Cancer Nano-Immunotherapy from the Injection to the Target: The Role of Protein Corona
Abstract
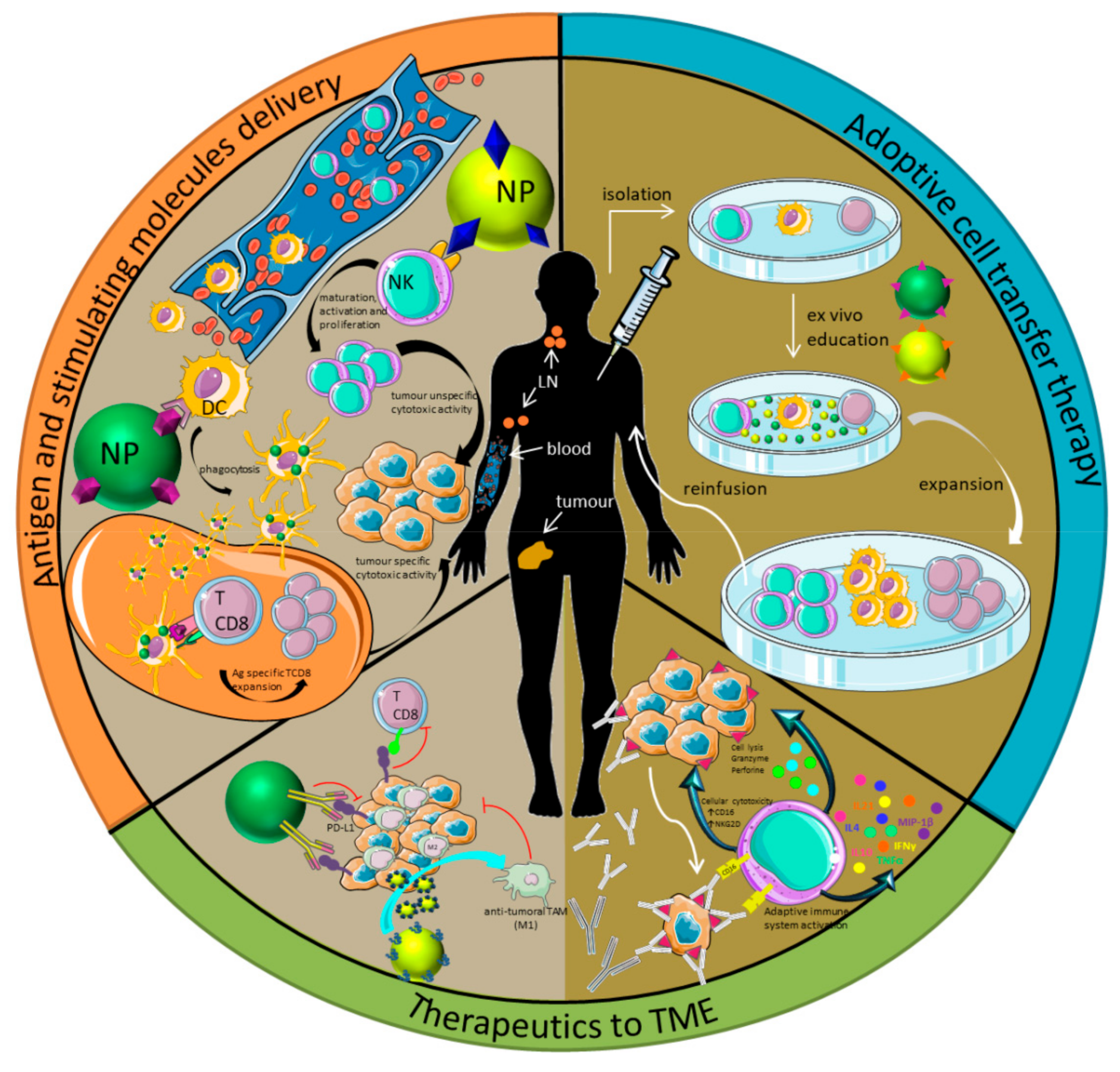
1. Introduction
2. Formation of Protein Corona: Effect on NP-Based Immunotherapy
2.1. Immune-Blinding as a Consequence of PC Formation
2.2. Immune-Response or Immune-Reactivity as a Consequence of PC Formation
3. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Couzin-Frankel, J. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H. Introduction to checkpoint inhibitors and cancer immunotherapy. Immunol. Rev. 2017, 276, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Maeng, H.; Terabe, M.; Berzofsky, J.A. Cancer vaccines: Translation from mice to human clinical trials. Curr. Opin. Immunol. 2018, 51, 111–122. [Google Scholar] [CrossRef]
- Vilgelm, A.E.; Johnson, D.B.; Richmond, A. Combinatorial approach to cancer immunotherapy: Strength in numbers. J. Leukoc. Biol. 2016, 100, 275–290. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Baker, J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef]
- Grabowski, N.; Hillaireau, H.; Vergnaud, J.; Tsapis, N.; Pallardy, M.; Kerdine-Römer, S.; Fattal, E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int. J. Pharm. 2015, 482, 75–83. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, 1–34. [Google Scholar] [CrossRef]
- Munro, N. Immunology and immunotherapy in critical care: An overview. AACN Adv. Crit. Care 2019, 30, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Blumberg, R.S. Correlation between early-life regulation of the immune system by microbiota and allergy development. J. Allergy Clin. Immunol. 2017, 139, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, L.J. Current and Newly Emerging Autoimmune Diseases. Vet. Clin. North Am. Small Anim. Pract. 2018, 48, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Ferro, K.; Peuß, R.; Yang, W.; Rosenstiel, P.; Schulenburg, H.; Kurtz, J. Experimental evolution of immunological specificity. Proc. Natl. Acad. Sci. USA 2019, 116, 20598–20604. [Google Scholar] [CrossRef]
- Natoli, G.; Ostuni, R. Adaptation and memory in immune responses. Nat. Immunol. 2019, 20, 783–792. [Google Scholar] [CrossRef]
- Wahren-Herlenius, M.; Dörner, T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013, 382, 819–831. [Google Scholar] [CrossRef]
- Moriyama, T. Diversity of food allergy. J. Nutr. Sci. Vitaminol. 2015, 61, S106–S108. [Google Scholar] [CrossRef]
- Vitallé, J.; Terrén, I.; Gamboa-Urquijo, L.; Orrantia, A.; Tarancón-Díez, L.; Genebat, M.; Ruiz-Mateos, E.; Leal, M.; García-Obregón, S.; Zenarruzabeitia, O.; et al. Altered expression of CD300a inhibitory receptor on CD4+ T cells from human immunodeficiency virus-1-infected patients: Association with disease progression markers. Front. Immunol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; Haanen, J.B.A.G.; Punt, C.J.A. Cancer immunotherapy-revisited. Nat. Rev. Drug Discov. 2011, 10, 591–600. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.-M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.-P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef]
