Specific Heat and Transport Functions of Water
Abstract
1. Introduction
2. Methods, Results and Discussion
2.1. Methods
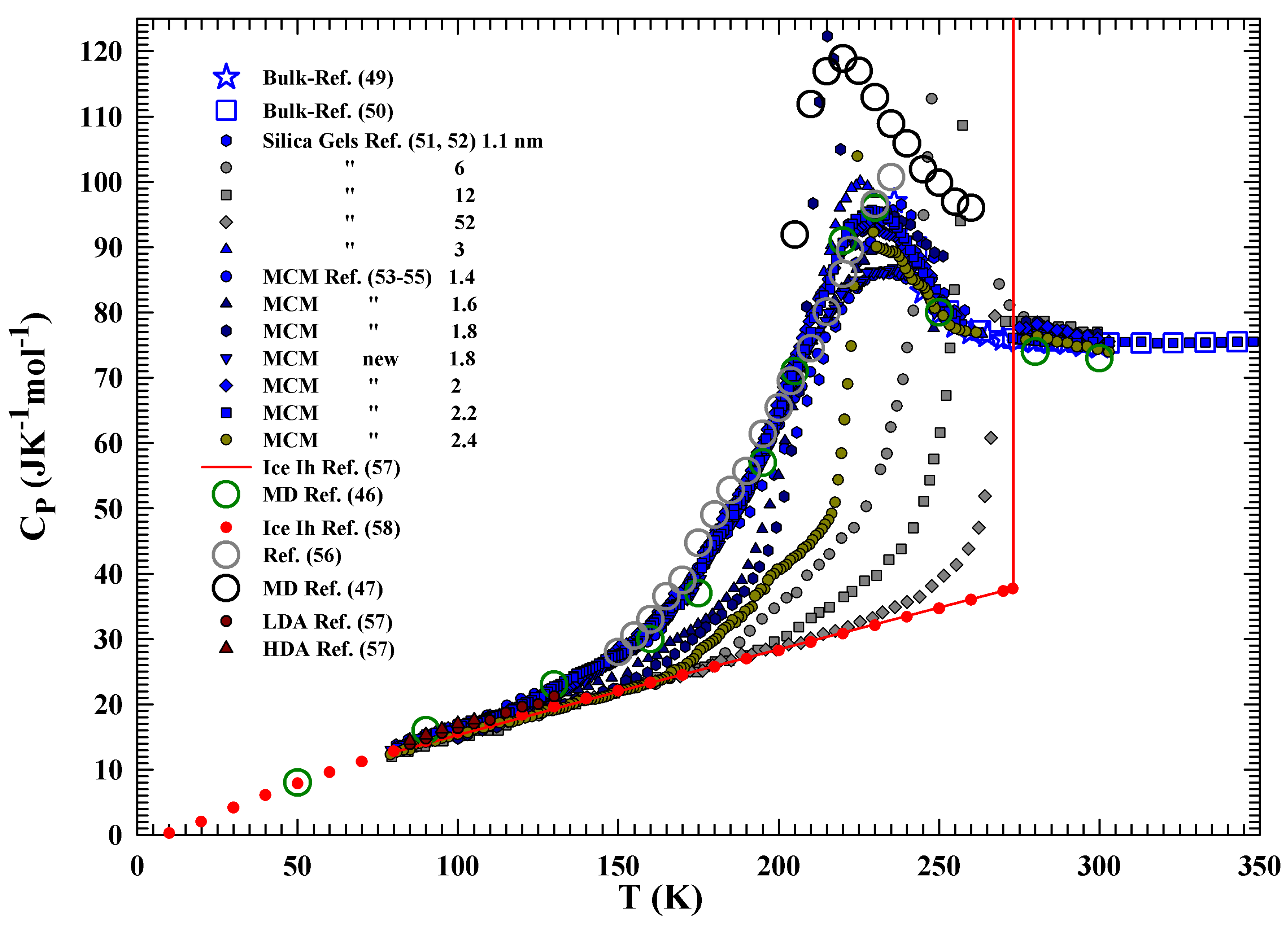
2.2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | Adam–Gibbs |
| HB | Hydrogen Bond |
| LDL | Low Density Liquid |
| HDL | High Density Liquid |
References
- Ball, P. Life’s Matrix: A Biography of Water; University of California Press: Berkeley, CA, USA, 2001. [Google Scholar]
- Galilei, G. Intorno Alle Cose, Che Stanno in sù L’acqua, ò Che in Quella si Muovono. Discorso al Serenissimo Don Cosimo II Gran Duca di Toscana; Cosimo Giunti: Florence, Italy, 1612. [Google Scholar]
- Magalotti, L. Saggi di Naturali Esperienze Fatte Nell’Accademia del Cimento Sotto la Protezione del Serenissimo Principe Leopoldo di Toscana e Descritte dal Segretario di essa Accademia; Vol. Esperienze Intorno agli Artificiali Agghiacciamenti; Accademia del Cimento: Florence, Italy, 1667; pp. 127–176. [Google Scholar]
- Debenedetti, P.G.; Stanley, H.E. Supercooled and Glassy Water. Phys. Today 2003, 56, 40–46. [Google Scholar] [CrossRef]
- Speedy, R.J.; Angell, C.A. Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at −45 °C. J. Chem. Phys. 1976, 65, 851–858. [Google Scholar] [CrossRef]
- Rapoport, E. Model for Melting-Curve Maxima at High Pressure. J. Chem. Phys. 1967, 46, 2891–2895. [Google Scholar] [CrossRef]
- Némethy, G.; Scheraga, H.A. Structure of Water and Hydrophobic Bonding in Proteins. I. A Model for the Thermodynamic Properties of Liquid Water. J. Chem. Phys. 1962, 36, 3382–3400. [Google Scholar] [CrossRef]
- Davis, C.M.; Litovitz, T.A. Two-State Theory of the Structure of Water. J. Chem. Phys. 1965, 42, 2563–2576. [Google Scholar] [CrossRef]
- Jhon, M.S.; Grosh, J.; Ree, T.; Eyring, H. Significant-Structure Theory Applied to Water and Heavy Water. J. Chem. Phys. 1966, 44, 1465–1472. [Google Scholar] [CrossRef]
- Kamb, B. Structure of Ice VI. Science 1965, 150, 205–209. [Google Scholar] [CrossRef]
- Palmer, J.; Martelli, F.; Liu, Y.; Car, R.; Panagiotopoulos, A.; Debenedetti, P. Metastable liquid–liquid transition in a molecular model of water. Nature 2014, 510, 385–388. [Google Scholar] [CrossRef]
- Pettersson, L.; Henchman, R.; Nilsson, A. Special issue on: Water—The Most Anomalous Liquid. Chem. Rev. 2016, 116, 7459–7462. [Google Scholar] [CrossRef]
- Palmer, J.; Poole, P.H.; Sciortino, F.; Debenedetti, P. Advances in Computational Studies of the Liquid-Liquid Transition in Water and Water-Like Models. Chem. Rev. 2018, 118, 9129–9151. [Google Scholar] [CrossRef]
