Inclusion of Soluble Fiber in the Gestation Diet Changes the Gut Microbiota, Affects Plasma Propionate and Odd-Chain Fatty Acids Levels, and Improves Insulin Sensitivity in Sows
Abstract
1. Introduction
2. Results
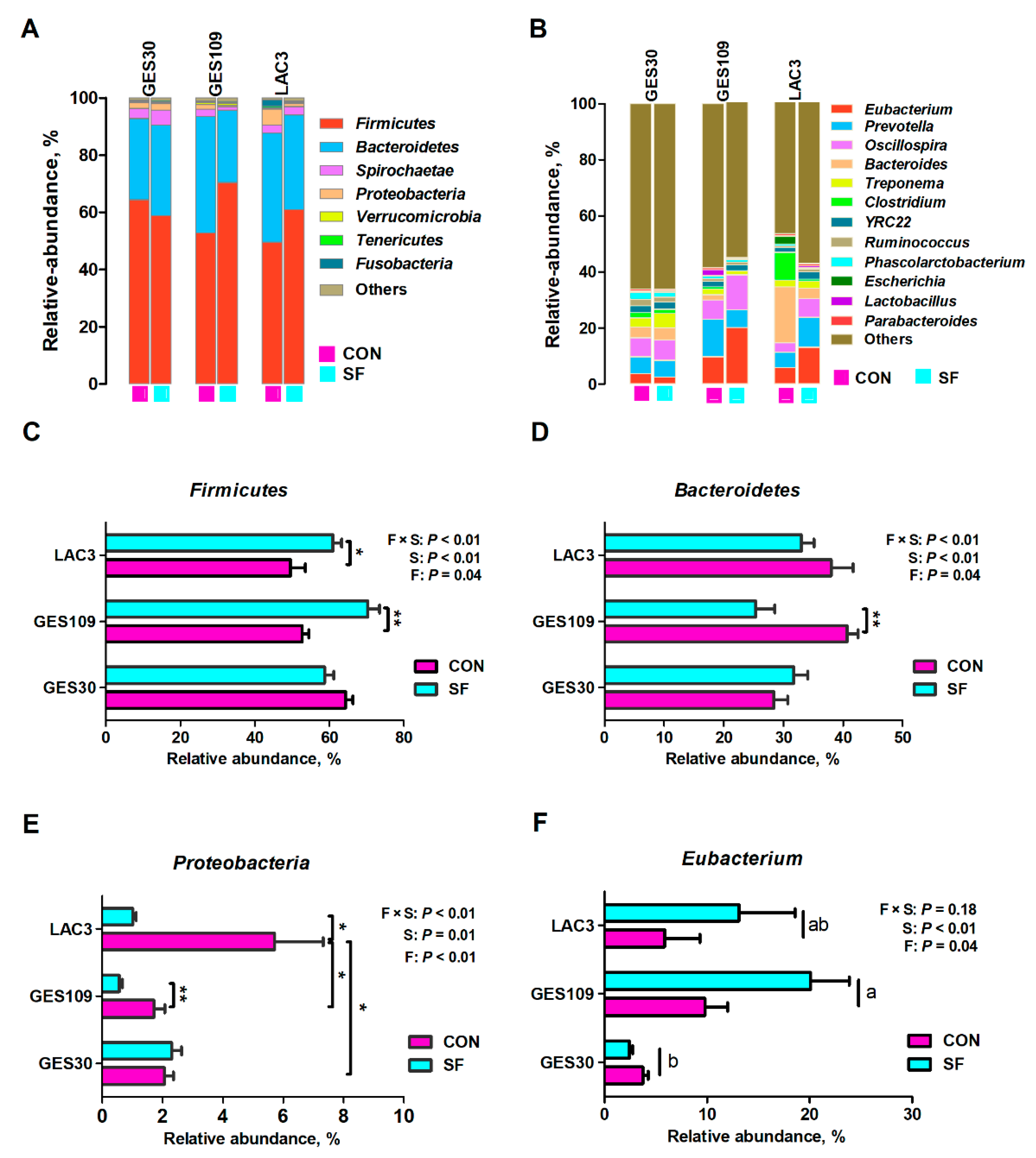
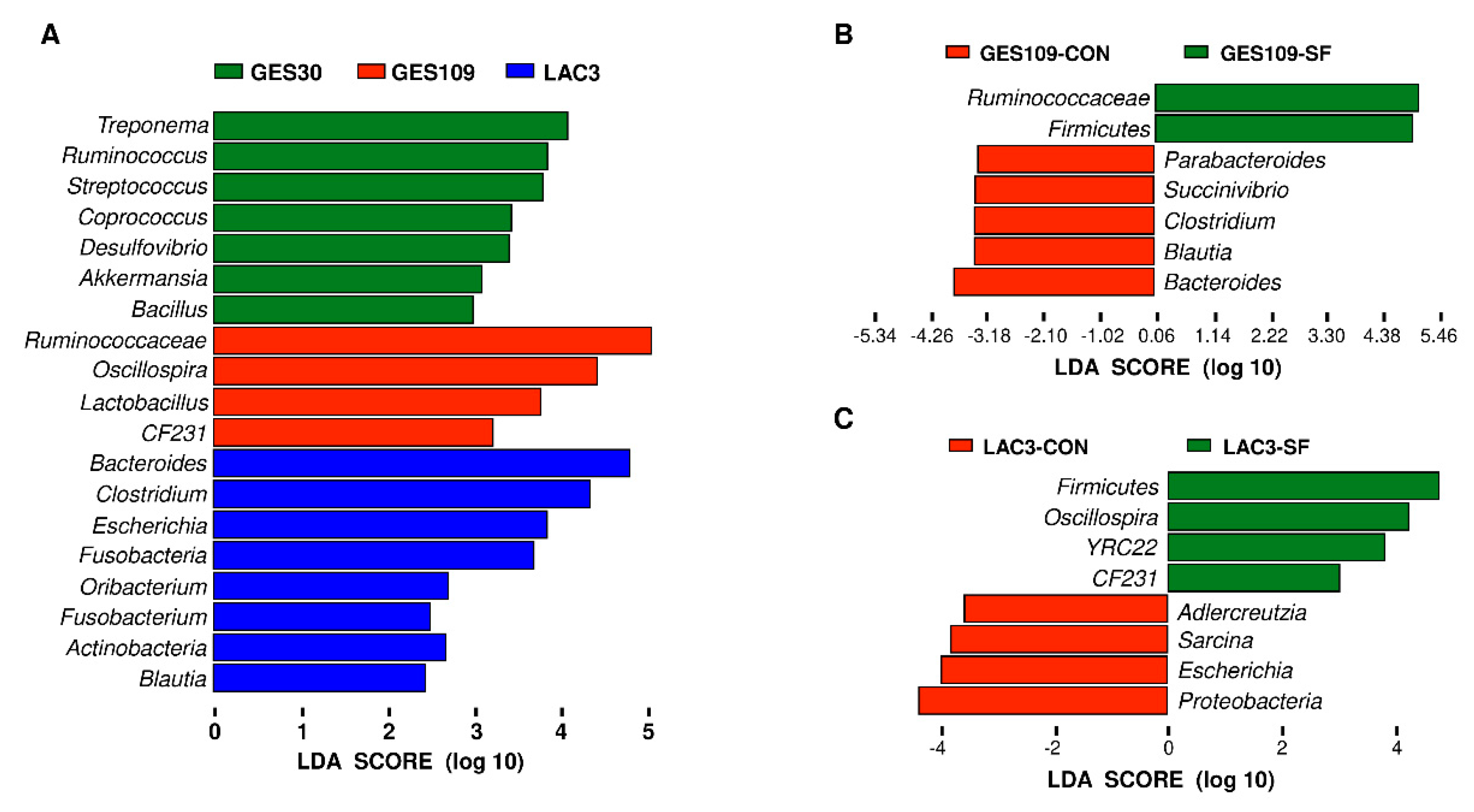
2.1. Dietary Soluble Fiber Changes the Gut Microbiota Diversity and Composition of Sows during Perinatal Period
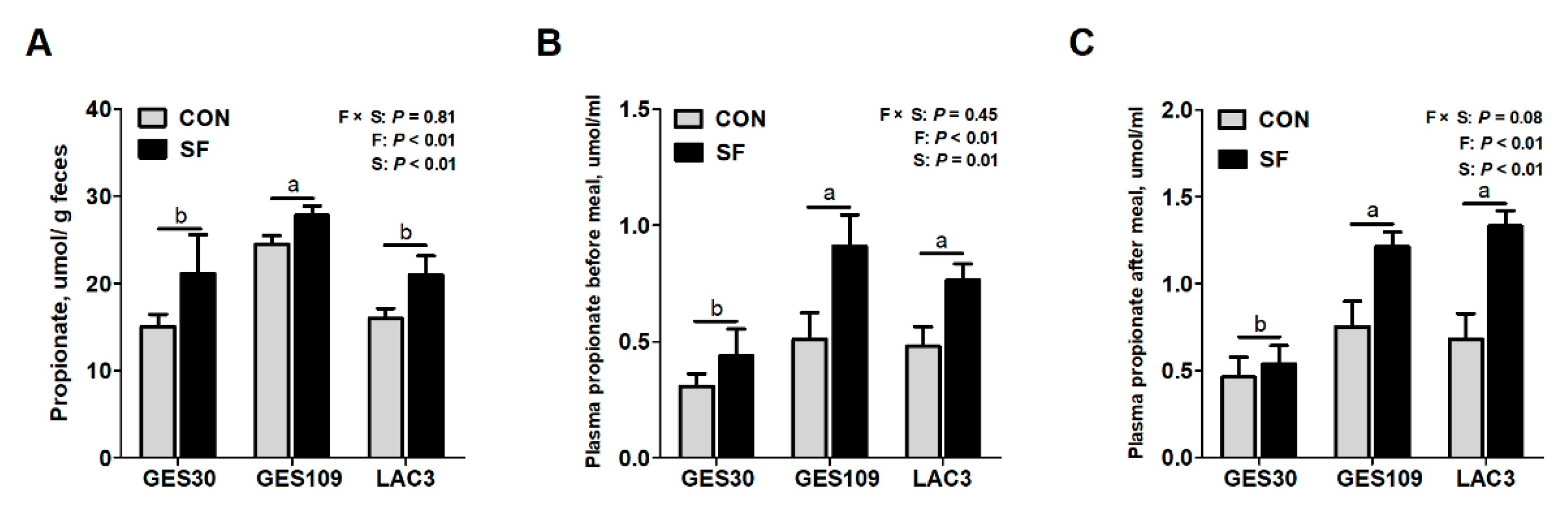
2.2. Dietary Soluble Fiber Increases the Intestinal Propionate Production and Plasma OCFAs Concentrations of Breeding Sows
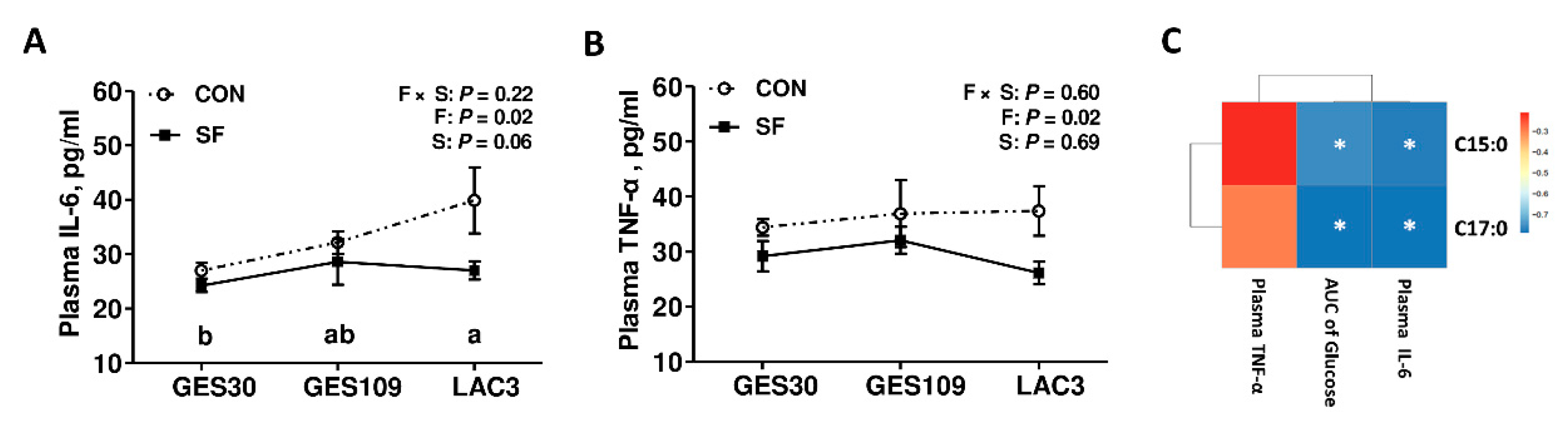
2.3. Dietary Soluble Fiber Ameliorates Insulin Desensitization and Systemic Low-Grade Inflammation in Sows at Perinatal Period
3. Discussion
4. Materials and Methods
4.1. Animals, Diets, Housing and Sample Collection
4.2. Surgical Procedure, Meal Test, Glucose Tolerance Test, and Sampling
4.3. Analysis of Fecal and Plasma Propionate
4.4. Analysis of Plasma Non-Esterified Fatty Acids
4.5. Plasma Glucose and Inflammatory Cytokine Analysis
4.6. Microbiota Analysis Based on 16s rRNA High-Throughput Sequencing
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nair, R.R.; Verma, P.; Singh, K. Immune-endocrine crosstalk during pregnancy. Gen. Comp. Endocrinol. 2017, 242, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Meng, N.; Liu, P.; Feng, Q.; Lin, S.; Fu, J.; Davidson, R.; Chen, X.; Rao, W.; Chen, F.; et al. Pregnancy-induced metabolic phenotype variations in maternal plasma. J. Proteome. Res. 2014, 13, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Pere, M.C.; Etienne, M. Insulin sensitivity during pregnancy, lactation, and postweaning in primiparous gilts. J. Anim. Sci. 2007, 85, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, E.; Le Floc’h, N.; Etienne, M.; Ramaekers, P.; Seve, B.; Pere, M.C. Reduced feed intake of lactating primiparous sows is associated with increased insulin resistance during the peripartum period and is not modified through supplementation with dietary tryptophan. J. Anim. Sci. 2010, 88, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Eissen, J.J.; Apeldoorn, E.J.; Kanis, E.; Verstegen, M.W.A.; de Greef, K.H. The importance of a high feed intake during lactation of primiparous sows nursing large litters. J. Anim. Sci. 2003, 81, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.S.; Anil, L.; Deen, J.; Baidoo, S.K.; Walker, R.D. Association of inadequate feed intake during lactation with removal of sows from the breeding herd. J. Swine Health Prod. 2006, 14, 296–301. [Google Scholar]
- Sun, H.Q.; Tan, C.Q.; Wei, H.K.; Zou, Y.; Long, G.; Ao, J.T.; Xue, H.X.; Jiang, S.W.; Peng, J. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim. Reprod. Sci. 2015, 152, 55–64. [Google Scholar] [CrossRef]
- Sun, H.Q.; Zhou, Y.F.; Tan, C.Q.; Zheng, L.F.; Peng, J.; Jiang, S.W. Effects of konjac flour inclusion in gestation diets on the nutrient digestibility, lactation feed intake and reproductive performance of sows. Animal 2014, 8, 1089–1094. [Google Scholar] [CrossRef]
- Tan, C.Q.; Wei, H.K.; Sun, H.Q.; Ao, J.T.; Long, G.; Jiang, S.W.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Backhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Yu, H.; Xu, C.; Jiang, S.; Peng, J. Metabolic Syndrome During Perinatal Period in Sows and the Link With Gut Microbiota and Metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Zhao, X.; Xu, C.; Zhou, Y.; Peng, J. Soluble Fiber with High Water-Binding Capacity, Swelling Capacity, and Fermentability Reduces Food Intake by Promoting Satiety Rather Than Satiation in Rats. Nutrients 2016, 8, 615. [Google Scholar] [CrossRef]
- Gray, L.E.K.; O’Hely, M.; Ranganathan, S.; Sly, P.D.; Vuillermin, P. The Maternal Diet, Gut Bacteria, and Bacterial Metabolites during Pregnancy influence Offspring Asthma. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; de Aguero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Louis, S.; Rings, A.; Messner, S.; Camarinha-Silva, A.; Seifert, J.; Bischoff, S.C.; et al. Intestinal Microbiota and Microbial Metabolites Are Changed in a Pig Model Fed a High-Fat/Low-Fiber or a Low-Fat/High-Fiber Diet. Plos One 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Vogt, J.; Wolever, T.M.S. Intravenous acetate elicits a greater free fatty acid rebound in normal than hyperinsulinaemic humans. Eur. J. Clin. Nutr. 2012, 66, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrin. Met. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Santaren, I.D.; Watkins, S.M.; Liese, A.D.; Wagenknecht, L.E.; Rewers, M.J.; Haffner, S.M.; Lorenzo, C.; Hanley, A.J. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am. J. Clin. Nutr. 2014, 100, 1532–1540. [Google Scholar] [CrossRef]
- Jenkins, B.; West, J.A.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Hornemann, S.; Petzke, K.J.; Schulze, M.B.; Pfeiffer, A.F.H.; Klaus, S. Odd-chain fatty acids as a biomarker for dietary fiber intake: A novel pathway for endogenous production from propionate. Am. J. Clin. Nutr. 2017, 105, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, B.R.; Tuncil, Y.E. A Perspective on the Complexity of Dietary Fiber Structures and Their Potential Effect on the Gut Microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Ao, J.; Long, G.; Peng, J. Inclusion of Konjac Flour in the Gestation Diet Changes the Gut Microbiota, Alleviates Oxidative Stress, and Improves Insulin Sensitivity in Sows. Appl. Environ. Microbiol. 2016, 82, 5899–5909. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef]
- Engels, C.; Ruscheweyh, H.J.; Beerenwinkel, N.; Lacroix, C.; Schwab, C. The Common Gut Microbe Eubacterium hallii also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016, 7, 713. [Google Scholar] [CrossRef]
- Wallace, R.J.; McKain, N.; McEwan, N.R.; Miyagawa, E.; Chaudhary, L.C.; King, T.P.; Walker, N.D.; Apajalahti, J.H.; Newbold, C.J. Eubacterium pyruvativorans sp. nov.; a novel non-saccharolytic anaerobe from the rumen that ferments pyruvate and amino acids, forms caproate and utilizes acetate and propionate. Int. J. Syst. Evol. Microbiol. 2003, 53, 965–970. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Petzke, K.J.; Blaut, M.; Loh, G.; Klaus, S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J. Nutr. Biochem. 2015, 26, 929–937. [Google Scholar] [CrossRef]