- McAllister, S.S.; Weinberg, R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.R.; Schmid, M.C. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc. R. Soc. Med. 1909, 3, 1–48. [Google Scholar] [CrossRef]
- Mulder, A.; Willey, S.; Schappe, T.; Berrien-Elliott, M.M.; Schneider, S.E.; Oh, S.T.; Wagner, J.A.; Yu, L.; Claas, F.; Neal, C.C.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357. [Google Scholar]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hu, Q.; Bellotti, A.; Gu, Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017, 29, 1–24. [Google Scholar] [CrossRef]
- Sahakyan, N.; Haddad, A.; Richardson, S.; Forcha-Etieundem, V.; Christopher, L.; Alharbi, H.; Campbell, R. Personalized Nanoparticles for Cancer Therapy: A Call for Greater Precision. Anticancer. Agents Med. Chem. 2017, 17, 1033–1039. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Coussens, L.M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Lizotte, P.H.; Wen, A.M.; Sheen, M.R.; Fields, J.; Rojanasopondist, P.; Steinmetz, N.F.; Fiering, S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016, 11, 295–303. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Pfannenstiel, L.W.; Lam, S.S.K.; Emens, L.A.; Jaffee, E.M.; Armstrong, T.D. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell. Immunol. 2010, 263, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Graziani, S.R.; Vital, C.G.; Morikawa, A.T.; Van Eyll, B.M.; Fernandes Junior, H.J.; Kalil Filho, R.; Maranhão, R.C. Phase II study of paclitaxel associated with lipid core nanoparticles (LDE) as third-line treatment of patients with epithelial ovarian carcinoma. Med. Oncol. 2017, 34, 151. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Chacón, J.I.; Antón, A.; Plazaola, A.; García-Martínez, E.; Seguí, M.A.; Sánchez-Rovira, P.; Palacios, J.; Calvo, L.; Esteban, C.; et al. Neoadjuvant Therapy with Weekly Nanoparticle Albumin-Bound Paclitaxel for Luminal Early Breast Cancer Patients: Results from the NABRAX Study (GEICAM/2011-02), a Multicenter, Non-Randomized, Phase II Trial, with a Companion Biomarker Analysis. Oncologist 2017, 22, 1301–1308. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, J.; Huang, M.; An, J.; Bai, P.; Wu, L.; Zhang, R. A phase 2 study of nanoparticle albumin-bound paclitaxel plus nedaplatin for patients with advanced, recurrent, or metastatic cervical carcinoma. Cancer 2017, 123, 420–425. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef]
- Higano, C.S.; Armstrong, A.J.; Sartor, A.O.; Vogelzang, N.J.; Kantoff, P.W.; McLeod, D.G.; Pieczonka, C.M.; Penson, D.F.; Shore, N.D.; Vacirca, J.; et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer 2019, 152, 4172–4180. [Google Scholar] [CrossRef]
