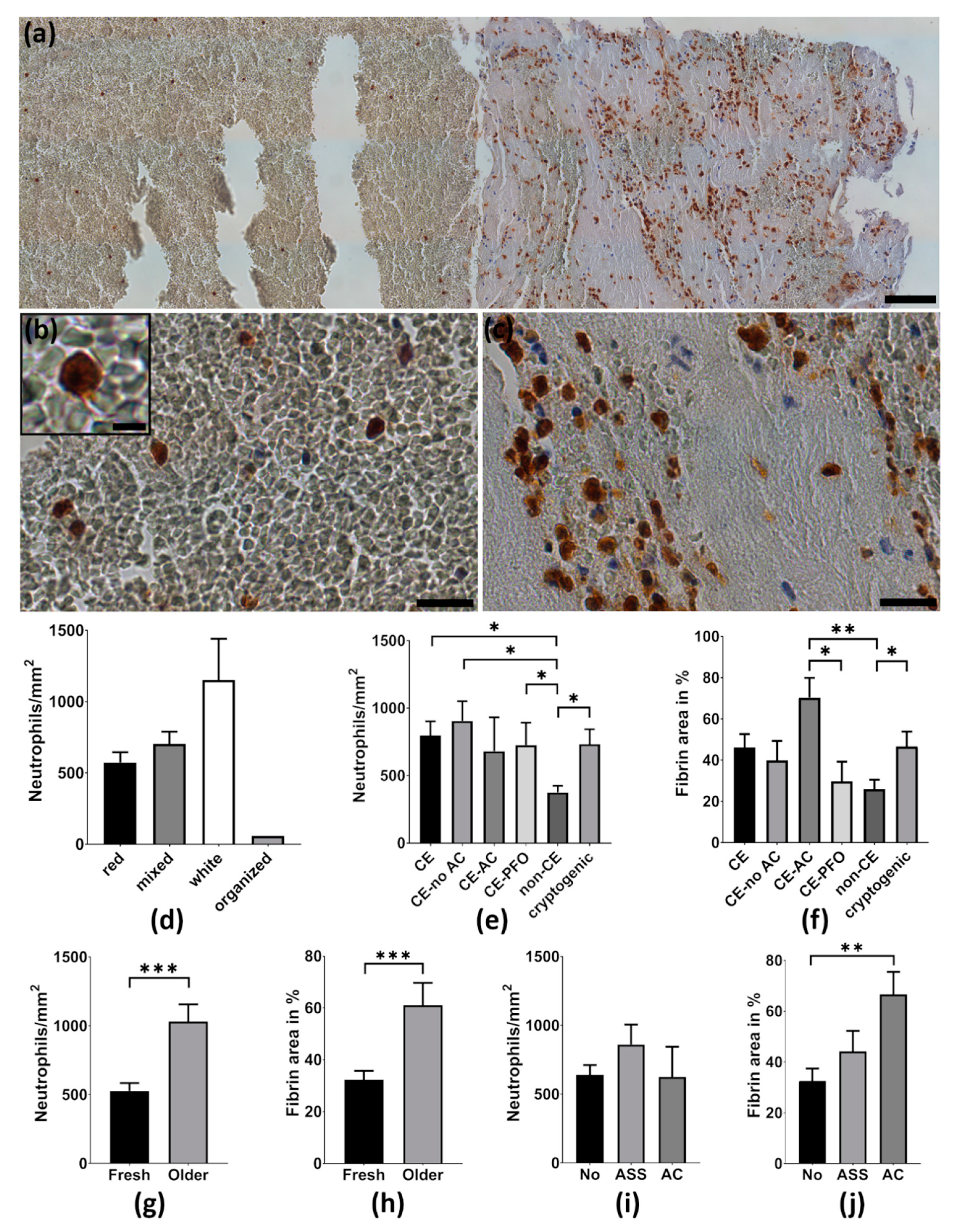
2.2. Neutrophils Are Abundant in Cerebral Thrombemboli
2.3. Thrombemboli Composition and Stroke Etiology
2.4. Thrombemboli Composition and Medication at Stroke Onset
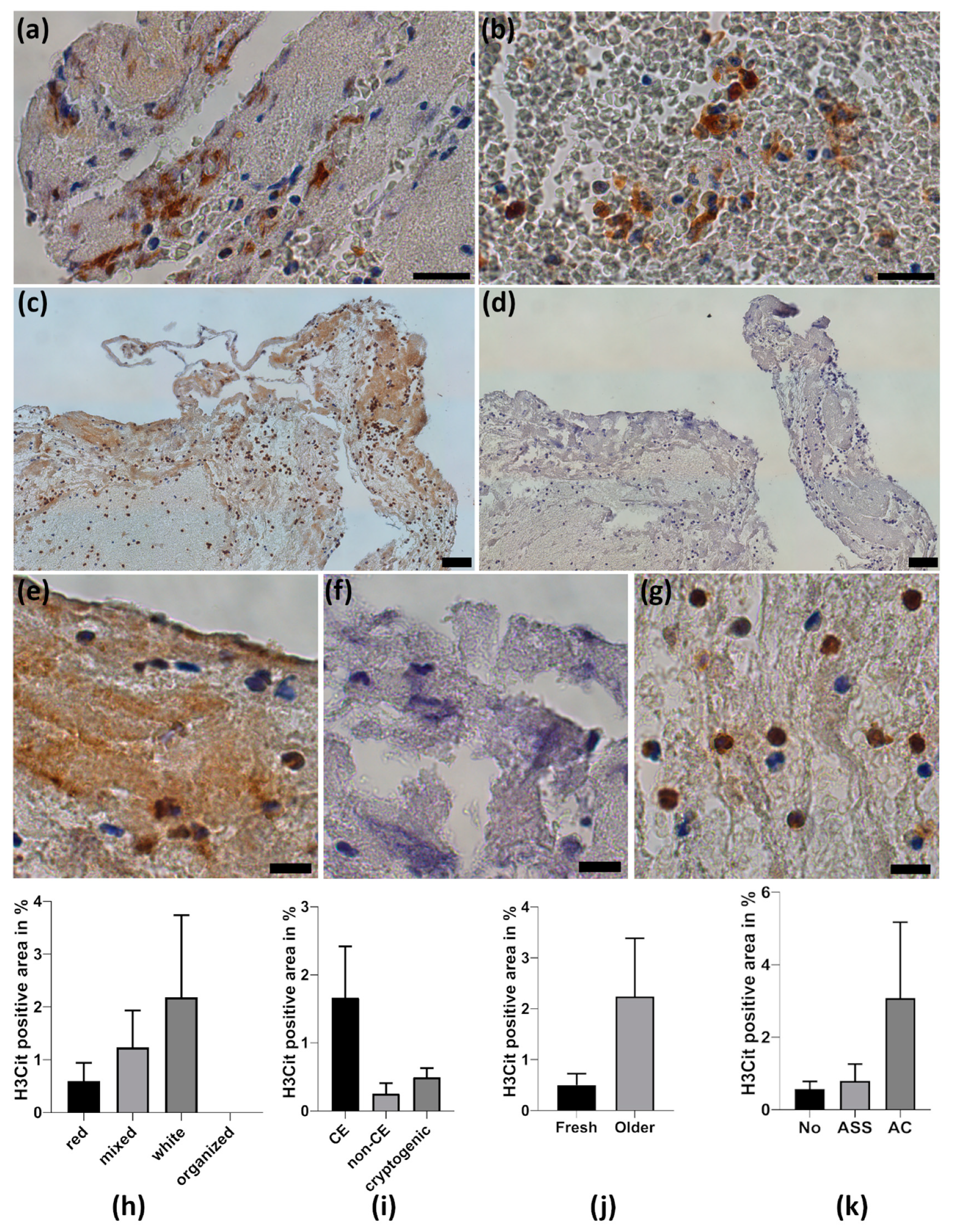
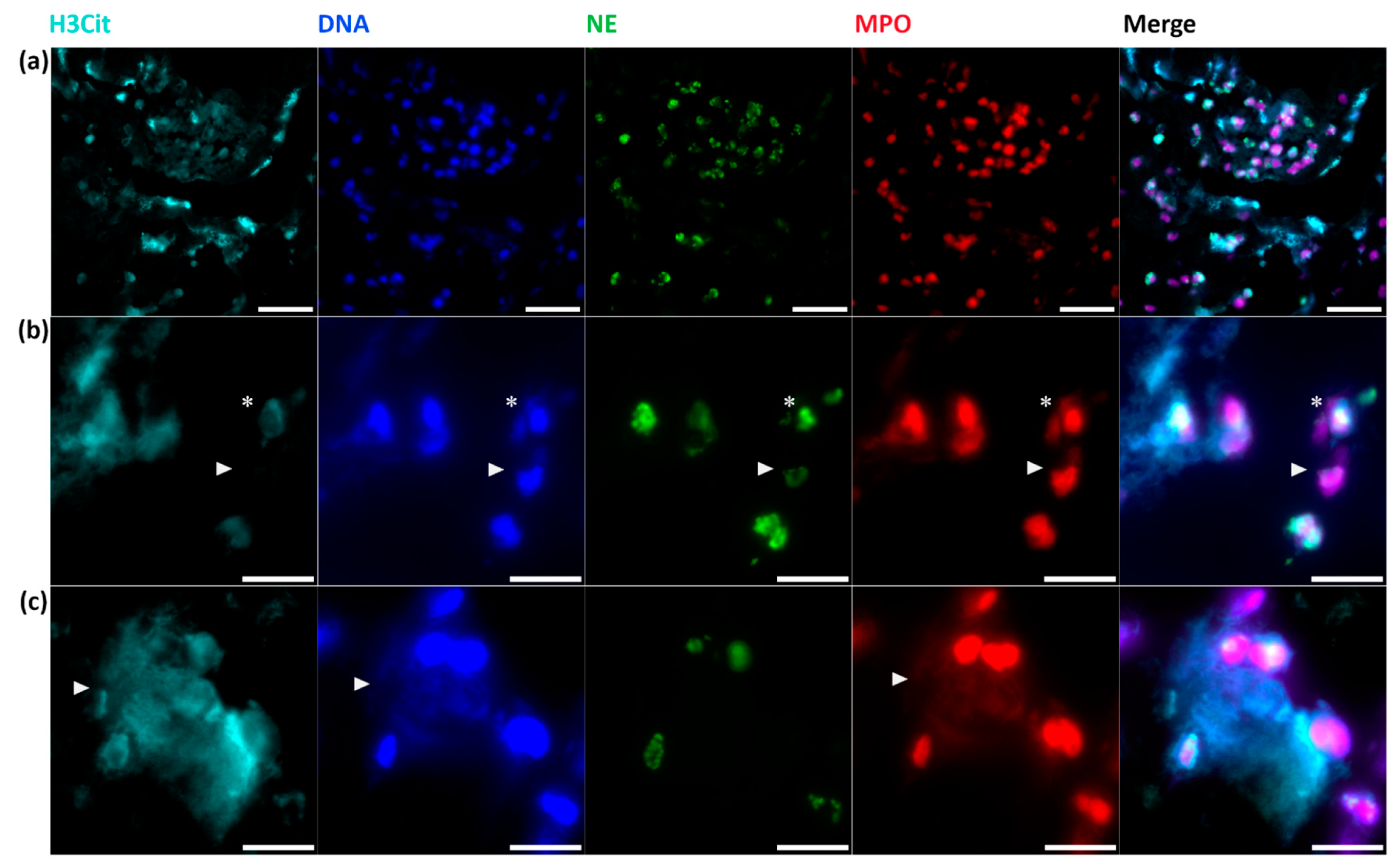
2.5. Neutrophil Extracellular Traps in Cerebral Thrombemboli
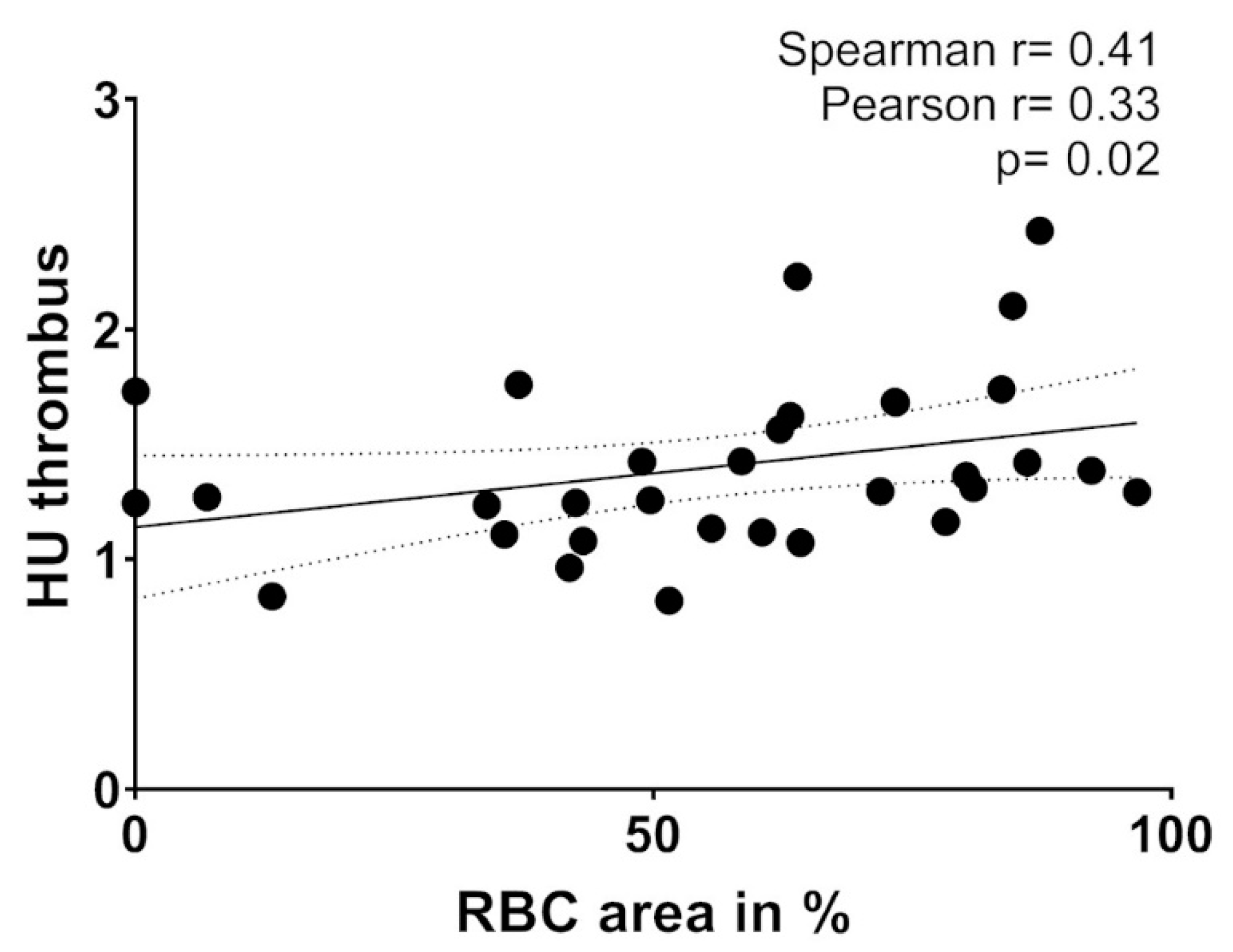
2.6. Thrombembolus Histopathology and Imaging Findings
3. Discussion
4. Materials and Methods
4.1. Patient Population and Study Design
4.2. Thrombectomy Procedure and Neuroradiological Analysis
4.3. Processing of Thrombemboli and Immunohistochemistry for Neutrophils and NETs
4.4. Immunofluorescence Staining of NETs
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | acute ischemic stroke |
| ASPECTS | Alberta Stroke Program Early CT Score |
| ASA | acetylsalicylic acid |
| CD | cluster of differentiation |
| DNA | deoxyribonucleic acid |
| DAPI | 4,6-diamidino-2-phenylindole |
| H&E | hematoxylin and eosin |
| HU | Hounsfield unit |
| ICA | internal carotid artery |
| IVT | intravenous thrombolysis |
| MT | mechanical thrombectomy |
| MCA | middle cerebral artery |
| MPO | myeloperoxidase |
| NCCT | non-contrast computer tomography |
| NIHSS | National Institutes of Health Stroke Scale |
| NE | neutrophil elastase |
| NET | neutrophil extracellular traps |