- Mishima, O.; Calvert, L.; Whalley, E. Melting ice I at 77 K and 10 kbar: A new method of making amorphous solids. Nature 1984, 310, 393–397. [Google Scholar] [CrossRef]
- Mishima, O.; Calvert, L.; Whalley, E. An apparently first-order transition between two amorphous phases of ice induced by pressure. Nature 1985, 314, 76–78. [Google Scholar] [CrossRef]
- Mishima, O. Relationship between melting and amorphization of ice. Nature 1996, 384, 76–78. [Google Scholar] [CrossRef]
- Burton, E.F.; Oliver, W.F.; McLennan, J.C. The crystal structure of ice at low temperatures. Proc. R. Soc. Lon. Ser. A Math. Phys. Sci. 1935, 153, 166–172. [Google Scholar] [CrossRef]
- Loerting, T.; Salzmann, C.; Kohl, I.; Mayer, E.; Hallbrucker, A. A second distinct structural “state” of high-density amorphous ice at 77 K and 1 bar. Phys. Chem. Chem. Phys. 2001, 3, 5355–5357. [Google Scholar] [CrossRef]
- Amann-Winkel, K.; Gainaru, C.; Handle, P.H.; Seidl, M.; Nelson, H.; Böhmer, R.; Loerting, T. Water’s second glass transition. Proc. Natl. Acad. Sci. USA 2013, 110, 17720–17725. [Google Scholar] [CrossRef]
- Amann-Winkel, K.; Böhmer, R.; Fujara, F.; Gainaru, C.; Geil, B.; Loerting, T. Colloquium: Water’s controversial glass transitions. Rev. Mod. Phys. 2016, 88, 011002. [Google Scholar] [CrossRef]
- Bruggeller, P.; Mayer, E. Complete vitrification in pure liquid water and dilute aqueous solutions. Nature 1980, 288, 569–571. [Google Scholar] [CrossRef]
- Poole, P.; Sciortino, F.; Essmann, U.; Stanley, H.E. Phase behaviour of metastable water. Nature 1992, 360, 324–328. [Google Scholar] [CrossRef]
- Santra, B.; Distasio, R.A., Jr.; Martelli, F.; Car, R. Local structure analysis in ab initio liquid water. Mol. Phys. 2015, 113, 2829–2841. [Google Scholar] [CrossRef]
- Sastry, S.; Debenedetti, P.G.; Sciortino, F.; Stanley, H.E. Singularity-free interpretation of the thermodynamics of supercooled water. Phys. Rev. E 1996, 53, 6144–6154. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kumar, P.; Buldyrev, S.V.; Chen, S.-H.; Poole, P.H.; Sciortino, F.; Stanley, H.E. Relation between the Widom line and the dynamic crossover in systems with a liquid–liquid phase transition. Proc. Natl. Acad. Sci. USA 2005, 102, 16558–16562. [Google Scholar] [CrossRef] [PubMed]
- Soper, A.K.; Ricci, M.A. Structures of High-Density and Low-Density Water. Phys. Rev. Lett. 2000, 84, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.H.; Carr, H.Y. Diffusion and Nuclear Spin Relaxation in Water. Phys. Rev. 1958, 111, 1201–1202. [Google Scholar] [CrossRef]