- Dufner, V.; Sayehli, C.M.; Chatterjee, M.; Hummel, H.D.; Gelbrich, G.; Bargou, R.C.; Goebeler, M.-E. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019, 3, 2491–2498. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.; Li, R.; Huang, X.; Liu, Q. Design of Outer Membrane Vesicles as Cancer Vaccines: A New Toolkit for Cancer Therapy. Cancers 2019, 11, 1314. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; Lorusso, P.M.; Burris, H.A.; Hart, L.L.; Low, S.C.; Parsons, D.M.; et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.A.; Dreicer, R.; Anderson, J.; Garcia, J.A.; Alva, A.; Hart, L.L.; Milowsky, M.I.; Posadas, E.M.; Ryan, C.J.; Graf, R.P.; et al. Safety and Efficacy of BIND-014, a Docetaxel Nanoparticle Targeting Prostate-Specific Membrane Antigen for Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Olofsson, H.; Stålberg, P.; Bergkvist, L.; Abdsaleh, S.; Wärnberg, F. Simplifying Logistics and Avoiding the Unnecessary in Patients With Breast Cancer Undergoing Sentinel Node Biopsy. A Prospective Feasibility Trial of the Preoperative Injection of Super Paramagnetic Iron Oxide Nanoparticles. Scand. J. Surg. 2018, 107, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Li, X.; Liang, X.; Luo, X. The influence of different long-circulating materials on the pharmacokinetics of liposomal vincristine sulfate. Int. J. Nanomedicine 2016, 11, 4187–4197. [Google Scholar]
- Wang, Z.; Zheng, Q.; Zhang, H.; Bronson, R.T.; Madsen, J.C.; Sachs, D.H.; Huang, C.A.; Wang, Z. Ontak-like human IL-2 fusion toxin. J. Immunol. Methods 2017, 448, 51–58. [Google Scholar] [CrossRef]
- Cobaleda-Siles, M.; Henriksen-Lacey, M.; De Angulo, A.R.; Bernecker, A.; Vallejo, V.G.; Szczupak, B.; Llop, J.; Pastor, G.; Plaza-Garcia, S.; Jauregui-Osoro, M.; et al. An iron oxide nanocarrier for dsRNA to target lymph nodes and strongly activate cells of the immune system. Small 2014, 10, 5054–5067. [Google Scholar] [CrossRef]
- Ruiz-De-Angulo, A.; Zabaleta, A.; Gómez-Vallejo, V.; Llop, J.; Mareque-Rivas, J.C. Microdosed lipid-coated 67Ga-magnetite enhances antigen-specific immunity by image tracked delivery of antigen and cpg to lymph nodes. ACS Nano 2016, 10, 1602–1618. [Google Scholar] [CrossRef]
- Houot, R.; Schultz, L.M.; Marabelle, A.; Kohrt, H. T-cell based immunotherapy: Adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 2015, 3, 1115–1122. [Google Scholar] [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Saibil, S.D.; Sotov, V.; Le, M.X.; Khoja, L.; Ghazarian, D.; Bonilla, L.; Majeed, H.; Hogg, D.; Joshua, A.M.; et al. Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2. Cancer Immunol. Immunother. 2019, 68, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Pusztai, L.; Pienkowski, T.; Im, Y.H.; Bianchi, G.V.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Galeota, E.; Magazzù, D.; et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann. Oncol. 2015, 26, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, X.; Ma, L.; Lin, A.; Gong, Y.; Yuan, G.; Liu, J. Core-shell structure QRu-PLGA-RES-DS nanocomposite with photothermal response induced M2 macrophage polarization for therapy rheumatoid arthritis. Nanoscale 2019, 11, 18209–18223. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Zhang, D.; Baldwin, P.; Leal, A.S.; Carapellucci, S.; Sridhar, S.; Liby, K.T. A nano-liposome formulation of the PARP inhibitor Talazoparib enhances treatment efficacy and modulates immune cell populations in mammary tumors of BRCA-deficient mice. Theranostics 2019, 9, 6224–6238. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Pombo, I.; Raposo, L.; Pedrosa, P.; Fernandes, A.R.; Baptista, P.V. Nanotheranostics Targeting the Tumor Microenvironment. Front. Bioeng. Biotechnol. 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Li, S.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Saikia, J.; Yazdimamaghani, M.; Hadipour Moghaddam, S.P.; Ghandehari, H. Differential Protein Adsorption and Cellular Uptake of Silica Nanoparticles Based on Size and Porosity. ACS Appl. Mater. Interfaces 2016, 8, 34820–34832. [Google Scholar] [CrossRef] [PubMed]
- Glancy, D.; Zhang, Y.; Wu, J.L.Y.; Ouyang, B.; Ohta, S.; Chan, W.C.W. Characterizing the protein corona of sub-10 nm nanoparticles. J. Control. Release 2019, 304, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Franqui, L.S.; De Farias, M.A.; Portugal, R.V.; Costa, C.A.R.; Domingues, R.R.; Souza Filho, A.G.; Coluci, V.R.; Leme, A.F.P.; Martinez, D.S.T. Interaction of graphene oxide with cell culture medium: Evaluating the fetal bovine serum protein corona formation towards in vitro nanotoxicity assessment and nanobiointeractions. Mater. Sci. Eng. C 2019, 100, 363–377. [Google Scholar] [CrossRef]
- Hühn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Riveragil, P.; Montenegro, J.M.; Braeckmans, K.; Müllen, K.; et al. Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef]
- Lai, W.; Wang, Q.; Li, L.; Hu, Z.; Chen, J.; Fang, Q. Interaction of gold and silver nanoparticles with human plasma: Analysis of protein corona reveals specific binding patterns. Colloids Surfaces B Biointerfaces 2017, 152, 317–325. [Google Scholar] [CrossRef]
- Giulimondi, F.; Digiacomo, L.; Pozzi, D.; Palchetti, S.; Vulpis, E.; Capriotti, A.L.; Chiozzi, R.Z.; Laganà, A.; Amenitsch, H.; Masuelli, L.; et al. Interplay of protein corona and immune cells controls blood residency of liposomes. Nat. Commun. 2019, 10, 3686. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.U.; Langer, K. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef]
- Aires, A.; Cabrera, D.; Alonso-Pardo, L.C.; Cortajarena, A.L.; Teran, F.J. Elucidation of the physicochemical properties ruling the colloidal stability of iron oxide nanoparticles under physiological conditions. ChemNanoMat 2017, 3, 183–189. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Fromell, K.; Mohlin, C.; Teramura, Y.; Nilsson, B. A human whole-blood model to study the activation of innate immunity system triggered by nanoparticles as a demonstrator for toxicity. Sci. Technol. Adv. Mater. 2019, 20, 688–698. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.; Li, P.Y.; Deng, J.; Arhontoulis, D.C.; Li, C.Y.; Bowne, W.B.; Cheng, H. Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles from Uptake by Liver Endothelial Cells for Long Blood Circulation. ACS Nano 2018, 12, 10130–10141. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulou, M.; Simon, J.; Landfester, K.; Mailänder, V.; Lieberwirth, I. Visualization of the protein corona: Towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale 2017, 9, 8858–8870. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moro, M.; Di Silvio, D.; Moya, S.E. Fluorescence correlation spectroscopy as a tool for the study of the intracellular dynamics and biological fate of protein corona. Biophys. Chem. 2019, 253, 106218. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrion, C.; Carril, M.; Parak, W.J. Techniques for the experimental investigation of the protein corona. Curr. Opin. Biotechnol. 2017, 46, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Carril, M.; Padro, D.; Del Pino, P.; Carrillo-Carrion, C.; Gallego, M.; Parak, W.J. In situ detection of the protein corona in complex environments. Nat. Commun. 2017, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Lindman, S.; Lynch, I.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Systematic investigation of the thermodynamics of HSA adsorption to N-iso-propylacrylamide/N-tert-butylacrylamide copolymer nanoparticles. Effects of particle size and hydrophobicity. Nano Lett. 2007, 7, 914–920. [Google Scholar] [CrossRef]
- Baier, G.; Costa, C.; Zeller, A.; Baumann, D.; Sayer, C.; Araujo, P.H.H.; Mailänder, V.; Musyanovych, A.; Landfester, K. BSA Adsorption on Differently Charged Polystyrene Nanoparticles using Isothermal Titration Calorimetry and the Influence on Cellular Uptake. Macromol. Biosci. 2011, 11, 628–638. [Google Scholar] [CrossRef]
- Bousquet, Y.; Swart, P.J.; Schmitt-Colin, N.; Velge-Roussel, F.; Kuipers, M.E.; Meijer, D.K.; Breton, P. Molecular Mechanisms of the Adsorption of a Model Protein (Human Serum Albumin) on Poly(Methylidene Malonate 2.1.2) Nanoparticles.pdf. Pharm. Res. 1999, 16, 141–147. [Google Scholar] [CrossRef]
- Milani, S.; Baldelli Bombelli, F.; Pitek, A.S.; Dawson, K.A.; Rädler, J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: Soft and hard corona. ACS Nano 2012, 6, 2532–2541. [Google Scholar] [CrossRef]
- Jiang, X.; Weise, S.; Hafner, M.; Röcker, C.; Zhang, F.; Parak, W.J.; Nienhaus, G.U. Quantitative analysis of the protein corona on FePt nanoparticles formed by transferrin binding. J. R. Soc. Interface 2010, 7. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Mulac, D.; Humpf, H.U.; Langer, K. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Gossmann, R.; Fahrländer, E.; Hummel, M.; Mulac, D.; Brockmeyer, J.; Langer, K. Comparative examination of adsorption of serum proteins on HSA- and PLGA-based nanoparticles using SDS-PAGE and LC-MS. Eur. J. Pharm. Biopharm. 2015, 93, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kah, J.C.Y.; Wong, K.Y.; Neoh, K.G.; Song, J.H.; Fu, J.W.P.; Mhaisalkar, S.; Olivo, M.; Sheppard, C.J.R. Critical parameters in the pegylation of gold nanoshells for biomedical applications: An in vitro macrophage study. J. Drug Target. 2009, 17, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Shanehsazzadeh, S.; Gruettner, C.; Lahooti, A.; Mahmoudi, M.; Allen, B.J.; Ghavami, M.; Daha, F.J.; Oghabian, M.A. Monoclonal antibody conjugated magnetic nanoparticles could target MUC-1-positive cells in vitro but not in vivo. Contrast Media Mol. Imaging 2015, 10, 225–236. [Google Scholar] [CrossRef]
- Wu, X.; McKay, C.; Pett, C.; Yu, J.; Schorlemer, M.; Ramadan, S.; Lang, S.; Behren, S.; Westerlind, U.; Finn, M.G.; et al. Synthesis and Immunological Evaluation of Disaccharide Bearing MUC-1 Glycopeptide Conjugates with Virus-Like Particles. ACS Chem. Biol. 2019, 14, 2176–2184. [Google Scholar] [CrossRef]