- Cheng, C.S.; Wei, H.K.; Xu, C.H.; Xie, X.W.; Jiang, S.W.; Peng, J. Maternal Soluble Fiber Diet during Pregnancy Changes the Intestinal Microbiota, Improves Growth Performance, and Reduces Intestinal Permeability in Piglets. Appl. Environ. Microb. 2018, 84. [Google Scholar] [CrossRef]
- Nestel, P.J.; Straznicky, N.; Mellett, N.A.; Wong, G.; De Souza, D.P.; Tull, D.L.; Barlow, C.K.; Grima, M.T.; Meikle, P.J. Specific plasma lipid classes and phospholipid fatty acids indicative of dairy food consumption associate with insulin sensitivity. Am. J. Clin. Nutr. 2014, 99, 46–53. [Google Scholar] [CrossRef]
- Chang, S.; Cui, X.; Guo, M.; Tian, Y.; Xu, W.; Huang, K.; Zhang, Y. Insoluble Dietary Fiber from Pear Pomace Can Prevent High-Fat Diet-Induced Obesity in Rats Mainly by Improving the Structure of the Gut Microbiota. J. Microbiol. Biotechnol. 2017, 27, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hong, T.; Li, N.; Zang, B.; Wu, X. Soluble dietary fiber improves energy homeostasis in obese mice by remodeling the gut microbiota. Biochem. Biophys. Res. Commun. 2018, 498, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Relman, D.A. The human microbiome: Ecosystem resilience and health. Nutr. Rev. 2012, 70, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Handelsman, J. Microbial symbiosis: In sickness and in health. DNA Cell Biol. 2009, 28, 359–360. [Google Scholar] [CrossRef]
- Chow, J.; Lee, S.M.; Shen, Y.; Khosravi, A.; Mazmanian, S.K. Host-bacterial symbiosis in health and disease. Adv. Immunol. 2010, 107, 243–274. [Google Scholar] [CrossRef]
- Henrissat, B.; Corthier, G.; Fontaine, E.; Dore, J.; Leclerc, M. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, Y.; Zhang, P.; Li, Y.; Gui, T.T.; Wang, J.; Jin, C.; Che, L.Q.; Li, J.; Lin, Y.; et al. Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front. Microbiol. 2017, 8, 2242. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Li, N.; Zhang, X.; Zhang, G.; Yang, F.; Zeng, X.; Liu, Z.; Qiao, S. Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation. FASEB J. 2019, 33, 4490–4501. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Ji, Y.J.; Li, H.W.; Zhu, Q.; Blachier, F.; Geng, M.M.; Chen, W.; Yin, Y.L. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: Effects of food composition at different times of pregnancy. Sci. Rep. Uk 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. Fems. Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria—From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microb. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [PubMed]
- Udayappan, S.; Manneras-Holm, L.; Chaplin-Scott, A.; Belzer, C.; Herrema, H.; Dallinga-Thie, G.M.; Duncan, S.H.; Stroes, E.S.G.; Groen, A.K.; Flint, H.J.; et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbi. 2016, 2, 16009. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; Michiels, J.; De Smet, S. Dietary fiber affects intestinal mucosal barrier function by regulating intestinal bacteria in weaning piglets. Commun. Agric. Appl. Biol. Sci. 2013, 78, 71–78. [Google Scholar]
- Eltweri, A.M.; Thomas, A.L.; Fisk, H.L.; Arshad, A.; Calder, P.C.; Dennison, A.R.; Bowrey, D.J. Plasma and erythrocyte uptake of omega-3 fatty acids from an intravenous fish oil based lipid emulsion in patients with advanced oesophagogastric cancer. Clin. Nutr. 2017, 36, 768–774. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microb. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12. [Google Scholar] [CrossRef] [PubMed]


| Item | Soluble Fiber Level | Reproductive Stage | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2% | GES30 | GES109 | LAC3 | F | S | F × S | ||
| Plasma NEFA before meal, µmol/L | |||||||||
| C14:0 | 1.53 | 0.97 | 0.59 b | 1.03 b | 1.87 a | 0.18 | 0.12 | < 0.01 | 0.24 |
| C15:0 | 1.34 B | 2.34 A | 1.91 | 2.12 | 1.63 | 0.21 | 0.02 | 0.58 | 0.66 |
| C16:0 | 123.46 A | 94.91 B | 53.67 b | 133.78 a | 128.32 a | 7.80 | 0.02 | < 0.01 | 0.33 |
| C17:0 | 1.66 B | 3.26 A | 1.57 | 2.51 | 3.18 | 0.32 | 0.01 | 0.09 | 0.22 |