- Adam, G.; Gibbs, J.H. On the Temperature Dependence of Cooperative Relaxation Properties in Glass-Forming Liquids. J. Chem. Phys. 1965, 43, 139–146. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, C.; Stanley, H.E. The thermodynamical response functions and the origin of the anomalous behavior of liquid water. Faraday Discuss. 2013, 167, 95–108. [Google Scholar] [CrossRef]
- Chen, S.-H.; Mallamace, F.; Mou, C.Y.; Broccio, M.; Corsaro, C.; Faraone, A.; Liu, L. The violation of the Stokes–Einstein relation in supercooled water. Proc. Natl. Acad. Sci. USA 2006, 167, 12974–12978. [Google Scholar] [CrossRef]
- Mallamace, F.; Baglioni, P.; Corsaro, C.; Spooren, J.; Stanley, H.E.; Chen, S.-H. Transport properties of supercooled confined water. Rivista del Nuovo Cimento 2011, 34, 253–388. [Google Scholar] [CrossRef]
- Xu, Y.; Petrik, N.G.; Smith, R.S.; Kay, B.D.; Kimmel, G.A. Growth rate of crystalline ice and the diffusivity of supercooled water from 126 to 262 K. Proc. Natl. Acad. Sci. USA 2016, 113, 14921–14925. [Google Scholar] [CrossRef]
- Bridgman, P. Water, in the Liquid and Five Solid Forms, under Pressure. Proc. Am. Acad. Art. Sci. 1912, 47, 441–558. [Google Scholar] [CrossRef]
- Cerveny, S.; Mallamace, F.; Swenson, J.; Vogel, M.; Xu, L. Confined Water as Model of Supercooled Water. Chem. Rev. 2016, 116, 7608–7625. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Branca, C.; Broccio, M.; Corsaro, C.; Mou, C.Y.; Chen, S.-H. The anomalous behavior of the density of water in the range 30 K < T < 373 K. Proc. Natl. Acad. Sci. USA 2007, 104, 18387–18391. [Google Scholar] [CrossRef] [PubMed]
- Erko, M.; Wallacher, D.; Hoell, A.; Hauß, T.; Zizak, I.; Paris, O. Density minimum of confined water at low temperatures: A combined study by small-angle scattering of X-rays and neutrons. Phys. Chem. Chem. Phys. 2012, 14, 3852–3858. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Corsaro, C.; Stanley, H.E. Possible relation of water structural relaxation to water characteristics. Proc. Natl. Acad. Sci. USA 2013, 110, 4899–4904. [Google Scholar] [CrossRef]
- Kim, K.H.; Späh, A.; Pathak, H.; Perakis, F.; Mariedahl, D.; Amann-Winkel, K.; Sellberg, J.A.; Lee, J.H.; Kim, S.; Park, J.; et al. Maxima in the thermodynamic response and correlation functions of deeply supercooled water. Science 2017, 358, 1589–1593. [Google Scholar] [CrossRef]
- Soper, A.K. Radical re-appraisal of water structure in hydrophilic confinement. Chem. Phys. Lett. 2013, 590, 1–15. [Google Scholar] [CrossRef]
- Soper, A.K. Density profile of water confined in cylindrical pores in MCM-41 silica. J. Phys. Cond. Matters 2013, 24, 1–11. [Google Scholar] [CrossRef]
- Caupin, F.; Holten, V.; Qiu, C.; Guillerm, E.; Wilke, M.; Frenz, M.; Teixeira, J.; Soper, A.K. Comment on “Maxima in the thermodynamic response and correlation functions of deeply supercooled water”. Science 2018, 360. [Google Scholar] [CrossRef]