- Bargheer, D.; Nielsen, J.; Gébel, G.; Heine, M.; Salmen, S.C.; Stauber, R.; Weller, H.; Heeren, J.; Nielsen, P. The fate of a designed protein corona on nanoparticles in vitro and in vivo. Beilstein J. Nanotechnol. 2015, 6, 36–46. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Riviere, J.E.; Choi, K. Biocorona formation on gold nanoparticles modulates human proximal tubule kidney cell uptake, cytotoxicity and gene expression. Toxicol. Vitr. 2017, 42, 150–160. [Google Scholar]
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle-cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles. J. Phys. Chem. B 2014, 118, 14017–14026. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, G.M.; Butcher, N.J.; Musumeci, A.W.; Deng, Z.J.; Martin, D.J.; Minchin, R.F. Cryptic epitopes of albumin determine mononuclear phagocyte system clearance of nanomaterials. ACS Nano 2014, 8, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Wilhelm, C.; Servais, J.; Factor, C.; Dencausse, A.; Bacri, J.C.; Luciani, N.; Gazeau, F. Nanomagnetic sensing of blood plasma protein interactions with iron oxide nanoparticles: Impact on macrophage uptake. ACS Nano 2012, 6, 2665–2678. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef]
- Dai, Q.; Guo, J.; Yan, Y.; Ang, C.S.; Bertleff-Zieschang, N.; Caruso, F. Cell-Conditioned Protein Coronas on Engineered Particles Influence Immune Responses. Biomacromolecules 2017, 18, 431–439. [Google Scholar] [CrossRef]
- Escamilla-Rivera, V.; Uribe-Ramírez, M.; González-Pozos, S.; Lozano, O.; Lucas, S.; De Vizcaya-Ruiz, A. Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages. Toxicol. Lett. 2016, 240, 172–184. [Google Scholar] [CrossRef]
- Visalakshan, R.M.; MacGregor, M.N.; Sasidharan, S.; Ghazaryan, A.; Mierczynska-Vasilev, A.M.; Morsbach, S.; Mailänder, V.; Landfester, K.; Hayball, J.D.; Vasilev, K. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses. ACS Appl. Mater. Interfaces 2019, 11, 27615–27623. [Google Scholar] [CrossRef]
- Wang, G.; Griffin, J.I.; Inturi, S.; Brenneman, B.; Banda, N.K.; Michael Holers, V.; Moghimi, S.M.; Simberg, D. In vitro and in vivo differences in murine third complement component (C3) opsonization and macrophage/leukocyte responses to antibody-functionalized iron oxide nanoworms. Front. Immunol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Chen, F.; Wang, G.; Griffin, J.I.; Brenneman, B.; Banda, N.K.; Holers, V.M.; Backos, D.S.; Wu, L.; Moghimi, S.M.; Simberg, D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat. Nanotechnol. 2017, 12, 387–393. [Google Scholar] [CrossRef]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Jackson, M.A.; Werfel, T.A.; Curvino, E.J.; Yu, F.; Kavanaugh, T.E.; Sarett, S.M.; Dockery, M.D.; Kilchrist, K.V.; Jackson, A.N.; Giorgio, T.D.; et al. Zwitterionic Nanocarrier Surface Chemistry Improves siRNA Tumor Delivery and Silencing Activity Relative to Polyethylene Glycol. ACS Nano 2017, 11, 5680–5696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Q.; Qu, H.; Sfyroera, G.; Tzekou, A.; Kay, B.K.; Nilsson, B.; Nilsson Ekdahl, K.; Ricklin, D.; Lambris, J.D. Protection of Nonself Surfaces from Complement Attack by Factor H-Binding Peptides: Implications for Therapeutic Medicine. J. Immunol. 