| PFO | patent foramen ovale |
| RBC | red blood cell |
| rt-PA | recombinant tissue plasminogen activator |
| SEM | standard error of the mean |
| TOAST | Trial of Org 10172 in Acute Stroke Treatment |
| vWF | von Willebrand Factor |
References
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- De Meyer, S.F.; Andersson, T.; Baxter, B.; Bendszus, M.; Brouwer, P.; Brinjikji, W.; Campbell, B.C.; Costalat, V.; Davalos, A.; Demchuk, A.; et al. Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int. J. Stroke 2017, 12, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Marder, V.J.; Chute, D.J.; Starkman, S.; Abolian, A.M.; Kidwell, C.; Liebeskind, D.; Ovbiagele, B.; Vinuela, F.; Duckwiler, G.; Jahan, R.; et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006, 37, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Niesten, J.M.; van der Schaaf, I.C.; van Dam, L.; Vink, A.; Vos, J.A.; Schonewille, W.J.; de Bruin, P.C.; Mali, W.P.; Velthuis, B.K. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE 2014, 9, e88882. [Google Scholar] [CrossRef]
- Sato, Y.; Ishibashi-Ueda, H.; Iwakiri, T.; Ikeda, Y.; Matsuyama, T.; Hatakeyama, K.; Asada, Y. Thrombus components in cardioembolic and atherothrombotic strokes. Thromb. Res. 2012, 130, 278–280. [Google Scholar] [CrossRef]