- Goy, C.; Potenza, M.A.C.; Dedera, S.; Tomut, M.; Guillerm, E.; Kalinin, A.; Voss, K.O.; Schottelius, A.; Petridis, N.; Prosvetov, A.; et al. Shrinking of Rapidly Evaporating Water Microdroplets Reveals their Extreme Supercooling. Phys. Rev. Lett. 2018, 120, 015501. [Google Scholar] [CrossRef]
- Kim, K.H.; Späh, A.; Pathak, H.; Perakis, F.; Mariedahl, D.; Amann-Winkel, K.; Sellberg, J.A.; Lee, J.H.; Kim, S.; Park, J.; et al. Response to Comment on “Maxima in the thermodynamic response and correlation functions of deeply supercooled water”. Science 2018, 360. [Google Scholar] [CrossRef]
- Pallares, G.; El Mekki Azouzi, M.; Gonzalez, M.A.; Aragones, J.L.; Abascal, J.L.F.; Valeriani, C.; Caupin, F. Anomalies in bulk supercooled water at negative pressure. Proc. Natl. Acad. Sci. USA 2014, 111, 7936–7979. [Google Scholar] [CrossRef] [PubMed]
- Starr, F.; Angell, C.A.; Stanley, H. Prediction of entropy and dynamic properties of water below the homogeneous nucleation temperature. Phys. A Stat. Mech. Appl. 2003, 323, 51–66. [Google Scholar] [CrossRef]
- Saito, S.; Bagchi, B. Thermodynamic picture of vitrification of water through complex specific heat and entropy: A journey through “no man’s land”. J. Chem. Phys. 2019, 150, 054502. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F. Unravelling the contribution of local structures to the characteristics of water: The synergistic action of several factors. J. Chem. Phys. 2019, 150, 094506. [Google Scholar] [CrossRef] [PubMed]
- Angell, C.A.; Sichina, W.J.; Oguni, M. Heat capacity of water at extremes of supercooling and superheating. J. Chem. Phys. 1982, 86, 998–1002. [Google Scholar] [CrossRef]
- Tombari, E.; Ferrari, C.; Salvetti, G. Heat capacity anomaly in a large sample of supercooled water. Chem. Phys. Lett. 1999, 300, 749–751. [Google Scholar] [CrossRef]
- Archer, D.G.; Carter, R.W. Thermodynamic Properties of the NaCl + H2O System. 4. Heat Capacities of H2O and NaCl(aq) in Cold-Stable and Supercooled States. J. Phys. Chem. B 2000, 104, 8563–8584. [Google Scholar] [CrossRef]
- Oguni, M.; Maruyama, S.; Wakabayashi, K.; Nagoe, A. Glass Transitions of Ordinary and Heavy Water within Silica-Gel Nanopores. Chem. Asian J. 2007, 2, 514–520. [Google Scholar] [CrossRef]
- Oguni, M.; Kanke, Y.; Namba, S. Thermal Properties of the Water Confined within Nanopores of Silica MCM-41. AIP Conf. Proc. 2008, 982, 34–38. [Google Scholar] [CrossRef]
- Nagoe, A.; Kanke, Y.; Oguni, M.; Namba, S. Findings of Cp Maximum at 233 K for the Water within Silica Nanopores and Very Weak Dependence of the Tmax on the Pore Size. J. Phys. Chem. B 2010, 114, 13940–13943. [Google Scholar] [CrossRef]