2011, 186, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Li, P.Y.; Fan, Z.; Cheng, H. Cell Membrane Bioconjugation and Membrane-Derived Nanomaterials for Immunotherapy. Bioconjug. Chem. 2018, 29, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, P.Y.; Deng, J.; Bady, S.C.; Cheng, H. Cell membrane coating for reducing nanoparticle-induced inflammatory responses to scaffold constructs. Nano Res. 2018, 11, 5573–5583. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Borgognoni, C.; Kim, J.H.; Zucolotto, V.; Fuchs, H.; Riehemann, K. Human macrophage responses to metal-oxide nanoparticles: A review. Artif. Cells, Nanomedicine Biotechnol. 2018, 46, 694–703. [Google Scholar] [CrossRef]
- Lundqvist, M.; Augustsson, C.; Lilja, M.; Lundkvist, K.; Dahlbäck, B.; Linse, S.; Cedervall, T. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Bros, M.; Nuhn, L.; Simon, J.; Moll, L.; Mailänder, V.; Landfester, K.; Grabbe, S. The protein corona as a confounding variable of nanoparticle-mediated targeted vaccine delivery. Front. Immunol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Tabatabaei, M.; Farokhzad, O.C.; Mahmoudi, M. Personalized protein corona on nanoparticles and its clinical implications. Biomater. Sci. 2017, 5, 378–387. [Google Scholar] [CrossRef]
- Lazarovits, J.; Chen, Y.Y.; Song, F.; Ngo, W.; Tavares, A.J.; Zhang, Y.N.; Audet, J.; Tang, B.; Lin, Q.; Cruz Tleugabulova, M.; et al. Synthesis of Patient-Specific Nanomaterials. Nano Lett. 2019, 19, 116–123. [Google Scholar] [CrossRef]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Cai, R.; Wang, J.; Daniyal, M.; Baimanov, D.; Liu, Y.; Yin, D.; Liu, Y.; Miao, Q.; Zhao, Y.; et al. Precision Nanomedicine Development Based on Specific Opsonization of Human Cancer Patient-Personalized Protein Coronas. Nano Lett. 2019, 19, 4692–4701. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef]
- Chan, J.K.; Horwood, N.; Nanchahal, J.; Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; et al. Alarmins: Awaiting a clinical response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef]
- Curtin, J.F.; Liu, N.; Candolfi, M.; Xiong, W.; Assi, H.; Yagiz, K.; Edwards, M.R.; Michelsen, K.S.; Kroeger, K.M.; Liu, C.; et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009, 6, e10. [Google Scholar] [CrossRef]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; Mckinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy HHS Public Access Author manuscript. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef]
- Wan, S.; Kelly, P.M.; Mahon, E.; Stöckmann, H.; Rudd, P.M.; Caruso, F.; Dawson, K.A.; Yan, Y.; Monopoli, M.P. The “sweet” Side of the protein corona: Effects of glycosylation on nanoparticle-cell interactions. ACS Nano 2015, 9, 2157–2166. [Google Scholar] [CrossRef]