| C18:0 | 68.37 A | 45.98 B | 32.07 b | 65.25 a | 67.63 a | 4.84 | < 0.01 | < 0.01 | 0.02 |
| C18:1n-9 | 79.32 | 64.88 | 30.98 b | 93.19 a | 84.41 a | 6.08 | 0.17 | < 0.01 | 0.91 |
| C18:2n-6 | 172.14 | 150.03 | 64.95 c | 216.04 a | 186.15 b | 11.67 | 0.13 | < 0.01 | 0.96 |
| C20:4n-6 | 85.21 | 68.29 | 51.32 b | 82.82 a | 89.41 a | 5.37 | 0.11 | < 0.01 | 0.99 |
| C22: 6n-3 | 0.78 | 0.48 | 0.23 | 1.01 | 0.60 | 0.14 | 0.23 | 0.07 | 0.09 |
| Even-SFA | 193.36 A | 141.86 B | 86.33 b | 200.06 a | 197.82 a | 12.16 | < 0.01 | < 0.01 | 0.08 |
| OCFAs | 3.00 B | 5.60 A | 3.48 | 4.63 | 4.81 | 0.45 | < 0.01 | 0.39 | 0.54 |
| Total-NEFA | 533.80 A | 431.14 B | 237.29 b | 597.75 a | 563.20 a | 31.08 | 0.01 | < 0.01 | 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Cheng, C.; Zhang, X.; Peng, J. Inclusion of Soluble Fiber in the Gestation Diet Changes the Gut Microbiota, Affects Plasma Propionate and Odd-Chain Fatty Acids Levels, and Improves Insulin Sensitivity in Sows. Int. J. Mol. Sci. 2020, 21, 635. https://doi.org/10.3390/ijms21020635
Xu C, Cheng C, Zhang X, Peng J. Inclusion of Soluble Fiber in the Gestation Diet Changes the Gut Microbiota, Affects Plasma Propionate and Odd-Chain Fatty Acids Levels, and Improves Insulin Sensitivity in Sows. International Journal of Molecular Sciences. 2020; 21(2):635. https://doi.org/10.3390/ijms21020635
Chicago/Turabian StyleXu, Chuanhui, Chuanshang Cheng, Xiu Zhang, and Jian Peng. 2020. "Inclusion of Soluble Fiber in the Gestation Diet Changes the Gut Microbiota, Affects Plasma Propionate and Odd-Chain Fatty Acids Levels, and Improves Insulin Sensitivity in Sows" International Journal of Molecular Sciences 21, no. 2: 635. https://doi.org/10.3390/ijms21020635
APA StyleXu, C., Cheng, C., Zhang, X., & Peng, J. (2020). Inclusion of Soluble Fiber in the Gestation Diet Changes the Gut Microbiota, Affects Plasma Propionate and Odd-Chain Fatty Acids Levels, and Improves Insulin Sensitivity in Sows. International Journal of Molecular Sciences, 21(2), 635. https://doi.org/10.3390/ijms21020635