- Oguni, M.; Kanke, Y.; Nagoe, A.; Namba, S. Calorimetric Study of Water’s Glass Transition in Nanoscale Confinement, Suggesting a Value of 210 K for Bulk Water. J. Phys. Chem. B 2011, 115, 14023–14029. [Google Scholar] [CrossRef] [PubMed]
- Tombari, E.; Salvetti, G.; Johari, G.P. Specific Heat and Transformations of Water in 1.4 and 1.8 nm Pore-MCMs. J. Phys. Chem. C 2012, 116, 2702–2709. [Google Scholar] [CrossRef]
- Cupane, A.; Fomina, M.; Piazza, I.; Peters, J.; Schirò, G. Experimental Evidence for a Liquid-Liquid Crossover in Deeply Cooled Confined Water. Phys. Rev. Lett. 2014, 113, 215701. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.P.; Mishima, O.; Whalley, E. High-density amorphous ice. III. Thermal properties. J. Chem. Phys. 1986, 84, 2766–2770. [Google Scholar] [CrossRef]
- Wagner, W.; Riethmann, T.; Feistel, R.; Harvey, A. New Equations for the Sublimation Pressure and Melting Pressure of H2O Ice Ih. J. Phys. Chem. Ref. Data 2011, 40, 043103. [Google Scholar] [CrossRef]
- De Michele, V.; Levantino, M.; Cupane, A. Hysteresis in the temperature dependence of the IR bending vibration of deeply cooled confined water. J. Chem. Phys. 2019, 150, 224509. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Fazio, E.; Chen, S.-H. Some considerations on the water polymorphism and the liquid–liquid transition by the density behavior in the liquid phase. J. Chem. Phys. 2019, 151, 044504. [Google Scholar] [CrossRef]
- Stillinger, F.H. Supercooled liquids, glass transitions, and the Kauzmann paradox. J. Chem. Phys. 1988, 88, 7818–7825. [Google Scholar] [CrossRef]
- Mallamace, F.; Branca, C.; Corsaro, C.; Leone, N.; Spooren, J.; Chen, S.-H.; Stanley, H.E. Transport properties of glass-forming liquids suggest that dynamic crossover temperature is as important as the glass transition temperature. Proc. Natl. Acad. Sci. USA 2010, 107, 22457–22462. [Google Scholar] [CrossRef]
- Yip, S.; Short, M.P. Multiscale materials modelling at the mesoscale. Nat. Mater. 2013, 12, 774–777. [Google Scholar] [CrossRef]
- Gillen, K.T.; Douglass, D.C.; Hoch, M.J.R. Self-Diffusion in Liquid Water to −31 °C. J. Chem. Phys. 1972, 57, 5117–5119. [Google Scholar] [CrossRef]
- Holz, M.; Heil, S.R.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]
- Mills, R. Self-diffusion in normal and heavy water in the range 1-45.deg. J. Phys. Chem. 1973, 77, 685–688. [Google Scholar] [CrossRef]
- Price, W.S.; Ide, H.; Arata, Y. Self-Diffusion of Supercooled Water to 238 K Using PGSE NMR Diffusion Measurements. J. Phys. Chem. A 1999, 103, 448–450. [Google Scholar] [CrossRef]