- Bacigaluppi, M.; Semerano, A.; Gullotta, G.S.; Strambo, D. Insights from thrombi retrieved in stroke due to large vessel occlusion. J. Cereb. Blood Flow Metab. 2019, 39, 1433–1451. [Google Scholar] [CrossRef]
- Schuhmann, M.K.; Gunreben, I.; Kleinschnitz, C.; Kraft, P. Immunohistochemical Analysis of Cerebral Thrombi Retrieved by Mechanical Thrombectomy from Patients with Acute Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 298. [Google Scholar] [CrossRef]
- Stoll, G.; Nieswandt, B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat. Rev. Neurol. 2019, 15, 473–481. [Google Scholar] [CrossRef]
- Laridan, E.; Martinod, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Laridan, E.; Denorme, F.; Desender, L.; Francois, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 2017, 82, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ducroux, C.; Di Meglio, L.; Loyau, S.; Delbosc, S.; Boisseau, W.; Deschildre, C.; Ben Maacha, M.; Blanc, R.; Redjem, H.; Ciccio, G.; et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke 2018, 49, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Staessens, S.; De Meyer, S.F. Thrombus heterogeneity in ischemic stroke. Platelets 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Pedraza, S.; Demchuk, A.; Daunis, I.E.J.; Termes, H.; Blasco, G.; Soria, G.; Boada, I.; Remollo, S.; Banos, J.; et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. AJNR Am. J. Neuroradiol. 