| Compound Name | Formulation Description | Chemotherapy | Immunotherapy Type | Route of Immunization | Clinical Trials | Approved by the FDA | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Immunotherapy | Ferumoxytol (Ferahem®) | IONP | No | Active | TME→ M2-like macrophages to M1-like | Yes, for anemia and kidney diseases | [29] | |
| eCPMV | VLP of cowpea mosaic virus | No | Active | Neutrophil activation in the TME | - | - | [30] | |
| RNA-LPX (Lipoplex®) | RNA-loaded liposomes | No | Active | DC maturation, Tcell response, inflammatory response | Phase I (2016) | [31] | ||
| PTX-LDE | Paclitaxel-loaded lipid core NPs | Yes | Active | DC maturation [32] | Phase II (2017) | [33] | ||
| MRX34 | miRNA-34a-loaded liposome | No | Passive | Downregulation of immune evasion tumor genes | Phase I (2016) | [34] | ||
| nab-Paclitaxel (Abraxane®) | Paclitaxel-loaded albumin NPs | Yes | Not applied right now | DC maturation | Phase III (2017) | Yes, for cancer treatment | [35,36] | |
| aCD47@CaCO3 | Anti CD47-loaded CaCO3 NP in fibrinogen solution | No | Active | After surgery, and with the addition of thrombin, aCD47@CaCO3 forms a immunotherapeutic gel in situ in the TME | - | - | [37] | |
| Sipuleucel-T (Provenge®) | ex vivo DCs | No | Active | Vaccine | Yes, for prostate cancer | [38] | ||
| Blinatumomab (Blincyto®) | Bi-specific T cell engager (BiTE). Specific to CD19 and CD3. | No | Passive | BiTE targeting CD19 (malignant B cell) and CD3 (T cell) and cytotoxicity effect against B cells. | Yes, for Philadelphia negative Acute lymphocytic leukaemia (ALL) | [39] | ||
| Talimogene Laherparepvec (T-VEC) | Injectable modified herpes virus | No | Active | Vaccine | Yes, for advanced melanoma | [40] | ||
| No immunotherapy | BIND-014 | Docetaxel-loaded Poly-Lactic Acid (PLA) NP and Prostate-Specific Membrane Antigen (PSMA) in the surface | Yes | No | - | Phase II(2018) | No | [41,42] |
| SPIO | Super paramagnetic iron oxide NPs | No. Only for imaging | - | - | Yes for imaging | [43] | ||
| Doxil® | Dox-loaded liposome | Yes | No | - | - | Yes | [44] | |
| Marqibo | Vincristine-loaded liposome | Yes | No | - | - | Yes, for Ph negative ALL | [45] | |
| Ontak® | Protein NPs | Yes | No | - | - | Yes, for cutaneous T cell lymphoma | [46] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikelez-Alonso, I.; Aires, A.; Cortajarena, A.L. Cancer Nano-Immunotherapy from the Injection to the Target: The Role of Protein Corona. Int. J. Mol. Sci. 2020, 21, 519. https://doi.org/10.3390/ijms21020519
Mikelez-Alonso I, Aires A, Cortajarena AL. Cancer Nano-Immunotherapy from the Injection to the Target: The Role of Protein Corona. International Journal of Molecular Sciences. 2020; 21(2):519. https://doi.org/10.3390/ijms21020519
Chicago/Turabian StyleMikelez-Alonso, Idoia, Antonio Aires, and Aitziber L. Cortajarena. 2020. "Cancer Nano-Immunotherapy from the Injection to the Target: The Role of Protein Corona" International Journal of Molecular Sciences 21, no. 2: 519. https://doi.org/10.3390/ijms21020519
APA StyleMikelez-Alonso, I., Aires, A., & Cortajarena, A. L. (2020). Cancer Nano-Immunotherapy from the Injection to the Target: The Role of Protein Corona. International Journal of Molecular Sciences, 21(2), 519. https://doi.org/10.3390/ijms21020519