- Prielmeier, F.X.; Lang, E.W.; Speedy, R.J.; Lüdemann, H.D. The Pressure Dependence of Self Diffusion in Supercooled Light and Heavy Water. Berichte der Bunsengesellschaft für Physikalische Chemie 1988, 92, 1111–1117. [Google Scholar] [CrossRef]
- Sjöström, J.; Swenson, J.; Bergman, R.; Kittaka, S. Investigating hydration dependence of dynamics of confined water: Monolayer, hydration water and Maxwell–Wagner processes. J. Chem. Phys. 2008, 128, 154503. [Google Scholar] [CrossRef]
- Ito, K.; Moynihan, C.; Angell, C.A. Thermodynamic determination of fragility in liquids and a fragile-to-strong liquid transition in water. Nature 1998, 398, 492–495. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Broccio, M.; Branca, C.; Gonzalez-Segredo, N.; Spooren, J.; Chen, S.-H.; Stanley, H.E. NMR evidence of a sharp change in a measure of local order in deeply supercooled confined water. Proc. Natl. Acad. Sci. USA 2008, 105, 12725–12729. [Google Scholar] [CrossRef]
- Mallamace, F.; Broccio, M.; Corsaro, C.; Faraone, A.; Majolino, D.; Venuti, V.; Liu, L.; Mou, C.Y.; Chen, S.-H. Evidence of the existence of the low-density liquid phase in supercooled, confined water. Proc. Natl. Acad. Sci. USA 2007, 104, 424–428. [Google Scholar] [CrossRef]
- Martelli, F.; Torquato, S.; Giovambattista, N.; Car, R. Large-Scale Structure and Hyperuniformity of Amorphous Ices. Phys. Rev. Lett. 2017, 119, 136002. [Google Scholar] [CrossRef]
- Stokely, K.; Mazza, M.G.; Stanley, H.E.; Franzese, G. Effect of hydrogen bond cooperativity on the behavior of water. Proc. Natl. Acad. Sci. USA 2010, 107, 1301–1306. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamace, F.; Corsaro, C.; Mallamace, D.; Fazio, E.; Chen, S.-H.; Cupane, A. Specific Heat and Transport Functions of Water. Int. J. Mol. Sci. 2020, 21, 622. https://doi.org/10.3390/ijms21020622
Mallamace F, Corsaro C, Mallamace D, Fazio E, Chen S-H, Cupane A. Specific Heat and Transport Functions of Water. International Journal of Molecular Sciences. 2020; 21(2):622. https://doi.org/10.3390/ijms21020622
Chicago/Turabian StyleMallamace, Francesco, Carmelo Corsaro, Domenico Mallamace, Enza Fazio, Sow-Hsin Chen, and Antonio Cupane. 2020. "Specific Heat and Transport Functions of Water" International Journal of Molecular Sciences 21, no. 2: 622. https://doi.org/10.3390/ijms21020622
APA StyleMallamace, F., Corsaro, C., Mallamace, D., Fazio, E., Chen, S.-H., & Cupane, A. (2020). Specific Heat and Transport Functions of Water. International Journal of Molecular Sciences, 21(2), 622. https://doi.org/10.3390/ijms21020622