2012, 33, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Staessens, S.; Denorme, F.; Francois, O.; Desender, L.; Dewaele, T.; Vanacker, P.; Deckmyn, H.; Vanhoorelbeke, K.; Andersson, T.; De Meyer, S.F. Structural analysis of ischemic stroke thrombi: Histological indications for therapy resistance. Haematologica 2020, 105, 498–507. [Google Scholar] [CrossRef]
- Denorme, F.; Langhauser, F.; Desender, L.; Vandenbulcke, A.; Rottensteiner, H.; Plaimauer, B.; Francois, O.; Andersson, T.; Deckmyn, H.; Scheiflinger, F.; et al. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood 2016, 127, 2337–2345. [Google Scholar] [CrossRef]
- Longstaff, C.; Varju, I.; Sotonyi, P.; Szabo, L.; Krumrey, M.; Hoell, A.; Bota, A.; Varga, Z.; Komorowicz, E.; Kolev, K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 2013, 288, 6946–6956. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef]
- Novotny, J.; Oberdieck, P.; Titova, A.; Pelisek, J.; Chandraratne, S.; Nicol, P.; Hapfelmeier, A.; Joner, M.; Maegdefessel, L.; Poppert, H.; et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 2020, 94, e2346–e2360. [Google Scholar] [CrossRef]
- Sporns, P.B.; Hanning, U.; Schwindt, W.; Velasco, A.; Minnerup, J.; Zoubi, T.; Heindel, W.; Jeibmann, A.; Niederstadt, T.U. Ischemic Stroke: What Does the Histological Composition Tell Us About the Origin of the Thrombus? Stroke 2017, 48, 2206–2210. [Google Scholar] [CrossRef]
- Boeckh-Behrens, T.; Kleine, J.F.; Zimmer, C.; Neff, F.; Scheipl, F.; Pelisek, J.; Schirmer, L.; Nguyen, K.; Karatas, D.; Poppert, H. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke 2016, 47, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Park, G.H.; Lee, J.S.; Lee, S.E.; Lee, S.J.; Kim, J.H.; Hong, J.M. Erythrocyte Fraction Within Retrieved Thrombi Contributes to Thrombolytic Response in Acute Ischemic Stroke. Stroke 2018, 49, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Jeong, H.S.; Kwon, H.J.; Song, K.S.; Kim, J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS ONE 2018, 13, e0197492. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, K.; Shibata, M.; Nakajima, H.; Mizutani, A.; Kitano, Y.; Seguchi, M.; Yamasaki, M.; Kobayashi, K.; Sano, T.; Mori, G.; et al. Erythrocyte-Rich Thrombus Is Associated with Reduced Number of Maneuvers and Procedure Time in Patients with Acute Ischemic Stroke Undergoing Mechanical Thrombectomy. Cerebrovasc. Dis. Extra 2018, 8, 39–49. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Value |
|---|---|
| Age, year, median (IQR) | 63 (56–77) |
| Gender, No. (%) | |
| Male | 18 (48.6) |
| Female | 19 (51.4) |
| Stroke etiology, No. (%) | |
| Cardioembolic | 21 (56.8) |
| CE-no AC | 10 (27.0) |
| CE-AC | 6 (16.2) |
| CE-PFO | 5 (13.5) |
| non-cardioembolic | 7 (18.9) |
| cryptogenic | 9 (24.3) |
| IV rt-PA, No. (%) | |
| Yes | 26 (70.3) |
| No | 11 (29.7) |
| NIHSS, median (IQR) | |
| Admission | 17 (13–22) |
| Dismissal | 8 (4–10) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essig, F.; Kollikowski, A.M.; Pham, M.; Solymosi, L.; Stoll, G.; Haeusler, K.G.; Kraft, P.; Schuhmann, M.K. Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7387. https://doi.org/10.3390/ijms21197387
Essig F, Kollikowski AM, Pham M, Solymosi L, Stoll G, Haeusler KG, Kraft P, Schuhmann MK. Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke. International Journal of Molecular Sciences. 2020; 21(19):7387. https://doi.org/10.3390/ijms21197387
Chicago/Turabian StyleEssig, Fabian, Alexander M. Kollikowski, Mirko Pham, László Solymosi, Guido Stoll, Karl Georg Haeusler, Peter Kraft, and Michael K. Schuhmann. 2020. "Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke" International Journal of Molecular Sciences 21, no. 19: 7387. https://doi.org/10.3390/ijms21197387
APA StyleEssig, F., Kollikowski, A. M., Pham, M., Solymosi, L., Stoll, G., Haeusler, K. G., Kraft, P., & Schuhmann, M. K. (2020). Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke. International Journal of Molecular Sciences, 21(19), 7387. https://doi.org/10.3390/ijms21197387




